Abstract
The phylogenetic relationships among tick species (Acari: Ixodida) have been revisited by several researchers over the last decades. Two subfamilies, Rhipicephalinae (Ixodidae) and Ornithodorinae (Argasidae), deserve special attention. The male reproductive system morphology, as well as the ultrastructure of the germ cells, may provide important information for phylogeny and systematics of metazoan groups, with spermatozoa exhibiting characters that can be used for this purpose. With that information in mind, this study aimed at evaluating, through a comparative analysis, the morphology of the male reproductive systems and germ cells of ticks species Rhipicephalus sanguineus and Ornithodoros rostratus. In order to do that, histology and scanning electron microscopy techniques were used. The results have shown that despite the similarities in the general morphology of the male reproductive system among studied Ixodida so far, there are morphological differences among the species studied herein, mainly the U-shaped testis (ancestral character) in O. rostratus and the pair testes (derived character) in R. sanguineus, and the general morphology of germ cells (spermatids V). Besides that, the morphological changes observed during the spermiogenesis appear to be different between the species studied here, probably characterizing the two families considered. The data generated in this study showed the importance of comparative internal morphology studies, mainly in regard to spermatology, despite the morphological data obtained herein not being enough to product a cladogram (sperm cladistics), it was already possible to observe clear differences among families Argasidae and Ixodidae in regard to the organization of their male reproductive systems and concerning the external morphology of spermatids. Data yet to be obtained through transmission electron microscopy techniques will allow the application of spermiocladistics and spermiotaxonomy as tools for tick systematics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phylogenetic relationships among tick species (Acari: Ixodida) have been reviewed by several researchers since the work published by Hoogstraal and Aeschlimann (1982), who proposed their phylogeny based on morphological characters and on the tick/host specificity, to the most recent reviews, which were based on their molecular systematics (Barker and Murrell 2002; Black et al. 1997; Burguer et al. 2012).
Two subfamilies, Rhipicephalinae (Ixodidae) and Ornithodorinae (Argasidae), deserve special attention. Rhipicephalinae includes polymorphic species of medical and veterinary importance, and the second one due to the fact it is not exactly known whether it is a taxonomically valid group (Black and Piesman 1994; Black et al. 1997, Dantas-Torres and Otranto 2015; Nava et al. 2015; Politi et al. 2013; Remedio et al. 2015).
Within the Rhipicephalinae, Rhipicephalus sanguineus (Latreille, 1806) is an important species which parasite domestic animals in urban and peripheral areas with a wide geographical distribution (Dantas-Torres 2010). Its taxonomic status, however, has been questioned, firstly due to the fact that its holotype was lost, and secondly, for its shallow and non-illustrated first description, which led to an erroneous identification of the individuals collected around the world. Because of that, R. sanguineus sl nowadays does not represent one species, but a complex of species (Dantas-Torres and Otranto 2015; Nava et al. 2015).
The representatives in the second subfamily mentioned herein (Ornithodorinae) have recently been more usual targets for phylogenetic reviews. Once, in the past, more relevance was given to studies with Ixodidae; there is still a knowledge void to be filled on Argasidae groups, including the Ornithodorinae species (Estrada-Peña et al. 2010). Thus, recent observations have been demonstrating that the variety of species in this groups and their geographical distribution is very different than what it was thought to be, and so is the new evidence on the medical and veterinary importance of those ectoparasites in the transmission of viruses and bacteria to livestock and humans (Ribeiro et al. 2013; Dantas-Torres et al. 2012; Hubálek and Rudolf 2012; Ravaomanana et al. 2010; Reck et al. 2013; Tavassoli et al. 2012).
Their male reproductive system morphology, as well as the ultrastructure of their germ cells, may provide important information for their phylogenetic systematics, as demonstrated by studies with insects, arachnids, and mites (Alberti et al. 2008; Dallai et al. 2005; Michalik et al. 2004). Many researchers assure that mature male germ cell morphology have species-specific characteristics; that is, each animal species has spermatozoa with typical characters, the reason by which they can be analyzed in taxonomic studies (Birkhead et al. 2009).
With that information in mind, this study aimed at studying, through a comparative analysis, the morphology of the male reproductive systems and the ultramorphology of germ cells in species R. sanguineus sl and Ornithodoros rostratus, and also at characterizing how the spermiogenesis process takes place, in order to identify possible characters for future taxonomic studies, and to confirm sperm taxonomy as an important tool to understand the systematics of Ixodida.
Materials and methods
Ticks
In order to conduct this study, 10 couples of R. sanguineus sl were used (10 males and 10 females). They were kept in a colony at the Biology Department of Unesp Rio Claro, SP state, Brazil. Rabbits (White New Zealand—Botucatu Variety), which had never been infested before, received the ticks infestation. The males were collected from the host after feeding for 6 days.
The individuals of species O rostratus were also placed on rabbits (White New Zealand—Botucatu Variety) which had never been infested before. The ticks were placed on the rabbits at their nymphal stage. After they molted into their adult stage, 10 males in the fasted state were collected.
Afterwards, males from each species were anesthetized through thermal shock in a freezer under −1 °C of temperature for 5 min, dissected in a petri dish with saline solution in PBS buffer. Then, their reproductive systems were taken out and fixed so that microscopy techniques could be applied.
This study was approved by the Ethical Committee in Animal Use (Comitê de Ética de Uso Animal-CEUA) at the Instituto de Biociências, UNESP, Rio Claro-SP, Brazil, under process number 017/2012 and protocol 1422.
Histology
The male reproductive systems of five individuals of each species were fixed in 2.5 % glutaraldehyde for 48 h and dehydrated in rising ethanol concentrations (70, 80, 90, and 95 %)—15 min in each concentration. After that, samples were embedded in Leica historesin (embedding) for 72 h, and then polymerized in this historesin, to later section of the blocks (3 μm) in microtome. The sections were then collected in glass slides and stained with hematoxylin-eosin (HE), to be photographed later by a DM750 Leica Microscope.
Scanning electron microscopy
The male reproductive systems of five individuals from each species were fixed in 2.5 % glutaraldehyde for 48 h. Then, the material was dehydrated in rising acetone concentrations (80, 85, 90, 95 %, and 100 %) in 5-min baths for each concentration. The systems were immersed twice in the 100 % concentration, for equal amounts of time. After the Critical Point Drying desiccation, the specimens were bonded with double-coated tape to aluminum brackets, plated with gold through sputtering, examined, and photographed in a Hitachi TM3000 scanning electron microscope.
Results
Rhipicephalus sanguineus sl
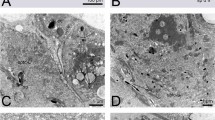
The male reproductive system in that species has a multilobed accessory gland complex that is located anteriorly to the body of the animal, and antero-dorsally are the seminal vesicles, which are connected to the vasa deferentia. These are connected to the independent testicles (right and left) with tubular shapes, which extend laterally to the tick body, increasing in diameter the more distal to the glandular complex they go (Fig. 1a).
Morphology and histology of the reproductive system and germ cells of Rhipicephalus sanguineus sl males. a Scanning photomicrograph of the reproductive system, comprising the glandular complex (GC), seminal vesicles (asterisk), and testicles (T); b-c Histological sections with an overview of the reproductive system, in which the glandular complex lobes and the testicles that are full of spermatids in different stages can be observed; d Cross-section of the proximal region of the testicle; e Spermatids I (spI); f-g Spermatids II (spII); h Spermatids III (spIII); i-j Spermatids IV (spIV); k-m Spermatids V (spV) showing the operculum without a rim in its base. c cisternae, sc spermatocyst, ep epithelium, DLL dorsolateral lobe, mc membranous complex, np nuclear process, n nucleus, op operculum, PDL posterodorsal lobe; r rim, SV seminal vesicle. Arrow indicates acrosomal vesicle
Throughout the testicles, the presence of the developing germ cells is observed. In their proximal region are the initial spermiogenesis stages, and in the distal region, the advanced stages of that process. In the seminal vesicles are the germ cells in their last stage, ready for mating (Fig. 1b, c).
The germ cells observed herein are spermatids I, the first stage of ticks spermiogenesis (spI); that is, they have already undergone the cell division processes (mitosis and meiosis). Those cells, sheltered inside the spermatocysts, have large nuclei, and no cell limits evident (Fig. 1d, e).
The spermatids II (spII) are located in the median region of the testes, and they are characterized for having large and round nuclei and also for having clear cell limits. Besides that, among cells in that stage, cytoplasmic bridges (between two or more cells) are noticed. Sets of cells are sheltered within spermatocysts (Fig. 1f, g).
The spermatids at stage III (spIII) are located more distally in the testicles. The presence of cisternae can be noticed in the cell limit, building up in its internal side. Besides that, the SpIII have an oval shape with chromatin in its periphery and a filamentous structure that is stained by hematoxylin, and it is located in the cytoplasm, anteriorly to the nucleus, next to the cell limit, herein called acrosomal vesicle (Fig. 1h).
The spermatids, now at stage IV (spIV), undergo expressive morphological changes, not being organized in spermatocysts anymore, going through lengthening, and suggesting the fusion of membranous cisternae. The acrosomal vesicle is not so evident anymore, and the nucleus, at this stage, is shaped like a half-moon, and it is located in the anterior region of the cell (Fig. 1i, j).
The last stage is the one of spermatids V (spV), observed in the most distal region of the testicles, and in the seminal vesicles. Scanning microscopy shows that those cells are filiform, with their anterior regions resembling heads and their posterior regions resembling tails. In the anterior region is the operculum, which does not have a rim around its base. Applying histological techniques, it is possible to notice the fusion of the cisternae into a membranous complex which runs along the cell limit. The nuclear process can also be observed with a screw-shaped aspect, located between the membranous complex and the cell limit (Fig. 1k, l, m).
Ornithodoros rostratus
The male reproductive system of O. rostratus has a multilobed accessory gland complex that is located anteriorly to the tick’s body. Dorsally, two large lobes are observed to be arranged parallel to one another (ADL). No seminal vesicles are observed. The testicle is observed as a single horse-shoe-shaped organ (Fig. 2a, b).
Morphology and histology of the reproductive system and germ cells of Ornithodoros rostratus males. a Scanning photomicrograph of the reproductive system, comprising the glandular complex (GC), and testicles (T); b-c Histological section with an overview of the reproductive system, in which the gland complex lobes and the testicles that are full of spermatids can be observed; c Spermatids I (spI); d Spermatids II (spII); e Spermatids III (spIII); f Spermatids IV (spIV); g-h Scanning photomicrograph of a spermatid V (spV) showing the operculum and the rim in its base. i Spermatids V. ADL anterodorsal lobe, c cisternae, sc spermatocyst, ep epithelium, mc membranous complex, np nuclear process, n nucleus, op operculum, PDL posterodorsal lobe, r rim. Arrow indicates acrosomal vesicle. Circle indicates nucleus
In the proximal region of the testicle, the germ cells are in more advanced development stages. In the distal portion are the cells in the early spermiogenesis stages (Fig. 2b). The spI in that species are globe-like cells that are found to be compacted inside the spermatocysts, with clear cell limits, large and round nuclei, and heterogeneous cytoplasm, due to the presence of granulation (Fig. 2c).
The next stage, the spII, is characterized by the formation of membranous cisternae which initially occupy the whole cell perimeter, and then they are grouped in the cell pole that is opposite to the nucleus, assuming a very peculiar shape. The nucleus is barely observed, suggesting that at this stage, this organelle loses its covering, and only some of the chromatin is stained. The acrosomal filament can be observed next to the nucleus. The cytoplasm, in a general way, has coarser granules, and, therefore, more evident (Fig. 2d).
In spIII, the cisternae, undergoing the fusion process, still occupy one pole of the cell and display subtriangular shape. The nucleus, the acrosomal filament, and the cytoplasm display the same characteristics of the previously described stage (Fig. 2e).
SpIV cells are under a lengthening process. At this stage, the cisternae have already fused, and they are now part of the membranous complex, which also follows the lengthening of the cell. At that time, the cell as a whole is hypertrophied, with a long cytoplasm area that is present between the cell limit and the membranous complex. The very small nucleus that is located in one of the poles is barely observed in most cells at this stage, but when it is, its color is mixed with the one of the cytoplasm (Fig. 2f).
The cells at the last spermiogenesis stage are found next to the accessory gland complex. They are long and, in their anterior regions, they have an operculum with a rim in its border. Next to the operculum, between the membranous complex and the cell limit, is the nuclear process, also lengthened and heavily stained by hematoxylin (Fig. 2g, h, i).
Discussion
Studies on the male reproductive system, germ cells, and the spermiogenesis process in ticks are scarce and limited to some species of small geographical distribution. Also, most published articles are outdated, which compromises image quality. Besides that, there are no comparative studies on the phylogeny and taxonomy of tick groups.
Still, available literature’s data enable some comparison and inference on the relationship between morphological data and the systematics of the group under study.
In this study, comparing the morphology of male reproductive system of two tick species that are classified in different families, it was possible to find some important characters. In R. sanguineus sl, two seminal vesicles were observed, and they were arranged parallel to and on the dorsal region of the accessory gland complex. In O. rostratus, in turn, the vesicles were not observed. In other Ixodidae species, these organs occupy the same region in the reproductive system as R. sanguineus sl (Anholeto et al. 2014; Sampieri et al. 2014). The last only vary in size, as they depend on the volume of germ cells that are in the lumen; in turn, for members of the Argasidae family, when they are observed, they are located ventrally to and below the gland complex (Roshdy 1961; Sonenshine 1970).
The morphological variation of tick testicles may also be considered important characters that can be used in taxonomy and in phylogeny. In species, in the Ixodidae family, testicles occur in pairs, and the right and the left ones are individualized—that is, they are not connected to each other. That pattern was also observed in Dermacentor andersoni and Dermacentor variabilis (Dumser and Oliver 1981), Amblyomma cajennense, Amblyomma sculptum, and Amblyomma aureolatum (Anholeto et al. 2014; Sampieri et al. 2014; Sampieri et al. personal information), in Rhipicephalus appendiculatus, Rhipicephalus evertsi evertsi, and Rhipicephalus simus (Wysoki and Bolland 1978), and now in R. sanguineus sl.
In a recent study, however, Sampieri et al. (unpublished data) observed the presence of a connection between the testicles of A. triste, under the format of a tubular isthmus at the distal region, a situation which is also found in Ornithodoros moubata (Wagner-Jevsenko 1958 cited in Sonenshine 1970). In O. rostratus, on the other hand, there is only one U-shaped testicle.
Generally, the single testicle is considered as a synapomorphic ancestor condition. Therefore, the “individualized testicle” would be a derived apomorphic character, and, consequently, “individualized testicles with distal connection” could be considered an intermediate one. If that hypothesis is correct, the condition regarding individualized testicles would be the most recent one, and it would have evolved more than once among the Ixodida, once individualized testicles have already been observed for Argasidae species (Pagenstecher, 1861 cited in Sonenshine, 1970). However, the access to that publication is compromised, as it is very old.
Concerning the morphology of germ cells and to the spermiogenesisprocess, some differences were observed among the species studied herein, which should be probably related to their biology. In R. sanguineus sl, spermatogenesis probable starts during the period in which nymphs are fed, and it is probably finished during the molting process. However, dividing spermatocytes have been observed in fasted R. appendiculatus, R. evertsi evertsi, and R. simus (Oliveira et al. 2012; Wysoki and Bolland 1978), indicating an interspecific variation at late spermatogenesis and early spermiogenesis. For R. sanguineus sl adult individuals with six feeding days, it is possible to observe spermiogenesis from the beginning; that is, when immature spermatids (spI) start to hypertrophy, which causes significant morphological alterations (five different stages).
For other Ixodidae, as in the previously mentioned Amblyomma, the end of spermatogenesis and beginning of spermiogenesis also undergo interspecific variations. Spermatogenesis ends either during maturing from nymph to adult, or already in the adult stage, after ticks start feeding from hosts. For adults, in advanced feeding stages, it is possible to observe all five stages of that process, making it clear that this is a character that may be common to the Ixodidae family.
For O. rostratus, the studies do not include consistent information regarding when spermiogenesis starts, but the first spermatid stage found is very distinct from the one observed in R. sanguineus sl and for other Ixodidae. The first cell is large with an already evident limit (formation of cisternae), also presenting large interphase nucleus—characteristics that indicate that spermiogenesis in this Argasidae starts at the nymphal stage. It is therefore considered to be a common character among species of the Ornithodoros genus, according to studies conducted by Feldman-Muhsam and Filshie (1976) with Ornithodoros tholozani, and Pinkerton et al. (1982) with O. moubata. Unlike what occurs with Ornithodoros, for Argas persicus, according to Monstasser et al. (2005), spermiogenesis usually lasts 2 weeks, after the adult individual feeds from the host. However, further studies are required to confirm whether it is a common characteristic of the Argas genus—and if so, which are the remaining existing patterns in that family.
Tick spermatozoa, as the ones in Acari as a whole, are aflagellate, and that type is also found in diplopoda and less frequently in other arthropods such as insects and crustaceans (Baccetti 1973; Krantz and Walter 2009; Reger 1963;). One of the characteristics of this sperm is the loss of its nuclear envelope, followed by morphological changes concerning the chromatin shape, which becomes highly condensed (nuclear process). After that, it is impossible to differentiate the nucleoplasm from cytoplasm (Reger 1962).
The so-called nuclear process can be clearly observed, albeit with differences between the two species. For R. sanguineus sl, the nuclear changes seem to have actually started in the spIII, when the nucleus still presented normal morphology. In the spIV, that morphology is lost, and the formation of the nuclear process is started, yet the organelle can still be seen. In the spV, the nuclear process formation has an end, and the genetic material, now called chromatin body, is arranged in the shape of a screw and is located in the anterior region of the cell, indicating that the nuclear envelope has be reabsorbed.
In turn, in O. rostratus, the nuclear process formation is started at the spII, where the nucleoplasm becomes indistinguishable from the cytoplasm, and the genetic material is weekly stained, a characteristic which is still maintained until the cell reaches stage IV. In the spV, the nuclear process is finished and the genetic material (chromatin body) is restricted to a filamentous structure that is strongly stained through the technique in the anterior region of the cell.
Finally, the histological and ultramorphology characterization of spV that were conducted in this study allowed comparing the different tick species already studied with that purpose and corroborated statements concerning the existence of a species-specific relationship between male germ cell morphology and the species which produced it. This fact became evident when the spVs of R. sanguineus sl and O. rostratus were observed and compared in this study, and to other previously described species, which characters such as the operculum shape, the presence or absence of a rim at the operculum base, and the shape of that rim would be specific to each species, as the study conducted by Wüest et al (1978) describing the spermatozoa of Amblyomma hebraeum.
In order to better visualize the information, the data from the comparative analysis of spermiogenesis processes and spermatid morphologies of R. sanguineus sl and O. rostratus are summarized in Fig. 3.
Schematic diagram of the spermiogenesis process of Rhipicephalus sanguineus sl (a-e) and Ornithodoros rostratus (f-j). a, f Spermatid I; b, g Spermatid II; c, h Spermatid III; d, i Spermatid IV; e, j Spermatid V. av acrosomal vesicle, c cisternae, mc membranous complex, np nuclear process, n nucleus
Conclusions
Generally speaking, tick sperm biology and evolution has not yet been fully explained, to the extent it could allow phylogenetic analysis per se, in order to compare it to the phylogenies of ticks in the current literature, especially in regard to the need to study other species which may generate enough data to allow the definition of common characters at subfamily and genus levels.
The data obtained in this study, besides contributing to the analysis of characters for future studies in the different tick groups, has shown that this kind of analysis is promising to solve problems that are of systematic and phylogenetic nature. Besides that, the use of transmission electron microscopy techniques may reveal new characters in a subcell level, at the same time that will allow the construction of cladograms, and the comparison with trees generated from the molecular systematics.
References
Anholeto A, Nunes PH, Remédio RN, Camargo-Mathias MI (2014) Testes of fed and unfed Amblyomma cajennense ticks (Acari: Ixodidae) first morphological data. Acta zool-stockholm. doi:10.1111/az o.12083
Alberti G, Carrera P, Martin P, Smit H (2008) Comparative spermatology of freshwater mites (Hydrachnidia, Acari). Soil Organ 80:155–169
Baccetti B, Dallai R, Fratello B (1973) The spermatozoon of arthropoda. XXII. The 12+0’, 14+0' or aflagellate sperm of protura. J Cell Sci 13:321–335
Barker SC, Murrell A (2002) Phylogeny, evolution and hitorical zoogeography of ticks: a review of recente progress. Exp Appl Acarol 28:55–68
Birkhead TR, Hosken DJ, Pitnick S (2009) Sperm biology: An evolutionary perspective. Academic Press, Oxford
Black WC IV, Piesman J (1994) Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc Natl Acad Sci U S A 91:10034–10038
Black WC IV, Klompen JSH, Keirans JE (1997) Phylogenetic relationships among tick sub-families (Ixodida: Ixodidae: Argasidae) based on the 18S nuclear rDNA gene. Mol Phylogenet Evol 7:129–144
Burguer TD, Shao R, Beati L, Miller H, Barker SC (2012) Phylogenetic analysis of ticks (Acari: Ixodida) using mitochondrial genomes and nuclear rRNA genes indicates that the genus Amblyomma is polyphyletic. Mol Phylogenet Evol 64:45–55
Dallai R, Machida R, Uchifune T, Lupetti P, Frati F (2005) The sperm structure of Galloisiana yuasai (Insecta, Grylloblattodea) and implications for the phylogenetic position of Grylloblattodea. Zoomorphology 124:205–212
Dantas-Torres F (2010) Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasitol Vector 26:1–11
Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onófrio VC, Marcili A, Bermudez SE, Ribeiro AF, Barros-Battesti DM, Labruna MB (2012) Description of a new species of bat-associated argasid tick (Acari: Argasidae) from Brazil. J Parasitol 98:36–45
Dantas-Torres F, Otranto D (2015) Further thoughts on the on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. doi:10.1016/j.vetpar.2014.12.014
Dumser JB, Oliver JH (1981) Kinetics of spermatogenesis, cell-cycle analysis, and testis development in nymphs of the tick Dermacentor variabilis. J Insect Physiol 27:743–753. doi: 10.1016/0022-1910(81)90064-0
Estrada-Peña A, Mangold AJ, Nava S, Venzal JM, Labruna MB, Guglielmone AA (2010) A review of the systematics of the tick family Argasidae (Ixodida). Acarologia 50:317–333
Feldman-Muhsam B, Filshie BK (1976) Scanning and transmission electron microscopy of the spermiophores of Ornithodoros ticks: an attempt to explain their motility. Tissue Cell 8:411–419
Hoogstraal H, Aeschlimann A (1982) Tick host specificity. Bull Soc Entomol Suisse 55:3–32
Hubálek Z, Rudolf I (2012) Tick-borne viruses in Europe. Parasitol Res 111:9–36
Krantz GW, Walter DE (2009) A manual of acarology. Texas Tech University Press, Lubock
Michalik P, Dallai R, Giusti F, Alberti G (2004) The ultrastructure of the peculiar synspermia of some Dysderidae (Aranae, Arachnida). Tissue Cell 36:447–460
Montasser A, Gadelhak GG, Tariq S (2005) Impact of ivermectin on the ultrastructure of the testis of Argas (Persicargas) persicus (Ixodoidea:Argasidae). Exp Appl Acarol 36:119–129. doi:10.1007/s10493-005-1270-2
Nava S, Estrada-Peña A, Petney T, Beati L, Labruna MB, Szabó MPJ, Venzal JM, Mastropaolo M, Mangold AJ, Guglielmone AA (2015) The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet Parasitol 208:2–8
Oliveira PR, Calligaris IB, Roma GC, et al (2012) Morphological characterization of the nymphs Rhipicephalus sanguineus ticks (Latreille, 1806) (Acari: Ixodidae). Description of the testes, integument, Malpighian tubules, and midgut on the detachment day. Microsc Res Tech 75:727–36. doi:10.1002/jemt.21118
Pinkerton AM, Hall JD, Shepherd J (1982) Scanning electron microscopy of post-ejaculatory spermiogenesis in the tick Ornithodoros moubata. Tissue Cell 14:785–797
Politi FAS, Souza-Moreira TM, Rodrigues ER, Queiroz GM, Figueira GM, Januário AH, Berenger JM, Scolovschi C, Parola P, Pietro RCLR (2013) Chemical characterization and acaricide potential of essential oil from aerial parts of Tagetes patula L. (Asteraceae) against engorged adult females of Rhipicephalus sanguineus (Latreille, 1806). Parasitol Res 112:2261–2268
Ravaomanana J, Michaud V, Jori F, Andriatsimahavandy A, Roger F, Albina E, Laurence V (2010) First detection of African swine fever vírus in Ornithodoros porcinus in Madagascar and new insights into tick distribution and taxonomy. Parasitol Vector 3:115
Reck J, Marks FS, Termignoni C, Guimarães JA, Martins JR (2013) Ornithodoros brasiliensis (mouro tick) salivary gland homogenates inhibit in vivo wound healing and in vitro endothelial cell proliferation. Parasitol Res 112:1749–1753
Reger JF (1962) A fine-structure study on spermiogenesis in the tick Amblyomma dissimili, with special reference to the motile process. J Ultrastruct Mol Struct R 7:550–565
Reger JF (1963) Spermiogenesis in the tick Amblyomma dissimili, as revealed by electron microscopy. J Ultrastruct Mol Struct R 8:607–621
Remedio RN, Nunes PH, Anholeto LA, Oliveira PR, Camargo-Mathias MI (2015) Morphological effects of neem (Azadirachta indica a. Juss) seed oil with known azadirachtin concentrations on the oocytes of semi-engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol Res 114:431–444
Ribeiro CCDU, Faccini JLH, Cançado PHD, et al (2013) Life cycle of Ornithodoros rostratus (Acari: Argasidae) under experimental conditions and comments on the host-parasite relationship in the Pantanal wetland region, Brazil. Exp Appl Acarol 61:139–46. doi: 10.1007/s10493-013-9669-7
Roshdy MA (1961) Comprataive internal morphology of subgenera of Argas ticks (Ixodidea, Argasidae). I. Subgenus Carios: Argas vespertilionis (Latreille, 1802). J Parasitol 47:987–994
Sampieri BR, Labruna MB, Bueno OC, Camargo-Mathias MI (2014) Dynamics of cell and tissue genesis in the male reproductive system of ticks (Acari: Ixodidae) Amblyomma cajennense (Fabricius, 1787) and Amblyomma aureolatum (Pallas, 1772): a comparative analysis. Parasitol Res 113:1511–1519
Sonenshine DE (1970) A contribution to the internal anatomy and histology of the bat-tick Ornithodoros Kelley Cooley and Kohls, 1941. II. The reproductive, muscular, respiratory, secretory and nervous system. J Med Entomol 7:298–312
Tavassoli M, Malekifard F, Soleimanzadeh A, Pourseyed SH, Bernousi I, Mardani K (2012) Susceptibility of different life stages of Ornithodoros lahorensis to entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Parasitol Res 111:1779–1783
Wüest J, Said AE, Swiderski Z, Aeschlimann K (1978) Morphology of spermatid and spermatozoon of Amblyomma hebraeum Koch (Acarina, Ixodidae). Z Parasitenkd 55:91–99
Wysoki M, Bolland HR (1978) Spermatogenesis, chromosomes and sex determination of four Rhipicephalus species (Acari: Ixodidae) from East Africa. Genetica 48: 233--238
Acknowledgments
This study was financially supported by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) through grant no. 2012/02384-8, by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) through research fellowships to M.I. Camargo-Mathias.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethical Committee in Animal Use (Comitê de Ética de Uso Animal-CEUA) at the Instituto de Biociências, UNESP, Rio Claro-SP, Brazil, under process number 017/2012 and protocol 1422.
Rights and permissions
About this article
Cite this article
Sampieri, B.R., Calligaris, I.B., da Silva Matos, R. et al. Comparative analysis of spermatids of Rhipicephalus sanguineus sensu lato (Ixodidae) and Ornithodoros rostratus ticks (Argasidae): morphophysiology aimed at systematics. Parasitol Res 115, 735–743 (2016). https://doi.org/10.1007/s00436-015-4797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4797-0