Abstract
The systematics of several ticks species (Acari: Ixodidae) remains controversial. Many species, including those of the Amblyomma cajennense complex and Rhipicephalus sanguineus s.l., are given special attention since they are cryptic species complexes and are also important in human and veterinary medicine. The A. cajennense complex was recently reorganized into six valid species, among which Amblyomma patinoi and Amblyomma mixtum have been confirmed in Colombia. On the other hand, the taxonomic status of R. sanguineus s.l. is controversial since it is a cosmopolitan cryptic species complex with a high reproductive capacity and a broad range of hosts (including man). To address this challenge, the germ cells of male ticks display a diverse morphology that offers novel opportunities for taxonomy. This study describes the events of spermatogenesis in A. mixtum and R. sanguineus s.l. individuals collected during active feeding on domestic hosts in the department of Caldas, Colombia. The individuals were identified using dichotomous keys and through PCR amplification of a fragment of the mitochondrial 16S ribosomal DNA gene. The male reproductive systems of A. mixtum and R. sanguineus s.l. were fixed in 2.5% glutaraldehyde for 48 h and dehydrated in increasing dilutions of ethanol. The samples were then embedded and mounted in historesin to obtain sections of 3 μm that were stained with hematoxylin-eosin (HE), photographed, and visualized through optical microscopy. The results show that the morphology of mature germ cells displays excellent diagnostic traits that can be used for tick taxonomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phylogenetic relationships among tick species (Acari: Ixodidae) have been discussed for decades by several authors based on morphological, physiological, and ecological variation studies, as well as reviews based on molecular systematics and biogeographical patterns (Hoogstraal and Aeschlimann 1982; Black et al. 1997; Barker and Murrell 2002; Burger et al. 2012; Dantas-Torres and Otranto 2013; Nava et al. 2017; Zemtsova et al. 2016). Hard ticks (Acari: Ixodidae) display cryptic species complexes; for example, the Amblyomma cajennense sensu lato complex, which until recently was considered a single tick species in the New World and is distributed from the southern United States to northern Argentina (Estrada-Peña et al. 2014). Recent studies based on genetic, reproductive, and morphological data reorganized the taxon into a complex of at least six valid species: Amblyomma cajennense sensu stricto, Amblyomma mixtum, Amblyomma sculptum, Amblyomma interandinum, Amblyomma tonelliae, and Amblyomma patinoi (Beati et al. 2013; Nava et al. 2014). A. cajennense s.s. is reported in the Amazon region of South America, A. interandinum in the northern inter-Andean valley of Peru, A. mixtum from Texas (USA) to western Ecuador, A. patinoi in the Eastern Andes mountain range of Colombia, and A. tonelliae in the Chaco region that extends from north-central Argentina to Bolivia and Paraguay. Finally, A. sculptum is distributed from the humid areas of northern Argentina to the adjacent regions of Bolivia and Paraguay, as well as the coast and central-western states of Brazil (Nava et al. 2014). In Colombia, there are reports of two species of the complex, namely, A. patinoi and A. mixtum (Nava et al. 2014; Rivera-Páez et al. 2016). However, Estrada-Peña et al. (2014) showed that Colombia has a high number of environmentally suited areas for A. cajennense s.s. and some favorable areas for A. sculptum based on habitability models in the Neotropics generated for four species of the complex.
R. sanguineus s.l. is the most widely distributed tick species in the world. It was considered a single taxon for more than two centuries (Dantas-Torres 2010; Dantas-Torres et al. 2018). Currently, R. sanguineus s.l. is not a single species but a complex with a difficult species differentiation due to a poor original species description and the lack of a type specimen. This leads to a misidentification of the collected individuals worldwide (Dantas-Torres and Otranto 2015; Nava et al. 2015). In the last decades, several studies have focused on the morphological, genetic, and biological differences among the populations of R. sanguineus s.l. to prove that, in some areas, there are more than one species classified under this name (Dantas-Torres and Otranto 2015; Nava et al. 2015). For example, based on morphometric and ultrastructural analyses, Coimbra-Dores et al. (2016) identified diagnostic traits to distinguish between Rhipicephalus turanicus and R. sanguineus. In Western Europe, these species share many phenotypic traits, and they are genetically related and sympatric; therefore, these can be mistakenly identified. Additionally, Coimbra-Dores et al. (2018) reconstructed a multigene phylogeny of 24 species of Rhipicephalus from the Afrotropical and Mediterranean regions, based on mitochondrial DNA genes (COI, 12S, and 16S). This analysis allowed improving species identification by elucidating cryptic species and demonstrating the suitability of mtDNA markers; therefore, it contributed to discriminative intraspecific and interspecific analyses. Genetic and breeding experiments show the existence of at least two distinct taxa, namely, the “temperate” and “tropical” lineages of R. sanguineus s.l. (Oliveira et al. 2005; Szabó et al. 2005; Burlini et al. 2010; Moraes-Filho et al. 2011; Levin et al. 2012; Nava et al. 2012; Dantas-Torres et al. 2013; Liu et al. 2013; Hekimoğlu et al. 2016; Sanches et al. 2016; Zemtsova et al. 2016; Caetano et al. 2017). Furthermore, the presence of different lineages in the R. sanguineus s.l. complex has certain implications not only from a taxonomical perspective but also from a medical-veterinary view since only certain populations of R. sanguineus s.l. are competent in pathogen transmission (Moraes-Filho et al. 2015). In this context, species such as A. mixtum, A. sculptum, and A. patinoi and some populations of R. sanguineus s.l. deserve special attention since they are medically important as known vectors of Rickettsia rickettsii, the causal agent of Rocky Mountain spotted fever (RMSF) or Tobias fever in Colombia (Dantas-Torres 2007; Faccini-Martínez et al. 2015; Rivera-Páez et al. 2018a, b). The transmission of R. rickettsii by R. sanguineus s.l. has been reported in Arizona, Baja California (USA), Mexico, Panama, Colombia, Brazil, and Argentina (Demma et al. 2005; Nicholson et al. 2006; Hidalgo et al. 2007; Silva et al. 2017; Eremeeva et al. 2011; Martínez-Caballero et al. 2018; Foley et al. 2019).
Currently, new tools and integrative studies are sought to address the taxonomical conflicts in ticks and discern some of the taxonomical issues. In this regard, the morphology of mature male germ cells displays species-specific traits since each animal species has spermatozoa with typical traits that can be analyzed in taxonomic studies (Birkhead et al. 2009; Dallai et al. 2016; de Oliveira et al. 2019). Several studies, mainly in insects, have used the external and ultrastructure morphologies of spermatozoa to estimate evolutionary rates and assess phylogenetic relationships (Dallai et al. 2016; Ravazi et al. 2016; Baffa et al. 2017; de Oliveira et al. 2019). In tick systematics, spermiotaxonomy has also proven promising for separating Ixodidae species since the analysis of the ultrastructure of the male reproductive system and its germ cells can help to better understand the phylogeny of these species (Sampieri et al. 2016a, b; Rivera-Páez et al. 2017). This study aimed to describe the spermatogenesis in A. mixtum and R. sanguineus s.l. collected while actively feeding on domestic hosts in the department of Caldas, Colombia and to compare the events of spermatogenesis with other populations and species. This research was conducted based on the public health relevance of these species and the need to elucidate their taxonomy, as well as the fact that some species of the A. cajennense complex are in sympatry or parapatry in several areas in the USA.
Materials and methods
In total, 267 adult ticks (Acari: Ixodidae) of the species A. mixtum (74 males and 21 females) and R. sanguineus s.l. (146 males and 26 females) were collected while actively feeding on domestic hosts, including 7 cows (Bos taurus), 10 domestic dogs (Canis lupus familiaris), and 5 horses (Equus caballus) in the municipalities of Neira, Manizales, and Victoria (Caldas, Colombia).
Study area and morphological and molecular identification
The ticks were collected while actively feeding on domestic hosts, including cows (Bos taurus), domestic dogs (Canis lupus familiaris), and horses (Equus caballus), in the municipalities of Neira (05° 09′ 59″ N, 75° 31′ 08″ W), Manizales (05° 03′ 58 ″ N, 75° 29′ 05″ W), and Victoria (05° 18′ 59″ N, 74° 54′ 45″ W) in the department of Caldas, Colombia between March and December of 2018. The ticks were deposited in wet traps and taken alive to the Laboratory of Genetics, Faculty of Exact and Natural Sciences (Universidad de Caldas, Manizales, Colombia), where they were taxonomically identified according to their external morphology using a Zeiss DV4 stereomicroscope and the available literature (Jones et al. 1972; Estrada-Peña et al. 2005; Barros-Battesti et al. 2006; Martins et al. 2010; Nava et al. 2014). Also, two male and two female specimens from each municipality (Manizales, Neira, and Victoria) were prepare for scanning electron microscopy (SEM) (FEI-QUANTA 250 scanning electron microscope), according to the techniques described by Corwin et al. (1979).
Five males and five females of each species (A. mixtum and R. sanguineus s.l.) from each municipality were individually processed for molecular analyses. The DNA extraction was performed using the DNeasy Blood & Tissue Kit (Qiagen), following the manufacturer’s instructions. A 460-bp fragment was amplified from the mitochondrial 16S rDNA gene using the primer pair 16SF 5’-CTGCTCAATGATTTTTTAAATTGCTGTGG-3′ and 16SR 5’-CCGGTCTGAACTCAGATCAAGT-3′ (Norris et al. 1996). The PCR products were visualized on 1% agarose gels stained with SYBR Safe DNA gel and run in 1 × TBE pH 8.0 running buffer at 110 V/50 mA. The gel was visualized on a GelDoc-It®2310 Image (UVP) photodocumenter. PCR products were purified with the QIAquick PCR Purification Kit (Qiagen®) and sent to Macrogen Advancing Through Genomics (South Korea) for DNA sequencing. Sequences obtained were evaluated and edited with the programs Geneious Trial v8.14 (Drummond et al. 2009). The 16S rDNA gene sequences were analyzed using Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990) to determine the closest similarities with other species of the A. cajennense s.l. complex and R. sanguineus s.l.
Histological analysis
We dissected 10 male individuals of A. mixtum and R. sanguineus s.l. each and fixed the reproductive systems in 2.5% glutaraldehyde for 48 h. The tissue samples were then dehydrated in increasing concentrations of ethanol (30, 50, 70, 80, 90, and 95%) for 30 min at each concentration. The samples were included in Leica historesin (inclusion) for 2 weeks (with a refill every 3 days) and polymerized in the same historesin to obtain 3 μm sections using a LEICA RM2235 microtome. The sections were placed on glass slides, stained with hematoxylin-eosin (HE), and photodocumented on a Nikon Eclipse E200 photomicroscope. The morphology of the germ cells of A. mixtum was compared with the descriptions of A. sculptum since the latter is the only species of the A. cajennense complex described to date (Sampieri et al. 2016b). Similarly, we compared the events of spermatogenesis between the individuals of the R. sanguineus s.l. complex from Caldas (Neira, Victoria, and Manizales) and with the descriptions made by Sampieri et al. (2016a) for R. sanguineus s.l.
Results
Morphological and molecular characterizations
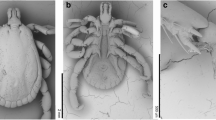
The taxonomic identification of the ticks was based on the external morphological traits of males and females, which corresponded to those of A. mixtum. According to Nava et al. (2014), very few external morphological traits can be consistently used to separate the species of the A. cajennense complex, A. mixtum – Colombia (Fig. 1a, c), and A. sculptum – Sao Paulo, Brazil (Fig. 1b, d). One of these traits is the female genital opening, which is “V” shaped in A. cajennense s.s., A. tonelliae, and A. interandinum, “U” shaped in A. mixtum and A. sculptum (Fig. 1e, f), and with short and bulging lateral flaps in A. patinoi. All the female specimens in this study showed a U-shaped genital opening (Fig. 1e). Also, as described by Nava et al. (2014), the males of A. mixtum differ from those of A. sculptum by the ornamentation and punctuations of A. sculptum (Fig. 1a, b). Likewise, we identified R. sanguineus s.l. males by traits such as an inornate scutum with festoons present, adanal plates in the ventral surface of the male, hexagonal basis capituli, and palps and hypostome of the same size (Dantas-Torres 2008; Martins et al. 2010).
Morphological analyses of the A. mixtum and A. sculptum. a, c Dorsal view of the male and female of A. mixtum (Caldas, Colombia). b, d Dorsal view of the male and female of A. sculptum (Sao Paulo, Brazil). e Genital aperture of the female of A. mixtum. f Genital aperture of the female of A. sculptum. (ga) genital aperture, (sc) scutum, (e) adjacent enamelled stripe, (fe) festoons, (h) hypostome, (pal) palps, (s) postero-median spot, (se) setae
The partial gene sequences for 16S rDNA of A. mixtum and R. sanguineus were 99% identical to the corresponding sequences available for each species in GenBank. GenBank nucleotide sequence accession numbers for the partial sequences generated in the present study are (MN365044 – MN365045) for A. mixtum and (MN396627 – MN396628) for R. sanguineus s.l.
Histological analysis
We were unable to describe the complete morphohistology of the reproductive system of the ticks collected in this study since the specimens were sampled in situ while feeding on their natural hosts, and the feeding was interrupted. In particular, the development of the reproductive system of ticks is directly related to feeding (Sampieri et al. 2014). Therefore, the germ cell development described here is spermatogenesis, which is the final stage of a series of morphological changes of the spermatids until they become a mature spermatozoon.
Spermiogenesis of A. mixtum
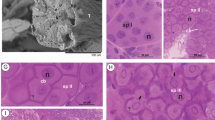
The male reproductive system of A. mixtum showed a basic morphology similar to other species of the genus (Sampieri et al. 2016b; Rivera-Páez et al. 2017) (Fig. 2). We could not observe if the testicles were connected to the distal region; however, the germ cells were organized into lined packages with a simple epithelium throughout the testicles until a certain stage of the spermiogenesis. We observed five morphologically distinct spermatids during spermiogenesis. Spermatids I (spI) are grouped in spermatocytes that display large nuclei and no cell limits evident, and spermatids II (spII) begin to show the formation of cell limits, and the presence of nucleoli within the cell nuclei is clearly observed (Fig. 2a). Spermatids III (spIII) display clear cell limits pentagonal in shape, a laterally located nucleus, and the presence of a filamentous structure in the anterior region, strongly stained by hematoxylin (acrosomal vesicle). We also observed cisternae bound to the cell membrane (Fig. 2b). Spermatids IV (spIV) undergo the marked morphological changes of spIII, including cell lengthening, high condensation of the nuclear material, fusion of the membranous cisternae, the development of the acrosomal vesicle, and the formation of the operculum (Fig. 2c). The attributes of the germ cells during the first four stages match the descriptions for other species of the genus Amblyomma (Sampieri et al. 2016b; Rivera-Páez et al. 2017). However, the last developmental stage of the germ cells, spermatids V (spV), shows marked differences among the study species (Fig. 2d). We compared the spermatogenesis of A. mixtum with A. sculptum (Sampieri et al. 2016b) since it is the only species of the complex described to date. We found differences regarding the mature spermatozoa (spV), such as the shape of the operculum, which as larger and more extended in A. mixtum compared with A. sculptum (Fig. 2d, e). Likewise, the shape of the nucleus in A. mixtum was helicoidal, while in A. sculptum, it was filiform (Fig. 2d, e). Therefore, in terms of the internal cell morphology, we propose a comparative scheme for stage spV between species (Fig. 2f, g) since the spV of A. mixtum is different from A. sculptum.
Spermatogenesis of A. mixtum. a Spermatids I and II. b Spermatids III. c Spermatids IV showing cell lengthening and the nuclear process. d Spermatids V with the helicoidal nucleus (n) of A. mixtum. e Spermatids V with the linear nucleus (n) of A. sculptum (Sampieri et al. 2016b). f SpV scheme of A. mixtum; g SpV scheme of A. sculptum. (av) acrosomal vesicle, (c) cisternae, (ep) epithelium, (n) nucleus, (op) operculum, (sc) spermatocytes, (spI) spermatid I, (spII) spermatid II, (spIII) spermatid III, (spIV) spermatid IV, (spV) spermatid V, (T) testicle
Spermiogenesis in R. sanguineus s.l.
Our observations confirmed the description made by Sampieri et al. (2016a) for R. sanguineus s.l. The first stage of spermiogenesis in ticks (spI) consists of cells with large nuclei and no cell limits evident, protected within the spermatocytes (Fig. 3a). Spermatids II (spII) have large rounded nuclei with clear cell limits and cytoplasmic bridges (between two or more cells) (Fig. 3c). In spermatids III (spIII), we observed cisternae along the cell limits, and the cells showed a circular and pentagonal shape in the periphery (Fig. 3b). Spermatids IV (spIV) undergo expressive morphological changes and are no longer organized in spermatocytes. SpIV are lengthened and the membranous cisternae are fused. In this stage, the nucleus shows the shape of a half-moon and is located in the anterior region of the cell (Fig. 3d). The last stage, spermatids V (spV), consists of filiform cells with head-like anterior regions and tail-like posterior regions (Fig. 3e–h). The anterior region contains the operculum with a rim around the base. We also observed fusion of membranous cisternae along the cell limits. The cells in this stage showed relevant differences between the R. sanguineus s.l. individuals from the different study areas of Caldas (Manizales, Neira, and Victoria) regarding traits such as the shape of the nucleus, size of the operculum, and shape of the posterior region of the mature spermatozoon (Fig. 3f). Accordingly, we propose a comparative scheme for stage spV between the R. sanguineus s.l. individuals from each municipality (Fig. 3i–k).
Spermatogenesis of R. sanguineus s.l. a Spermatids I grouped into spermatocytes (circle). b Spermatids III. c Spermatids II. d Spermatids IV during the cell lengthening (arrow), including the nuclear process (n). e Spermatids V (Neira). f Posterior region of Spermatids V (Neira). g Spermatids V (Victoria). h Spermatids V (Manizales). i SpV scheme (Neira). j SpV scheme (Victoria). k SpV scheme (Manizales). (c) cisternae, (ep) epithelium, (mc) membranous complex, (n) nucleus, (op) operculum, (pr) posterior region, (sc) spermatocyte, (spI) spermatid I, (spII) spermatid II, (spIII) spermatid III, (spIV) spermatid IV, (spV) spermatid V, (T) testicle
Discussion
The taxonomic analysis provides important information for the phylogeny of ticks of the A. cajennense and R. sanguineus s.l. complexes, given the constant taxonomic controversies and revalidations in recent years. The external morphology of A. mixtum agreed with the descriptions made by Nava et al. (2014). Also, the external morphology of R. sanguineus s.l. corresponded to the one proposed by Estrada-Peña et al. (2005).
Currently, spermiotaxonomy is considered a promising tool for distinguishing Ixodidae species, and the phylogeny of this group is improved by the analysis of the ultrastructure of the male reproductive system and germ cells (Sampieri et al. 2016a, b; Rivera-Páez et al. 2017). Rivera-Páez et al. (2017) reported the first cladogram for the genus Amblyomma based on morphological traits of the male reproductive system and germ cells in several species, which provided valuable phylogenetic information. This study complements the previous research by demonstrating that the mature spermatozoa (spV) of the analyzed individuals show distinct morphological traits that provide relevant information for separating the species of the A. cajennense complex and the genus Amblyomma. Furthermore, we describe two novel traits that complement the list of diagnostic characters in tick spermiotaxonomy, including the shape of the tail end piece and disposition of the acrosomal vesicle throughout the spermatozoon (Fig. 1e, d). We clarify that this study compared the morphology of germ cells of A. mixtum and A. sculptum since these are cryptic species and display a sympatric distribution (Nava et al. 2014; Estrada-Peña et al. 2014). However, it would be important to study the morphological plasticity of male germ cells of the other species of the A. cajennense complex to guarantee that these differences are fixed traits.
For R. sanguineus s.l., we found differences regarding the shape and size of the operculum, the shape of the nuclear process, and the shape of the tail end piece of mature germ cells in ticks collected from the different municipalities (Neira, Victoria, and Manizales) of Caldas, Colombia. Several authors report that in Colombia, as in other tropical countries of the USA, there is a single species of the R. sanguineus complex (Nava et al. 2012; Nava et al. 2018). Therefore, to date, it is not possible to infer that germ cell morphology is useful for discriminating the members of this complex at the interspecific level. However, the morphological differences of male germ cells found in this study are supported by variations in the shape of the capitulum, genital opening, and spiracular plates of the specimens of R. sanguineus analyzed here (data not shown).
Nonetheless, a robust spermiotaxonomy analysis requires broader sampling and experimentation (to include all species of the A. cajennense complex and R. sanguineus from different regions), as well as controlling the feeding times of the ticks on the vertebrate hosts. The analysis of male germ cells, however, is a useful tool that complements phylogenetic studies in ticks. This is especially relevant for studies on cryptic species complexes for which DNA analysis is often ambiguous or inconclusive.
Also, as previously mentioned, the species of the A. cajennense and R. sanguineus complexes are vectors of disease-causing pathogens of medical and veterinary importance, such as Rickettsia spp. For this reason, accurate species discrimination is crucial not only to gain better knowledge on their phenology, taxonomy, and ecology but also to specifically and better understand the ecology of tick-borne pathogens, their transmission dynamics, epidemiology, and control of tick-borne diseases (Coimbra-Dores et al. 2016).
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Baffa AF, Camara DCP, Santos-Mallet JR, da Silva ER, Costa J, Freitas SP (2017) Sperm dimorphism in the Triatoma brasiliensis species complex and its applications. Med Vet Entomol 31:192–199
Barker SC, Murrell A (2002) Phylogeny, evolution and historical zoogeography of ticks: A review of recent progress. Exp Appl Acarol. 28:55–68
Barros-Battesti DM, Arzua M, Bechara GH, 2006 Carrapatos de importância médico veterinária da Região Neotropical: um guia ilustrado para identificação de espécies. Vox/ICTTD-3/Butantan, São Paulo 223 p.
Beati L, Nava S, Burkman EJ, Barros-Battesti DM, Labruna MB, Guglielmone AA, Faccini JL (2013) Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: Phylogeography and evidence for allopatric speciation. BMC Evol Biol 13:267
Birkhead TR, Hosken DJ, Pitnick S (2009) Sperm biology: an evolutionary perspective. Anim Biol 59:457–458
Black WC, Klompen JSH, Keirans JE (1997) Phylogenetic relationships among tick subfamilies (ixodida: ixodidae: argasidae) based on the 18S nuclear rDNA gene. Mol Phylogenet Evol 7:129–144
Burger TD, Shao R, Beati L, Miller H, Barker SC (2012) Phylogenetic analysis of ticks (Acari: Ixodida) using mitochondrial genomes and nuclear rRNA genes indicates that the genus Amblyomma is polyphyletic. Mol Phylogenet Evol 64:45–55
Burlini L, Teixeira KRS, Szabó MPJ, Famadas KM (2010) Molecular dissimilarities of Rhipicephalus sanguineus (Acari: Ixodidae) in Brazil and its relation with samples throughout the world: is there a geographical pattern? Exp Appl Acarol 50:361–374
Caetano RL, Vizzoni VF, Bitencourth K, Carriço C, Sato TP, Pinto ZT, Gazeta GS (2017) Ultrastructural morphology and molecular analyses of tropical and temperate “species” of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in Brazil. J Med Entomol 54:1201–1212
Coimbra-Dores MJ, Nunes T, Dias D, Rosa F (2016) Rhipicephalus sanguineus (Acari: Ixodidae) species complex: morphometric and ultrastructural analyses. Exp Appl Acarol. 70(4):455–468
Coimbra-Dores MJ, Maia-Silva M, Marques W, Oliveira AC, Rosa F, Dias D (2018) Phylogenetic insights on Mediterranean and Afrotropical Rhipicephalus species (Acari: Ixodida) based on mitochondrial DNA. Exp Appl Acarol. 75(1):107–128
Corwin D, Clifford CM, Keirans JE (1979) An improved method for cleaning and preparing ticks for examination with the scanning electron microscope. J MedEntomol 16:352–353
Dallai R, Gottardo M, Beutel RG (2016) Structure and evolution of insect sperm: new interpretations in the age of phylogenomics. Annu Rev Entomol 61:1–23
Dantas-Torres F (2007) Rocky Mountain spotted fever. Lancet Infect Dis 7:724–732
Dantas-Torres F (2008) The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 152:173–185
Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors 3:26
Dantas-Torres F, Otranto D (2013) Species diversity and abundance of ticks in three habitats in southern Italy. Ticks Tick Borne Dis 4:251–255
Dantas-Torres F, Otranto D (2015) Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol 208:9–13
Dantas-Torres F, Latrofa MS, Annoscia G, Giannelli A, Parisi A, Otranto D (2013) Morphological and genetic diversity of Rhipicephalus sanguineus sensu lato from the New and Old Worlds. Parasit Vectors 6:1–17
Dantas-Torres F, Latrofa MS, Ramos RAN, Lia RP, Capelli G, Parisi A, Otranto D (2018) Biological compatibility between two temperate lineages of brown dog ticks, Rhipicephalus sanguineus (sensu lato). Parasit Vectors 11:1–10
de Oliveira MLR, Camara DCP, Freitas SPC, Santos-Mallet JR (2019) Spermatological morphology of Triatoma species (Hemiptera: Reduviidae: Triatominae). J Med Entomol 56(4):959–966
Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH (2005) Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 353:587–594
Drummond AJ, Ashton B, Cheung M, Heled J, Kearse M, Moir R, Thierer T, Wilson A, 2009 Geneious 8. pp. 14. p. disponible en: https://www.geneious.com
Eremeeva ME, Zambrano ML, Anaya L, Beati L, Karpathy SE, Santos-Silva MM, Salceda B, Macbeth D, Olguin H, Dasch GA, Aranda CA (2011) Rickettsia rickettsii in Rhipicephalus ticks, Mexicali, Mexico. J Med Entomol 48:418–421
Estrada-Peña A, Venzal JM, Mangold AJ, Cafrune MM, Guglielmone AA (2005) The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst Parasitol 60:99–112
Estrada-Peña A, Tarragona EL, Vesco U, de Meneghi D, Mastropaolo M, Mangold AJ, Nava S (2014) Divergent environmental preferences and areas of sympatry of tick species in the Amblyomma cajennense complex (Ixodidae). Int J Parasitol 44:1081–1089
Faccini-Martínez ÁA, Costa FB, Hayama-Ueno TE, Ramírez-Hernández A, Cortés-Vecino JA, Labruna MB, Hidalgo M (2015) Rickettsia rickettsii in Amblyomma patinoi ticks. Colombia Emerg Infect Dis 21:537–539
Foley J, Tinoco-Gracia L, Rodriguez-Lomelí M, Estrada-Guzmán J, Fierro M, Mattar-Lopez E, Peterson A, Pascoe E, Gonzalez Y, Hori-Oshima S, Armstrong PA, Lopez G, Jacome-Ibarra M, Paddock CD, Zazueta OE (2019) Unbiased assessment of abundance of Rhipicephalus sanguineus sensu lato ticks, canine exposure to spotted fever group Rickettsia, and risk factors in Mexicali, México. Am J Trop Med Hyg 101:22–32
Hekimoğlu O, Sağlam İK, Özer N, Estrada-Peña A (2016) New molecular data shed light on the global phylogeny and species limits of the Rhipicephalus sanguineus complex. Ticks Tick Borne Dis 7:798–807
Hidalgo M, Orejuela L, Fuya P, Carrillo P, Hernandez J, Parra E, Keng C, Small M, Olano JP, Bouyer D, Castaneda E, Walker D, Valbuena G (2007) Rocky mountain spotted fever, Colombia. Emerg Infect Dis 13:1058–1060
Hoogstraal H, Aeschlimann A (1982) Tick-host specificity. Bulletin de La Societe Entomologique Suisse 5:5–32
Jones EK, Clifford CM, Keirans JE, Kohls GM (1972) The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young University Science Bulletin-Biological Series 17:1–40
Levin ML, Studer E, Killmaster L, Zemtsova G, Mumcuoglu KY (2012) Crossbreeding between different geographical populations of the brown dog tick, Rhipicephalus sanguineus (Acari: Ixodidae). Exp Appl Acarol. 58:51–68
Liu GH, Chen F, Chen YZ, Song HQ, Lin RQ, Zhou DH, Zhu XQ (2013) Complete mitochondrial genome sequence data provides genetic evidence that the brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) represents a species complex. Int J Biol Sci 9:361–369
Martínez-Caballero A, Moreno B, González C, Martínez G, Adames M, Pachar JV, Varela-Petrucelli JB, Martínez-Mandiche J, Suárez JA, Domínguez L, Zaldívar Y, Bermúdez S (2018). Descriptions of two new cases of Rocky Mountain spotted fever in Panama, and coincident infection with Rickettsia rickettsii in Rhipicephalus sanguineus sl in an urban locality of Panama City, Panama Epidemiol Infect 146:875–878
Martins TF, Onofrio VC, Barros-Battesti DM, Labruna MB (2010) Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: descriptions, redescriptions, and identification key. Ticks Tick Borne Dis 1:75–99
Moraes-Filho J, Marcili A, Nieri-Bastos FA, Richtzenhain LJ, Labruna MB (2011) Genetic analysis of ticks belonging to the Rhipicephalus sanguineus group in Latin America. Acta Trop 117:51–55
Moraes-Filho J, Krawczak FS, Costa FB, Soares JF, Labruna MB (2015) Comparative evaluation of the vector competence of four South American populations of the Rhipicephalus sanguineus group for the bacterium Ehrlichia canis, the agent of canine monocytic ehrlichiosis. PLoS One 10(9):e0139386
Nava S, Mastropaolo M, Venzal JM, Mangold AJ, Guglielmone AA (2012) Mitochondrial DNA analysis of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) in the Southern Cone of South America. Vet Parasitol 190:547–555
Nava S, Beati L, Labruna MB, Cáceres AG, Mangold AJ, Guglielmone AA (2014) Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1. Ticks Tick Borne Dis 5:252–276
Nava S, Estrada-Peña A, Petney T, Beati L, Labruna MB, Szabó MPJ, Guglielmone AA (2015) The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet Parasitol 208:2–8
Nava S, Venzal JMM, González-Acuña DG, Martins TFF, Guglielmone AA, (2017) Ticks of the Southern Cone of America: diagnosis, distribution, and hosts with taxonomy, ecology and sanitary importance. In ticks of the Southern Cone of America: diagnosis, distribution, and hosts with taxonomy, ecology and sanitary importance (1st). San Diego, Cambridge: Elsevier, London.
Nava S, Beati L, Venzal JM, Labruna MB, Szabó MP, Petney T, Saracho-Bottero MN, Tarragona EL, Dantas-Torres F, Santos MM, Mangold AJ, Guglielmone AA, Estrada-Peña A (2018) Rhipicephalus sanguineus (Latreille, 1806): Neotype designation, morphological re-description of all parasitic stages and molecular characterization. Ticks Tick Borne Dis 9(6):1573–1585
Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D (2006) Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann Ny Acad Sci 1078: 338–341
Norris DE, Klompen JS, Keirans JE, Black WC (1996) Population genetics of Ixodes scapularis (Acari: Ixodidae) based on mitochondrial 16S and 12S genes. J Med Entomol 33:78–89
Oliveira PR, Bechara GH, Denardi SE, Saito KC, Nunes ET, Szabó MPJ, Mathias MIC (2005) Comparison of the external morphology of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks from Brazil and Argentina. Vet Parasitol 129:139–147
Ravazi A, Oliveira J, Rosa JA, Azeredo-Oliveira MTV, Alevi KCC (2016) Spermiotaxonomy of the tribe Rhodniini (Hemiptera, Triatominae). Genet Mol Res 15(1). https://doi.org/10.4238/gmr.15017366
Rivera-Páez FA, Labruna MB, Martins TF, Sampieri BR, Camargo-Mathias MI (2016) Amblyomma mixtum Koch, 1844 (Acari: Ixodidae): First record confirmation in Colombia using morphological and molecular analyses. Ticks Tick Borne Dis 7:842–848
Rivera-Páez FA, Sampieri BR, Labruna MB, da Silva Matos R, Martins TF, Camargo-Mathias MI (2017) Comparative analysis of germ cells and DNA of the genus Amblyomma: adding new data on Amblyomma maculatum and Amblyomma ovale species (Acari: Ixodidae). Parasitol Res 116:2883–2892
Rivera-Páez FA, Labruna MB, Martins TF, Perez JE, Castaño-Villa GJ, Ossa-López PA, Camargo-Mathias MI (2018a) Contributions to the knowledge of hard ticks (Acari: Ixodidae) in Colombia. Ticks Tick Borne Dis 9:57–66
Rivera-Páez FA, Martins TF, Ossa-López PA, Sampieri BR, Camargo-Mathias MI (2018b) Detection of Rickettsia spp. in ticks (Acari: Ixodidae) of domestic animals in Colombia. Ticks Tick Borne Dis 9:819–823
Sampieri BR, Labruna MB, Bueno OC, Camargo-Mathias MI (2014) Dynamics of cell and tissue genesis in the male reproductive system of ticks (Acari: Ixodidae) Amblyomma cajennese (Fabricius, 1787) and Amblyomma aureolatum (Pallas, 1772): A comparative analysis. Parasitol Res 113:1511–1519
Sampieri BR, Calligaris IB, da Silva Matos R, Rivera-Páez FA, Bueno OC, Camargo-Mathias MI (2016a) Comparative analysis of spermatids of Rhipicephalus sanguineus sensu lato (Ixodidae) and Ornithodoros rostratus ticks (Argasidae): morphophysiology aimed at systematics. Parasitol Res 115:735–743
Sampieri BR, Moreira JCS, Páez FAR, Camargo-Mathias MI (2016b) Comparative morphology of the reproductive system and germ cells of Amblyomma ticks (Acari: Ixodidae): A contribution to Ixodidae systematics. JMAU. 4:95–107
Sanches GS, Évora PM, Mangold AJ, Jittapalapong S, Rodriguez-Mallon A, Guzmán PEE, Camargo-Mathias MI (2016) Molecular, biological, and morphometric comparisons between different geographical populations of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae). Vet Parasitol 215:78–87
Silva AB, Duarte MM, da Costa Cavalcante R, de Oliveira SV, Vizzoni VF, de Lima Duré AÍ, de Melo Iani FC, Machado-Ferreira E, Gazêta GS (2017) Rickettsia rickettsii infecting Rhipicephalus sanguineus sensu lato (Latreille 1806), in high altitude atlantic forest fragments, Ceará state, Brazil. Acta Trop 173:30–33
Szabó MPJ, Mangold AJ, João CF, Bechara GH, Guglielmone AA (2005) Biological and DNA evidence of two dissimilar populations of the Rhipicephalus sanguineus tick group (Acari: Ixodidae) in South America. Vet Parasitol 130:131–140
Zemtsova GE, Apanaskevich DA, Reeves WK, Hahn M, Levin ML (2016) Phylogeography of Rhipicephalus sanguineus sensu lato and its relationships with climatic factors. Exp Appl Acarol. 69:191–203
Acknowledgments
We would like to thank the Vicerrectoría de Investigaciones y Posgrados (Universidad de Caldas), Colombia and Colciencias Convocatoria 775 Joven investigador, 2017.
Funding
The project was funded by COLCIENCIAS (code 112777758193); execution contract: 858 of 2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors have no conflict of interest to declare.
Statement of informed consent/Human and animal rights and informed consent
We requested signed informed consent from the animal owner or land administrator prior to collecting the ectoparasite samples. Also, this research was conducted under the “Framework permit granted to Universidad de Caldas by the Autoridad Nacional de Licencias Ambientales (ANLA) of Colombia, according to the Resolution 02497 of December 31st of 2018” and the “Approval of the Bioethics Committee of the Faculty of Exact and Natural Sciences – Universidad de Caldas (June 2nd of 2017).
Additional information
Section Editor: Neil Bruce Chilton
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ospina-Pérez, E.M., Mancilla-Agrono, L.Y. & Rivera-Páez, F.A. Germ cells: a useful tool for the taxonomy of Rhipicephalus sanguineus s.l. and species of the Amblyomma cajennense complex (Acari: Ixodidae). Parasitol Res 119, 1573–1582 (2020). https://doi.org/10.1007/s00436-020-06662-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06662-y