Abstract
Human babesiosis, a worldwide emerging tick-borne disease, is caused by the intraerythrocytic apicomplexan parasite, babesia. In recent years, the number of infected patients globally has continued to rise, and thus human babesiosis poses a significant public health threat. Therefore, stronger initiatives should be undertaken to prevent further spread and development of this disease. In the present review, we summarize the epidemiology of reported human babesiosis cases in China from 1993 until now. The data show that Babesia microti is the dominant species causing human babesiosis in China and has led to more than 100 human infections thus far, where Babesia crassa-like is the second-most common. Moreover, Guangxi province is the second-most infected area after the Heilongjiang province. We also review the babesia life cycle, manifestation, diagnosis, and treatment. Additionally, we discuss babesiosis prevention strategies to raise public awareness, and also provide suggestions for improved babesiosis control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Babesia, an intraerythrocytic protozoan, was first identified in cattle and sheep erythrocytes by Victor Babes in 1888 (Babes 1888). As an apicomplexan parasite, Babesia spp. belongs to the suborder Piroplasmida and family Babesiidae, which is the second-most common blood-borne parasite (Conrad et al. 2006; Gray et al. 2010). Interestingly, this parasite can potentially infect all vertebrate mammals, including humans, and causes a zoonotic vector-borne disease—babesiosis—which can lead to both livestock losses and significant harm to general public health (Farber et al. 2015; Schnittger et al. 2012). As a tick-borne pathogen, babesia has invaded new geographic areas in tandem with the expansion of tick habitats, causing babesiosis to become an increasing global problem (Beugnet and Moreau 2015). Human babesiosis is not well known to many physicians, so it is difficult for them to come up with the diagnosis. Several patients have been identified with babesia infection; however, in China, few received standard treatment. In recent years, the number of human babesiosis cases in China has been increasing, but relatively little importance is attached to the disease. In the present review, we summarize the reported human cases of babesiosis in China from 1993 until now, which we hope will provide practical information for clinicians, and which may help with diagnosis.
Etiology of human babesiosis
Among Babesia species, four phylogenetic clades can cause human infections. The first is Babesia microti and B. microti-like parasites, which mostly infect humans (Goethert 2003). The second clade consists of Babesia duncani and B. duncani-type parasites, which mainly infect dogs (Conrad et al. 2006; Kjemtrup and Conrad 2000). The third clade is composed of Babesia divergens, B. divergens-like parasites, and Babesia venatorum; the former two mainly infect cattle, and the latter mainly causes deer infection (Herwaldt et al. 2003; Jiang et al. 2015). These three clades are all small parasites (trophozoites found in erythrocytes that measure less than 3 μm); however, the third is also phylogenetically related to large babesia (trophozoites larger than 3 μm). The last clade comprises large babesia, such as Babesia bovis and Babesia bigemina, which mainly infect ungulates (Criado-Fornelio et al. 2003). Since the first human B. divergens infection was confirmed in the former Yugoslavia in 1956, increasing numbers of B. divergens and B. microti human infections have been reported worldwide, including in the America, Europe, Africa, and Asia (Gray et al. 2010; Hunfeld et al. 2008; Skrabalo and Deanovic 1957; Vannier and Krause 2012).
Babesia life cycle
Babesia has a complex lifecycle with developmental stages occurring both within the mammalian host and the tick vector (Fig. 1). One of its critical developmental steps initially takes place within mammalian host red blood cells, where babesia sporozoites attach to erythrocytes by docking onto glycosaminoglycans and sialoglycoproteins (Lobo et al. 2012; Yokoyama et al. 2006). There, they mature into trophozoites and pullulate to form merozoites. Upon leaving the erythrocytes, the progeny merozoites again invade new erythrocytes and thus an asexual growth cycle is formed (Kumar et al. 2004; Yokoyama et al. 2002). When ticks take blood meals from an infected mammalian host, the parasite enters the tick gut together with the blood in the form of gametocytes, which cannot be commonly found in human erythrocytes. Then, it completes its life cycle within tick vectors to enable effective transmission. In the tick gut, the parasites develop into gametes and fuse to form zygotes, which subsequently migrate across the tick gut barrier into the hemolymph via unknown mechanisms, where they mature into ookinetes (Liu and Bonnet 2014). Finally, they arrive at the salivary glands with the flow of hemolymph and become dormant sporoblasts (Karakashian et al. 1986). When the infected ticks feed on mammalian host, the sporoblasts become active and enter the host’s bloodstream, which may result in a host babesia infection. Interestingly, humans are accidental hosts and are most commonly infected via bites of ixodid ticks, the predominant babesia vector. In addition, blood transfusions and trans-placental transmission can also play significant roles in the infection process (Spielman et al. 1985; Swanson et al. 2006).
Epidemiology of human babesiosis in China
According to the reported cases, B. microti, B. venatorum, and B. divergens are thought to be the main pathogens causing human babesiosis in China, including in Zhejiang, Yunnan, and Guangxi provinces (Table 1 and Fig. 2). It is asserted that the first Chinese case of babesiosis could happen in 1944 (Qu 2007); subsequently, antibody against B. microti was detected by serological test in Taiwan in the year of 1977 (Hsu and Cross 1977). No actual human babesiosis cases were reported, until two patients were diagnosed with non-identified babesiosis in China in 1993 and 2000 (Shi et al. 1996; Su et al. 2002).
Over the past 20 years, more than 100 patients from Zhejiang, Yunnan, and Guangxi provinces have contracted B. microti babesiosis; therefore, B. microti appears to be the dominant pathogen causing human babesiosis in China. The first confirmed human B. microti infection was reported in 2011 in Zhejiang province, and was confirmed with blood smears and PCR (Yao et al. 2012). B. microti has since been identified in ten patients residing along the China-Myanmar border in 2012 and 2013; interestingly, two patients were also co-infected with Plasmodium (Zhou et al. 2013). In 2013, it was reported that the positive B. microti infection rate in blood donors from Guangxi province was 2.53% (48/1900) (Wang et al. 2016). In addition, B. microti infection was also investigated in Guangxi province citizens, with a 33.6% (40/121) infection rate (Qiao et al. 2015). Moreover, two sporadic cases in southern Taiwan and Yunnan with an unknown species resembling B. microti infection were also reported (Shih et al. 1997; Wang and Huang 2014).
Babesia crassa-like, ranking only second to B. microti, is a novel pathogen that causes human babesiosis in Heilongjiang, China, during 2015 and 2016. Jia and colleagues recruited 1125 participants with a recent history of tick bites, and found 58 people were infested by B. crassa-like, among which, 27 were suspected cases for the subclinical symptoms (Jia et al. 2018). It is the first time to identify babesiosis caused by B. crassa-like pathogen in China.
B. venatorum is another pathogen that causes human babesiosis in China. Of 2912 individuals from Heilongjiang province who sought medical care after a tick bite, Jiang and colleagues reported that 48 people had contracted a B. venatorum infection. Among them, 32 were confirmed and 16 were probable cases (Jiang et al. 2015). An additional case of B. venatorum infection concerned an 8-year-old boy in Xinjiang Uygur Autonomous Region, diagnosed using microscopy, PCR, and animal inoculation (Sun et al. 2014).
Thus far, B. divergens has been a rare cause of human babesiosis infection in China. Only two males from Shandong province have had a B. divergens infection, which was confirmed by PCR amplification and sequence analysis (Qi et al. 2011). In addition to the more common babesia species that can infect humans, a 42-year-old male in Hangzhou, Zhejiang province, was diagnosed with a novel babesia species infection in 2015, which was subsequently named Babesia sp. XXB/Hangzhou (Man et al. 2016).
A common geographical feature linking these widely distributed human babesiosis cases is abundant vegetation. The southern rural areas of Zhejiang and Guangxi have significant vegetative cover, the China-Myanmar border is hilly and extensively covered by primary and secondary rainforests, and Heilongjiang in northeastern China is a forested mountainous area (Jiang et al. 2015; Zhou et al. 2013), which all provide a natural habitat for ticks. In addition, most patients with babesiosis had a history of tick bite or blood transfusion (Jiang et al. 2015; Su et al. 2002; Yao et al. 2012; Zhou et al. 2013), consistent with the established human babesiosis transmission route.
Clinical manifestations of human babesiosis
Clinical symptoms do not manifest immediately after babesiosis infection, and the incubation period depends upon the infection route. Patients often present symptoms 1–4 weeks following a bite from an infected tick. Interestingly, the appearance of symptoms can take 1–9 weeks or longer when infection occurs via the transfusion of contaminated blood products (Vannier et al. 2008). Infected individuals often exhibit malaise and fatigue, followed by sporadic fever which is the most consistent and obvious symptom, and which can last 10 years with repetitive occurrences and/or reach 40.9 °C in some individuals (Man et al. 2016). Moreover, chills and sweats are also common symptoms, which can be accompanied by headache, myalgia, anorexia, cough, sore throat, arthralgia, and nausea (Krause et al. 1996b). Occasional symptoms such as breathing difficulties, eye redness, and dark-colored urine are also reported. However, these symptoms are non-specific and could be related to other similar diseases, resulting in unsuitable treatment (Reubush 2nd et al. 1977).
Parasitemia is the main laboratory finding in babesia-infected individuals, and can persist for several months following the initiation of standard therapy in asymptomatic individuals, and may continue for more than a year in those who do not receive proper treatment. Additionally, parasitemia is often difficult to detect in immunocompetent patients (Martinot et al. 2011). In contrast, immunocompromised individuals, who have undergone splenectomy, been troubled by cancer or chronic liver or heart disease, and who have taken immunosuppressive drugs, always suffer from severe infection and present typical clinical symptoms such as high fever, high parasitemia, and severe anemia (Hunfeld et al. 2008). In addition, parasitemia is usually associated with hemolytic anemia, characterized by decreased hematocrit, low hemoglobin and haptoglobin levels, elevated reticulocyte count, and elevated lactate dehydrogenase levels (Joseph et al. 2011). Moreover, raised liver enzymes, variable leukocyte count, and thrombocytopenia are beneficial in facilitating the differentiation of babesiosis from other diseases that also cause fever (Vannier and Krause 2012).
Diagnosis of human babesiosis
Individuals with unexplained febrile illness who have settled in or traveled to areas with endemic babesiosis, or who have received a blood transfusion within the last 6 months, should be considered for a babesiosis diagnosis (Krause et al. 2002). Diagnostic methods mainly involve blood smear microscopy, parasite culture, serological tests, and molecular detection; their respective advantages and disadvantages are summarized in detail in Table 2.
Blood smear microscopy
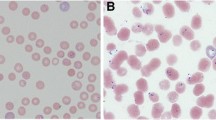
Blood smear microscopy is the classic effective diagnostic method for human babesiosis. After Giemsa staining, parasites can be identified within erythrocytes as pleomorphic ring forms, such as round, oval, pear-shaped, amoeboid, and arranged in singles, pairs, or rarely in tetrads—termed as the “Maltese-cross appearance”—with light blue cytoplasm (Hildebrandt et al. 2013). Interestingly, infected erythrocytes remain normal size, and the cytoplasm of the ring form remains clear, especially in the infection of large babesia due to vacuole presence (Parija et al. 2015). The most pathognomonic characteristics of B. microti and B. duncani merozoites is the Maltese cross pattern, while B. divergens and B. venatorum merozoites typically appear as paired pear-shaped forms and only occasionally as tetrads (Conrad et al. 2006; Herwaldt et al. 2003). However, it is difficult to identify specific babesia species with blood smear microscopy (Hoare 1980). Parasitemia generally ranges from 1 to 10%, but can also reach 80% in severe infections. In the early stages of illness, parasitemia is often less than 1%, and parasites may not be noticed; thus, smears from serial blood collections must be investigated in at least 300 microscopic fields to avoid overlooking the parasite (Bruckner et al. 1985). Babesia spp. ring forms in erythrocytes are also quite similar to those of Plasmodium spp. and requires careful observation for correct identification. Compared to Plasmodium, babesia possesses pleomorphic ring forms in infected erythrocytes but lacks hemozoin pigments, identifiable gametocytes, and schizonts (Pruthi et al. 1995). Altogether, blood smear microscopy is the first step of diagnosis and additional evaluation should be performed to increase detection sensitivity and specificity.
Parasite culture
Parasite cultivation could be divided into two types including animal inoculation (in vivo) and artificial medium cultivation (in vitro). And cultivation has been used in the diagnosis of babesiosis in animals but not typically used for human babesia infections because it is time-consuming and unable to give precise results (Parija et al. 2015). However, cultivation can be helpful in the identification of asymptomatic/low-parasitemic individuals, defining phylogenetic relationships, and antimicrobial susceptibility testing. Following the appearance of visible parasitemia, the cultivation period usually takes 7–10 days by animal inoculation (Parija et al. 2015). Gerbils, splenectomized calves and hamsters, and SCID (severe combined immunodeficiency) mice have been used to detect the infection of Babesia spp., e.g., B. duncani, B. divergens, and B. microti (Bloch et al. 2012; Entrican et al. 1979; Wei et al. 2001). In addition, M199, NCTC-135, and RPMI 1640 supplied with various factors including HEPES and TES have also been used as medium for cultivation of Babesia spp., e.g., B. bigemina, B. bovis, and B. divergens (Erp et al. 1980; Grande et al. 1997; Levy and Ristic 1980; Vega et al. 1985). Interestingly, B. microti can only be cultured in vitro for short term and not applicable for diagnostic purposes (Shikano et al. 1995).
Serological tests
Serological tests are also widely used to examine asymptomatic or low-parasitema blood donors who are infected by Babesia spp., and include immunofluorescence assays (IFA), enzyme linked immune-sorbent assay (ELISA), immunoblot, and immunochromatography (Hildebrandt et al. 2013). Although IFA is the gold standard for diagnosing babesia infection (Krause et al. 1996a), there is currently no universal antigen to enable screening for all babesia species that infect humans, and antigen cross-reactivity also exists between different Babesia species, and between Babesia spp. and other parasites (Hildebrandt et al. 2013). The closer the phylogenetic relationship, the more frequent the chance of non-specific cross-reactivity (Gabrielli et al. 2012). Hence, a seropositive result would indicate babesia infection, but would not be able to identify which species. It is worth noting that lowered antibody production states may provide a serologically negative escape hatch, especially in immunocompromised patients. False-negative test results can also be obtained in the early stages of B. microti and B. venatorum infection (Herwaldt et al. 2011; Hunfeld et al. 2008). Therefore, seronegative results may result in unsuitable treatment or delay appropriate antimicrobial therapy. Serological tests are thus unable to confirm the diagnosis under most circumstances due to no available universal antigen and the presence of false negatives; therefore, patients should undergo further investigation.
Molecular detection
Polymerase chain reaction (PCR) is one of the most common molecular detection methods and has been widely used in Babesia spp. phylogeny identification and epidemiology studies, and especially for discovering new babesia species (Hildebrandt et al. 2008; Johnson et al. 2012). PCR is more sensitive and specific for babesia detection than the above described methods (Krause 2003). Babesia PCR identification is often based on amplified 18S RNA, and the limits of pathogen identification are ~ 100 gene copies, equivalent to approximately 5–10 parasites/μL (Bloch et al. 2013; Teal et al. 2012). PCR detection of babesia DNA strongly supports an active and therefore ongoing infection (Haselbarth et al. 2007; Krause et al. 1998). In the future, molecular detection will play an even greater role in Babesia spp. diagnosis.
Treatment of human babesiosis
Anti-babesia drugs
Two classical combinations of different drugs can effectively treat human babesiosis, including azithromycin in combination with atovaquone, and clindamycin combined with quinidine (Weiss 2002; Wormser et al. 2006). For immunocompetent patients with mild to moderate babesiosis symptoms, oral azithromycin and atovaquone is a good choice for fewer side effects, and is also effective for moderate to severe babesiosis (Kletsova et al. 2017; Krause et al. 2000). Clindamycin and quinidine is only recommended for patients with severe clinical manifestations, although it was traditionally the first choice for all degrees of babesiosis (Krause et al. 2000). It is noteworthy that in critical cases, clindamycin or azithromycin is more effective when administered intravenously instead of orally (Simonsen et al. 2011; Wormser et al. 2006). In severely immunocompromised patients who have undergone splenectomy or an immunocompromising therapy, the antimicrobial treatment regimen should persist for at least 6 weeks, and then continue for another 2 weeks after parasites are no longer apparent in blood smears (Krause et al. 2008; Ord and Lobo 2015). Atovaquone-proguanil or artesunate therapy could also be an option when prescribing a treatment course (Goo et al. 2010; Vyas et al. 2007). Unfortunately, the standard treatment course is seldom applied in China.
Blood transfusion
Exchange transfusion partially removes babesia-infected erythrocytes and reduces vasoactive elements such as cytokines or procoagulant substances in the circulation, which can improve symptoms (Krause 2003). Blood transfusion should be considered in individuals with severe babesiosis who are not reacting to various drug combinations, as well as in patients with high-grade parasitemia (≥ 10%), significant hemolysis, or who are in danger of renal, hepatic, or pulmonary injury (Wormser et al. 2006). Moreover, blood transfusion may contribute to rapid clinical improvement in severe and sudden cases (Dorman et al. 2000). Blood transfusion is an important adjuvant therapy when treating infants with babesiosis. Simonsen and colleagues suggested that double-volume exchange blood transfusion is necessary for premature infants suffering from fulminant babesiosis with nearly 20% parasitemia (Simonsen et al. 2011).
Prevention of human babesiosis
To prevent human babesiosis, two main pathways based on the Babesia spp. transmission route—tick bite and blood transfusion—should be considered. Tick bites are the most common cause of Babesia spp. infection; thus, to limit tick bite risk, it is necessary to reduce the amount of exposed skin when traveling to tick-infested areas (Piesman and Eisen 2008). Furthermore, applying DEET-containing tick repellent to the skin and impregnating protective clothing with acaricides are also worthwhile preventative measures (Appel et al. 2008).
Blood transfusion can also expose recipients to Babesia spp. infection risk. With the number of babesiosis cases increasing, it is vital to strengthen the supervision and management of blood sample screening tests. The Guangxi study which identified babesia in multiple blood donor samples is a potent reminder about blood sample safety (Wang et al. 2016). It is therefore imperative to implement rigorous standardized blood sample babesia detection procedures in blood banks.
Conclusion
Human babesiosis, an emerging tick-borne disease, poses a significant public health threat worldwide. In China, B. microti, B. venatorum, and B. divergens are thought to be the main pathogens causing human babesiosis, which are distributed in a variety of areas including Zhejiang, Yunnan, and Guangxi provinces. In the present review, we summarized the epidemiology of reported human babesiosis cases in China from 1993 until now. And we also review the babesia life cycle, manifestation, diagnosis, treatment, and prevention strategies, which provide suggestions for improved babesiosis control in China.
Availability of data and material
The datasets used and/or analyzed in the present study are available from the authors on reasonable request.
References
Appel KE, Gundert-Remy U, Fischer H, Faulde M, Mross KG, Letzel S, Rossbach B (2008) Risk assessment of Bundeswehr (German Federal Armed Forces) permethrin-impregnated battle dress uniforms (BDU). Int J Hyg Environ Health 211:88–104. https://doi.org/10.1016/j.ijheh.2007.10.005
Babes V (1888) Sur l’hemoglobinurie bacterienne du boeuf. C R Acad Sci 107:692–694
Beugnet F, Moreau Y (2015) Babesiosis. Rev Sci Tech 34:627–639. https://doi.org/10.20506/rst.34.2.2385
Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, Reed W, Hunter R, Ryals R, Hagar W, Xayavong MV, Slemenda SB, Pieniazek NJ, Wilkins PP, Kjemtrup AM (2012) The third described case of transfusion-transmitted Babesia duncani. Transfusion 52:1517–1522. https://doi.org/10.1111/j.1537-2995.2011.03467.x
Bloch EM, Lee TH, Krause PJ, Telford SR III, Montalvo L, Chafets D, Usmani-Brown S, Lepore TJ, Busch MP (2013) Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion 53:2299–2306. https://doi.org/10.1111/trf.12098
Bruckner DA, Garcia LS, Shimizu RY, Goldstein EJC, Murray PM, Lazar GS (1985) Babesiosis: problems in diagnosis using autoanalyzers. Am J Clin Pathol 83:520–521. https://doi.org/10.1093/ajcp/83.4.520
Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford III SR, Herwaldt BL (2006) Description of Babesia duncanin. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 36:779–789. https://doi.org/10.1016/j.ijpara.2006.03.008
Criado-Fornelio A, Martinez-Marcos A, Buling-Sarana A, Barba-Carretero JC (2003) Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part I Epizootiol Aspects Vet Parasitol 113:189–201. https://doi.org/10.1016/S0304-4017(03)00078-5
Dorman SE, Cannon ME, Telford SR 3rd, Frank KM, Churchill WH (2000) Fulminant babesiosis treated with clindamycin, quinine, and whole-blood exchange transfusion. Transfusion 40:375–380. https://doi.org/10.1046/j.1537-2995.2000.40030375.x
Entrican JH, Williams H, Cook IA, Lancaster WM, Clark JC, Joyner LP, Lewis D (1979) Babesiosis in man: report of a case from Scotland with observations on the infecting strain. J Infection 1:229–234. https://doi.org/10.1016/S0163-4453(79)91219-2
Erp EE, Smith RD, Ristic M, Osorno BM (1980) Optimization of the suspension culture method for in vitro cultivation of Babesia bovis. Am J Vet Res 41:2059–2062. https://doi.org/10.1258/002367779780943189
Farber FR, Muehlenbachs A, Robey TE (2015) Atraumatic splenic rupture from Babesia: a disease of the otherwise healthy patient. Ticks Tick Borne Dis 6:649–652. https://doi.org/10.1016/j.ttbdis.2015.05.010
Gabrielli S, Galuppi R, Marcer F, Marini C, Tampieri MP, Moretti A, Pietrobelli M, Cancrini G (2012) Development of culture-based serological assays to diagnose Babesia divergens infections. Vector Borne Zoonotic Dis 12:106–110. https://doi.org/10.1089/vbz.2011.0706
Goethert HK (2003) What is Babesia microti? Parasitology 127:301–309. https://doi.org/10.1017/S0031182003003822
Goo YK, Terkawi MA, Jia H, Aboge GO, Ooka H, Nelson B, Kim S, Sunaga F, Namikawa K, Igarashi I, Nishikawa Y, Xuan X (2010) Artesunate, a potential drug for treatment of Babesia infection. Parasitol Int 59:481–486. https://doi.org/10.1016/j.parint.2010.06.004
Grande N, Precigout E, Ancelin ML, Moubri K, Carcy B, Lemesre JL, Vial H, Gorenflot A (1997) Continuous in vitro culture of Babesia divergens in a serum-free medium. Parasitology 115:81–89. https://doi.org/10.1016/j.jalgebra.2009.03.019
Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L (2010) Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis 1:3–10. https://doi.org/10.1016/j.ttbdis.2009.11.003
Haselbarth K, Tenter AM, Brade V, Krieger G, Hunfeld KP (2007) First case of human babesiosis in Germany—clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol 297:197–204. https://doi.org/10.1016/j.ijmm.2007.01.002
Herwaldt BL, Cacciò S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga PP, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S, Löschenberger K, Tura S, Pieniazek NJ (2003) Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis 9:942–948. https://doi.org/10.3201/eid0908.020748
Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M (2011) Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 155:509–519. https://doi.org/10.7326/0003-4819-155-8-201110180-00362
Hildebrandt A, Gray JS, Hunfeld KP (2013) Human Babesiosis in Europe: what clinicians need to know. Infection 41:1057–1072. https://doi.org/10.1007/s15010-013-0526-8
Hildebrandt A, Tenter A, Straube E, Hunfeld K (2008) Human babesiosis in Germany: just overlooked or truly new? Int J Med Microbiol 298:336–346. https://doi.org/10.1016/j.ijmm.2007.11.001
Hoare CA (1980) Comparative aspects of human babesiosis. Trans R Soc Trop Med Hyg 74:143–148. https://doi.org/10.1016/0035-9203(80)90230-8
Hsu NH, Cross JH (1977) Serologic survey for human babesiosis on Taiwan (in Chinese). J Formos Med Assoc 76:950–954
Hunfeld KP, Hildebrandt A, Gray JS (2008) Babesiosis: recent insights into an ancient disease. Int J Parasitol 38:1219–1237. https://doi.org/10.1016/j.ijpara.2008.03.001
Jia N, Zheng YC, Jiang JF, Jiang RR, Jiang BG, Wei R, Liu HB, Huo QB, Sun Y, Chu YL, Fan H, Chang QC, Yao NN, Zhang WH, Wang H, Guo DH, Fu X, Wang YW, Krause PJ, Song JL, Cao WC (2018) Human babesiosis caused by a Babesia crassa-like pathogen: a case series. Clin Infect Dis 67:1110–1119. https://doi.org/10.1093/cid/ciy212
Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, Sun Y, Jia N, Wang YW, Ma L, Liu HB, Chu YL, Ni XB, Liu K, Song YD, Yao NN, Wang H, Sun T, Cao WC (2015) Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: a descriptive study. Lancet Infect Dis 15:196–203. https://doi.org/10.1016/S1473-3099(14)71046-1
Johnson N, Voller K, Phipps LP, Mansfield K, Fooks AR (2012) Rapid molecular detection methods for arboviruses of livestock of importance to northern. Eur J Biomed Biotechnol 2012:719402–719418. https://doi.org/10.1155/2012/719402
Joseph JT, Roy SS, Shams N, Visintainer P, Nadelman RB, Hosur S, Nelson J, Wormser GP (2011) Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis 17:843–847. https://doi.org/10.3201/eid1705.101334
Karakashian SJ, Rudzinska MA, Spielman A, Lewengrub A, Campbell J (1986) Primary and secondary ookinetes of Babesia microti in the larval and nymphal stages of the tick Ixodes dammini. Can J Zool 64:328–339. https://doi.org/10.1139/z86-053
Kjemtrup AM, Conrad PA (2000) Human babesiosis: an emerging tick-borne disease. Int J Parasitol 30:1323–1337. https://doi.org/10.1016/S0020-7519(00)00137-5
Kletsova EA, Spitzer ED, Fries BC, Marcos LA (2017) Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob 16:26. https://doi.org/10.1186/s12941-017-0198-9
Krause PJ (2003) Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis 3:45–51. https://doi.org/10.1089/153036603765627451
Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A (2008) Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 46:370–376. https://doi.org/10.1086/525852
Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A (2000) Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343:1454–1458. https://doi.org/10.1056/NEJM200011163432004
Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T, Closter L, Christianson D, Telford SR, Persing D, Radolf JD, Spielman A, the Deer‐Associated Infection Study Group (2002) Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis 34:1184–1191. https://doi.org/10.1086/339813
Krause PJ, Ryan R, Telford S 3rd, Persing D, Spielman A (1996a) Efficacy of immunoglobulin M serodiagnostic test for rapid diagnosis of acute babesiosis. J Clin Microbiol 34:2014–2016
Krause PJ, Spielman A, Telford SR, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH (1998) Persistent parasitemia after acute babesiosis. N Engl J Med 339:160–165. https://doi.org/10.1056/NEJM199807163390304
Krause PJ, Telford SR 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH (1996b) Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 275:1657–1660. https://doi.org/10.1001/jama.1996.03530450047031
Kumar S, Yokoyama N, Kim JY, Huang X, Inoue N, Xuan X, Igarashi I, Sugimoto C (2004) Expression of Babesia equi EMA-1 and EMA-2 during merozoite developmental stages in erythrocyte and their interaction with erythrocytic membrane skeleton. Mol Biochem Parasitol 133:221–227. https://doi.org/10.1016/j.molbiopara.2003.10.010
Levy MG, Ristic M (1980) Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science 207:1218–1220. https://doi.org/10.1126/science.7355284
Liu XY, Bonnet SI (2014) Hard tick factors implicated in pathogen transmission. PLoS Negl Trop Dis 8:e2566. https://doi.org/10.1371/journal.pntd.0002566
Lobo CA, Rodriguez M, Cursino-Santos JR (2012) Babesia and red cell invasion. Curr Opin Hematol 19:170–175. https://doi.org/10.1097/MOH.0b013e328352245a
Man SQ, Qiao K, Cui J, Feng M, Fu YF, Cheng XJ (2016) A case of human infection with a novel Babesia species in China. Infect Dis Poverty 5:28. https://doi.org/10.1186/s40249-016-0121-1
Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, Jouglin M, Chauvin A, de Briel D (2011) Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis 17:114–116. https://doi.org/10.3201/eid1701.100737
Ord RL, Lobo CA (2015) Human Babesiosis: pathogens, prevalence, diagnosis and treatment. Curr Clin Microbiol Rep 2:173–181. https://doi.org/10.1007/s40588-015-0025-z
Parija SC, Kp D, Venugopal H (2015) Diagnosis and management of human babesiosis. Trop Parasitol 5:88–93. https://doi.org/10.4103/2229-5070.162489
Piesman J, Eisen L (2008) Prevention of tick-borne diseases. Annu Rev Entomol 53:323–343. https://doi.org/10.1146/annurev.ento.53.103106.093429
Pruthi RK, Marshall WF, Wiltsie JC, Persing DH (1995) Human babesiosis. Mayo Clin Proc 70:853–862. https://doi.org/10.1016/S0025-6196(11)63943-8
Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, Wang Z, Yang D, Wang S, Chai T (2011) Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res 109:241–245. https://doi.org/10.1007/s00436-011-2382-8
Qiao Y, Peng H, Zhu HM, Yan JZ (2015) Nest-PCR identification of one human infected of Babesia microti in Guangxi and investigation on his colleagues (in Chinese). Int J Parasit Dis 42:152–155
Reubush TK 2nd, Cassaday PB, Marsh HJ, Lisker SA, Voorhees DB, Mahoney EB, Healy GR (1977) Human babesiosis on Nantucket Island. Clinical features. Ann Intern Med 86:6–9. https://doi.org/10.7326/0003-4819-86-1-6
Sanchez E, Vannier E, Wormser GP, Hu LT (2016) Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and Babesiosis: a review. JAMA 315:1767–1777. https://doi.org/10.1001/jama.2016.2884
Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA (2012) Babesia: a world emerging. Infect Genet Evol 12:1788–1809. https://doi.org/10.1016/j.meegid.2012.07.004
Schuster FL (2002) Cultivation of Babesia and Babesia-like blood parasites: agents of an emerging zoonotic disease. Clin Microbiol Rev 15:365–373. https://doi.org/10.1128/CMR.15.3.365-373.2002
Shi ZB, Li ZZ, Gao QR (1996) A case of human infection with babesia (in Chinese). Chin J Parasitol Parasit Dis 14:1
Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC (1997) Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol 35:450–454
Shikano S, Nakada K, Hashiguchi R, Shimada T, Ono K (1995) A short term in vitro cultivation of Babesia rodhaini and Babesia microti. J Vet Med Sci 57:955–957. https://doi.org/10.1292/jvms.57.955
Simonsen KA, Harwell JI, Lainwala S (2011) Clinical presentation and treatment of transfusion-associated babesiosis in premature infants. Pediatrics 128:e1019–e1024. https://doi.org/10.1542/peds.2010-0502
Skrabalo Z, Deanovic Z (1957) Piroplasmosis in man; report of a case. Doc Med Geogr Trop 9:11–16
Spielman A, Wilson ML, Levine JF, Piesman J (1985) Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol 30:439–460. https://doi.org/10.1146/annurev.en.30.010185.002255
Su GG, Zhao NF, Ye XX (2002) a case report of babesiosis (in Chinese). Chin J Zoonoses 18:112
Sun Y, Li SG, Jiang JF, Wang X, Zhang Y, Wang H, Cao WC (2014) Babesia venatorum infection in child, China. Emerg Infect Dis 20:896–897. https://doi.org/10.3201/eid2005.121034
Swanson SJ, Neitzel D, Reed KD, Belongia EA (2006) Coinfections acquired from Ixodes ticks. Clin Microbiol Rev 19:708–727. https://doi.org/10.1128/CMR.00011-06
Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S (2012) A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol 50:903–908. https://doi.org/10.1128/JCM.05848-11
Vannier E, Gewurz BE, Krause PJ (2008) Human babesiosis. Infect Dis Clin N Am 22:469–488. https://doi.org/10.1016/j.idc.2008.03.010
Vannier E, Krause PJ (2012) Human babesiosis. N Engl J Med 366:2397–2407. https://doi.org/10.1056/NEJMra1202018
Vega CA, Buening GM, Green TJ, Carson CA (1985) In vitro cultivation of Babesia bigemina. Am J Vet Res 46:416–420. https://doi.org/10.2754/avb198554010091
Vyas JM, Telford SR, Robbins GK (2007) Treatment of refractory Babesia microti infection with atovaquone-proguanil in an HIV-infected patient: case report. Clin Infect Dis 45:1588–1590. https://doi.org/10.1086/523731
Wang H, Huang F (2014) Babesia infection in the southwest of China, a case report. Jundishapur J Microbiol 7:e13504. https://doi.org/10.5812/jjm.13504
Wang HS, Peng H, Zhu HM, Xue SL (2016) Investigation of Babesia spp. infections in blood donors in Guangxi, China (in Chinese). Acad J Second Mil Med Univ 37:1
Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, Telford SR, Ishihara C (2001) Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol 39:2178–2183. https://doi.org/10.1128/JCM.39.6.2178-2183.2001
Weiss LM (2002) Babesiosis in humans: a treatment review. Expert Opin Pharmacother 3:1109–1115. https://doi.org/10.1517/14656566.3.8.1109
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB (2006) The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. https://doi.org/10.1086/508667
Qu FY (2007) Historical review on the development of medical parasitology in China during the years of 1871–2006 (in Chinese). Chin J Parasitol Parasit Dis 25:259–273
Yao LN, Ruan W, Zeng CY, Li ZH, Zhang X, Lei YL, Lu QY, Che HL (2012) Pathogen identification and clinical diagnosis for one case infected with Babesia (in Chinese). Chin J Parasitol Parasit Dis 30:118–121
Yokoyama N, Okamura M, Igarashi I (2006) Erythrocyte invasion by Babesia parasites: current advances in the elucidation of the molecular interactions between the protozoan ligands and host receptors in the invasion stage. Vet Parasitol 138:22–32. https://doi.org/10.1016/j.vetpar.2006.01.037
Yokoyama N, Suthisak B, Hirata H, Matsuo T, Inoue N, Sugimoto C, Igarashi I (2002) Cellular localization of Babesia bovis merozoite rhoptry-associated protein 1 and its erythrocyte-binding activity. Infect Immun 70:5822–5826. https://doi.org/10.1128/IAI.70.10.5822-5826.2002
Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, Ge HX, Chen JH, Hu W (2013) Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty 2:24. https://doi.org/10.1186/2049-9957-2-24
Acknowledgements
We thank Miss Yixiang Wang for help with drawing of the picture.
Funding
This work was supported by grants from the China Postdoctoral Science Foundation (Grant No. 2015 M580472), the Natural Science Foundation of Jiangsu Province (Grant No. BK20150212), the Research Foundation of Xuzhou Medical University (Grant No. D2015006), the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions (Grant No. PPZY2015B161), and the Jiangsu Students’ Platform for innovation and entrepreneurship training program (Grant No. 201810313064X).
Author information
Authors and Affiliations
Contributions
ZTC, HQL, HRY, and ANB carried out the literature search and drafted the first version of the manuscript. XGG, DLK, and XYL were responsible for designing, coordinating, and revising the review.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Additional information
Section Editor: Dana Mordue
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Z., Li, H., Gao, X. et al. Human Babesiosis in China: a systematic review. Parasitol Res 118, 1103–1112 (2019). https://doi.org/10.1007/s00436-019-06250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06250-9