Abstract
Although best known as an animal disease, human babesiosis is attracting increasing attention as a worldwide emerging zoonosis. Humans are commonly infected by the bite of ixodid ticks. Rare ways of transmission are transplacental, perinatal and transfusion-associated. Infection of the human host can cause a very severe host-mediated pathology including fever, and hemolysis leading to anemia, hyperbilirubinuria, hemoglobinuria and possible organ failure. In recent years, apparently owing to increased medical awareness and better diagnostic methods, the number of reported cases in humans is rising steadily worldwide. Hitherto unknown zoonotic Babesia spp. are now being reported from geographic areas where babesiosis was not previously known to occur and the growing numbers of travelers and immunocompromised individuals suggest that the frequency of cases in Europe will also continue to rise. Our review is intended to provide clinicians with practical information on the clinical management of this rare, but potentially life-threatening zoonotic disease. It covers epidemiology, phylogeny, diagnostics and treatment of human babesiosis and the potential risk of transfusion-transmitted disease with a special focus on the European situation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tick-transmitted hemoparasites of the protozoan genus Babesia (phylum Apicomplexa) are the second most common blood-borne parasites of mammals after trypanosomes [1]. The disease shows a worldwide distribution and affects a wide variety of many mammalian species, occasionally including man. The major impact of the disease, however, occurs in the cattle industry and in companion animals, and the species affecting cattle and dogs are the most studied. Human disease due to babesia was first confirmed in Europe with the description of a fatal Babesia divergens infection in 1956 in the former Yugoslavia [2] and, ever since, babesiosis has been viewed as a potentially life-threatening zoonotic disease in humans [3–5]. Since the late 1950s, two species of babesia in particular, the cattle species B. divergens in Europe and the rodent species B. microti in North America have been shown to cause significant numbers of human infections [5–7]. Although, recently several other Babesia species have also been involved in human infections worldwide [7], the major public health burden in humans still occurs in North America and is due to B. microti, especially in the eastern parts of the US (see Table 1) [5–7]. Molecular analysis of the implicated pathogens suggests that the host ranges of many Babesia spp. are less restricted than previously believed and that hitherto unrecognized species can cause infections in a variety of animal hosts, as well as in humans, especially in those with immunologically compromising conditions [6–15]. Most importantly, many facts pertaining to the epidemiology and pathogenesis of this parasitic infection remain unclear, especially in Europe, and the disease may have previously been overlooked in many European countries due to a lack of medical awareness and microbiological detection methods. Moreover, the growing numbers of travelers returning from areas where the disease is potentially endemic and the steady increase of immunocompromised individuals suggest that the frequency of cases in Europe will continue to rise steadily. This review covers aspects of epidemiology, phylogeny, diagnostics and treatment of human babesiosis, with a special focus on the European situation, in order to provide clinicians with practical information on the causative agents and clinical management of this rare, but potentially life-threatening zoonotic disease.
Epidemiology
Human cases of babesiosis are difficult to quantify because many cases are not detected and diagnosed correctly, and others have not been reported or published. Although babesial parasites, in principle, show a world-wide distribution, only a few publications report human disease cases outside the United States and Europe (see Table 1) [16–23]. However, since the 1950s approximately 50 cases have been published reported in Europe [5–7, 24]. Most of them were attributed to B. divergens or closely related parasites (43 cases). In a few cases B. venatorum (3 cases [10, 14]) and B. microti (1 autochthonous case [25], 6 imported cases [26–31]) were identified as causative agents (see Table 1). More than half of European cases have occurred in France and the British Isles. However, within the last 10 years confirmed disease has been reported in several other European countries, including Austria [10], the Czech Republic [27], Finland [32], Germany [14], Italy [10], Montenegro [33], Portugal [34], Poland [35], and Switzerland [26]. Two asymptomatic cases of B. microti infections in forestry workers in Poland were presented at the XII International Jena Symposium on Tick-borne Diseases 21–23 March 2013 in Weimar (Welc-Falęciak et al., Risk of human babesiosis due to Babesia microti in forest ecosystems from North-Eastern Poland. Oral presentation). It remains to be seen whether the parasites detected in this study belong to the same zoonotic genotype described in the US [36] and Jena [25]. Furthermore, there are some additional suspected cases published with insufficient diagnostic confirmation [37–39]. The more severe cases have involved the steadily growing population of travelers and patients with immunocompromising or hemato-oncological disorders [6, 14, 25, 40–42]. However, some severe influenza-like infections in immunocompetent individuals have also been described recently [24].
Seroepidemiology
Seroprevalence studies tested samples from individuals located in the Rhine–Main area of midwestern Germany for babesia antibodies and reported seroprevalence rates of 5.4–8 % for B. microti and of 3.6 % for B. divergens [43, 44]. Positivity rates in these studies were significantly higher among patients exposed to ticks than among a population of healthy blood donors. Similarly, a recent Polish serosurvey revealed antibodies against B. microti in 5 % of the forestry workers tested [45]. A study involving 396 blood donors from Eastern Switzerland identified 5 (1.5 %) donors with B. microti antibodies [46]. Since many samples were collected outside the normal tick season, these data may represent conservative seroprevalence estimates. Thus, while relatively few studies have been conducted in Europe, there is growing evidence of locally acquired babesia infections [47].
Vectors, lifecycles, and phylogeny
Babesia spp. are classified as apicomplexan parasites of the suborder Piroplasmida and family Babesiidae on the basis of their exclusive invasion of erythrocytes, multiplication by budding rather than schizogony, and a lack of hemozoin.
Life-cycles
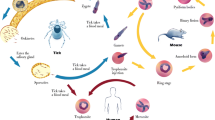
The life-cycles of the parasites are very similar (see Fig. 1). Babesia spp. are naturally transmitted by the bite of infected ticks (almost all ixodids rather than argasids) and the main life-cycle difference among them is the presence of transovarial transmission in some species (Babesia sensu stricto species) and not in others (B. microti-like).
Simplified general life cycle of Babesia spp. (modified and adapted from Hunfeld et al. [6]). Babesia life cycles consist of merogony, gamogony and sporogony. Infection is acquired when sporozoites (Sz) are transferred during tick feeding. Sporozoites then invade erythrocytes and develop into trophozoites (T). Trophozoites divide by binary fission and produce merozoites (M) which continue infection and reinitiate the replicative cycle in the host. Some trophozoites develop into gametocytes (G) which can initiate infection in the tick vector. In the tick gut gametocytes develop into “Strahlenkörper” (Sk) which fuse to form a zygote (Z) developing into a kinete (K). Kinetes gain access to the hemolymph of the tick, replicate and invade various organs. Note that members of Babesia sensu stricto spp. groups can infect the ovaries and be transmitted transovarially via eggs so that all instars (larvae, nymphs and adult females) are potentially infective, whereas members of the Babesia microti-like groups are only transmitted from one instar to the next (transstadially), so that larvae are rarely if ever infected. In all Babesia spp., sporogony is initiated when kinetes invade the salivary glands (Sg). Here, the parasite forms a multinucleated sporoblast (St). Newly developed sporozoites (Sz) are then inoculated into the host with tick saliva at the next blood meal
Phylogeny
To date, more than 100 Babesia species have been identified, infecting many mammalian and some avian species [5, 7]. Traditionally, Babesia spp. were mainly grouped on the basis of their morphology host/vector specificity and susceptibility to drugs. Pragmatically, they are divided into the small Babesia spp. (trophozoites of 1.0–2.5 μm diameter) and large Babesia spp. (2.5–5.0 μm diameter) [6, 7, 48]. These morphological classifications are generally consistent with phylogenetic characterization based on nuclear ssrRNA gene (18S rRNA) sequences [3]. At least 2 different groups of Babesia spp. cause human babesiosis in Europe (see Table 1): Firstly, B. microti-like and secondly, B. divergens, B. divergens-like parasites and B. venatorum, sometimes referred to as belonging to the Babesia sensu stricto spp. group [7].
Vectors and reservoirs
In Europe, many ixodid tick species can transmit babesia to their natural hosts, however, I. ricinus is the most important human-biting tick involved and is the only species thought to transmit the main Babesia spp. (B. microti, B. divergens and B. venatorum), that cause human babesiosis in Europe [5, 7, 47, 49]. Dermacentor reticulatus was recently implicated as a vector in a Polish study [50], but this conclusion appears to have been based only on the presence of B. microti DNA in adult ticks collected from vegetation. Since the preceding nymphal stages will almost certainly have fed on rodents, it is not surprising that B. microti DNA persisted into the next stage. This finding is emphatically not evidence for vector competence, which can best be determined by carefully controlled transmission experiments. In fact Walter (1982) determined in just such controlled experiments that D. reticulatus is not a vector of B. microti. Walter (1982) also showed that several other common European ticks (D. marginatus, Haemaphysalis punctata, Rhipicephalus sanguineus, Ixodes hexagonus) do not transmit B. microti [51]. The only proven vector in Europe, apart from I. ricinus, is I. trianguliceps; however this species rarely if ever bites humans, and the strains of B. microti it transmits are probably not infectious for humans [7].
The typical host reservoirs for the medically most important Babesia spp. in Europe are cattle (B. divergens), roe deer (B. venatorum) and small mammals (B. microti) [5, 7]. Table 1 provides a detailed overview of the currently most medically important Babesia spp., their geographical distribution and the corresponding vectors.
Prevalence of Babesia spp. in ticks
In Europe, data determining risk areas for acquisition of babesiosis via tick infestation are not available. Infection rates of Babesia spp. in ticks are usually rather low, but published values range from 0.9 to 20 % [52–68], though one study in Austria reported the extraordinarily high infection rate of 51 % [69]. However, not all studies differentiated between B. microti and B. divergens, and only a few of them included characterization of B. venatorum [57, 59, 62–65, 67]. Babesia divergens and B. microti were reported from ticks feeding on birds in two different regions of Germany recently [70, 71] and B. venatorum was detected in ticks feeding on birds in Norway and Russia [72, 73], suggesting that birds may play a role in the dispersal of babesia-infected ticks. A recent study on questing I. ricinus from Norway identified 17 (0.9 %) positives out of 1,908 tested ticks, with B. venatorum being the most prevalent Babesia spp. [68].
However, the great variety of pathogens—e.g. Borrelia spp. Anaplasma spp., Rickettsia spp., Coxiella burnetii and Francisella tularensis—that have been detected in coinfected ticks suggests the possibility of multiple human infections acquired after a single tick infestation [56, 58, 65, 70, 74–77].
Common clinical features of human babesiosis in Europe
In the northern hemisphere, peak transmission by ticks occurs from May to September and incubation periods vary from 5 to 33 days after a tick bite [6]. However, most individuals do not remember tick infestation [14, 49, 78]. Other modes of transmission that occur more rarely are transplacental, perinatal and via contaminated blood products [47]. So far only five confirmed congenital human cases due to B. microti have been documented in the United States [79–83] whereas more than 160 cases of transfusion-transmitted babesiosis have been recognized [4, 47].
Patient population and common co-morbidities
In general, patients of all ages including children are affected, but most present clinically at 40–60 years of age [78, 84]. Most European patients infected with Babesia spp. share splenectomy or immunocompromising conditions as risk factors for acquiring the disease. In addition, for all babesia infections, advanced age and depressed cellular immunity are associated with a higher risk of symptomatic infection and more severe illness [85]. The rising number of HIV-positive individuals and the increasing population of immunocompromised individuals may therefore serve to boost the number of human babesiosis cases [6, 14, 40, 41, 49, 86–88]. Transfusion-transmitted cases may arise at any time of the year and incubation periods can be much longer than when infection is transmitted by ticks [14, 49, 89, 90].
In cases of coinfections with Babesia spp. and other tick-borne pathogens, patients often experience a greater number of symptoms for a longer period of time [91, 92]. Most documented cases have so far involved B. microti, but one fatal case of coinfection with B. divergens and Borrelia spp., in a patient with a rudimentary spleen, was reported from Finland recently [32].
Immunocompetent patients
In immunocompetent individuals parasitemia is often hard to detect [24]. Patients may present with non-specific symptoms such as fever, flu-like disease, headache, chills, sweats and myalgia [6, 24, 47]. Interestingly, however, two moderate cases of babesiosis in immunocompetent patients were reported very recently from France, one of which was attributed to B. divergens [24]. Clinical diagnosis of human babesiosis can be further complicated by long-term persistence of subclinical infections, notably by B. microti [93], which may underlie other tick-borne diseases, particularly Lyme borreliosis [78, 91, 92]. Symptoms in immunocompetent individuals usually abate spontaneously within a few weeks [94], but in some cases may persist at a low level [93, 95].
Immunocompromised patients
In immunocompromised, patients, typical clinical symptoms include high fever (up to 40 °C), high parasitemia (20–80 %) diaphoresis, severe anemia, shortness of breath, weakness and fatigue. Patients may later develop jaundice, dark urine, CNS involvement, or complications such as congestive heart failure and respiratory distress syndrome [5, 6]. In severe cases, monitoring of parasitemia by blood smear examination and PCR analysis, and clinical long-term follow-up is important [14, 25]. Clinicians should be aware that in these patients relapse and persistence of the parasite may occur despite treatment [5, 6].
Babesia microti infections
Babesia microti infections are rare outside the Americas but infection can occur also in Asia (Taiwan and Japan) [18, 96] and Australia [23]. However, infections have been repeatedly reported in travelers returning from North America [26–31]. A recent case report described B. microti-infection in an 82-year old man returning to France after traveling in the United States, who presented with severe fever and hemophagocytosis [29]. So far, only one well documented autochthonous case of B. microti has been described in Europe, which was obviously transfusion-associated and occurred in a German patient suffering from a hematological malignancy [25].
B. divergens infections
Symptoms appear rapidly as a result of fulminant, life-threatening infections within 1–3 weeks p.i. with septic fever, hemoglobinuria or jaundice due to severe hemolysis, and up to 42 % of patients die [6, 97]. To date, about 43 cases of B. divergens infections have been reported in Europe, predominantly in asplenic patients [5–7, 24, 32]. Many severe B. divergens infections in the past ended fatally with general organ failure 4–7 days after the manifestation of hemoglobinuria [5, 6, 32, 98, 99].
B. venatorum (EU1-3) infection
Clinical symptoms of the first cases of B. venatorum babesiosis in Italy and Austria, which occurred in two asplenic men with Hodgkin′s disease and large B cell lymphoma, ranged from mild to moderately severe and both patients were cured after successful chemotherapy with clindamycin and/or quinine [10, 14]. The third case of B. venatorum occurred in a German asplenic man with Hodgkin′s disease. This case however, was unique in that the patient remained seronegative for specific antibodies for several months and relapsed after initial treatment, possibly due to the previous combined application of rituximab and prednisolone, which have highly immunosuppressive effects [14]. Otherwise there is no reliable evidence that B. divergens or B. divergens-like parasites including B. venatorum cause chronic disease [7].
Clinical chemistry and hematology
Clinical laboratory testing in apparent cases of human babesiosis may show non-specific findings such as elevated transaminases, alkaline phosphatases, unconjugated bilirubin and lactic dehydrogenase. Normochromia, normocytic anemia, thrombocytopenia and, occasionally, leucopenia may also be observed [5, 10, 14, 24, 25, 29, 30, 32, 35]. Importantly, clinicians should be aware that diagnosis can be missed by automated blood analyzers [100]. A positive Coombs test in combination with hemolytic anemia and elevated procalcitonin levels is highly indicative of babesiosis [14, 49] and should prompt further diagnostic tests.
Direct detection of pathogens
Thus far, rapid microbiological diagnosis of human babesiosis in Europe has been based mainly on the direct detection of the parasites in blood smears and by PCR.
Microscopical findings
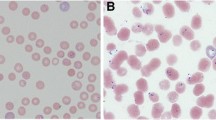
In symptomatic patients the parasites can usually be seen in Giemsa-stained blood smears. They appear within erythrocytes as ring forms or piriform inclusions with light blue cytoplasm (see Fig. 2). However, early in the course of infection or because of a low level parasitemia, parasites may not be visualised and smears from serial blood collection must be investigated [6, 97, 101]. Malaria is the most important differential diagnosis as Plasmodium spp. can also appear as intraerythrocytic ring forms (see Fig. 2) and early malaria stages may lack parasitic pigment (hemozoin). Reliable Babesia spp. identification is not possible microscopically unless paired pyriforms are seen.
a–d Photomicrographs of babesia parasites (denoted by black arrows) on Giemsa-stained peripheral blood smears smear of peripheral blood showing Babesia microti (a), Babesia divergens (b), and Babesia venatorum (c) in infected erythrocytes. Parasites are about 1.5–2.5 μm in diameter Photomicrographs showing P. falciparum (d) on Giemsa-stained peripheral blood smears (modified and adapted from Hunfeld et al. [6])
Molecular detection
In patients with suspected babesiosis, PCR should support microscopic detection of the parasites. Studies have shown that PCR targeting the 18S rRNA gene is more sensitive (5–10 parasites/μmol) [102] and equally as specific as blood smear evaluation in the detection of acute Babesia spp. infections [103–107]. Moreover, several other molecular test formats including DNA probes, reverse line blot hybridization, loop mediated isothermal amplification and real-time PCR-assays technology have been developed, mainly for veterinary diagnostic laboratories [47, 108, 109]. Detection of babesia DNA suggests a parasitemia, and persistent DNA detection clearly points to ongoing infection [3, 14, 93]. Nevertheless, reversion to PCR negativity may lag significantly behind a clinical response to antimicrobial therapy. So far no standardized molecular detection methods are available for routine use in European laboratories. The development of sensitive and specific multiplex PCR assays may be an important future improvement in the laboratory diagnosis of human disease following single or multiple infections with tick-borne pathogens [49, 92].
Molecular pathogen identification
For the purposes of epidemiology and phylogeny, PCR technology and sequence analyses of the amplicons has proved powerful in more exact species identification, especially in newly recognized organisms [7, 49, 110]. However, it should be borne in mind that there are no rules about the degree of homology of fragments of single genes, such as 18s rRNA, required for conclusions on the identity of novel pathogens and biological parameters are also required.
Animal culture
For confirmation of babesiosis it is also possible to directly inoculate 1.0 ml ETDA whole blood into the peritoneum of golden hamsters, jirds, or mice [3, 5–7, 111]. Such bioassays, however, may take 2–4 weeks to become positive and are not useful in emergency situations [14, 85, 112].
Indirect detection methods (antibody testing)
In cases of suspected babesiosis immunofluorescence assays are currently the most frequently applied test system for the detection of specific IgG and IgM antibodies.
Immunofluorescence assay (IFA)
To date, no standardized IFA for diagnosis of babesiosis is available for diagnostic purposes in diagnostic laboratories. As with IFAs used for diagnosis of other diseases, reliable interpretation clearly depends on the experience of the investigator, the type of conjugate used, and the type and preparation of the antigen. Clinical microbiological studies, however, suggest IFA for the detection of anti-Babesia antibodies to be specific, sensitive, and reproducible [44, 45, 113, 114]. The serologic cross-reactivity between B. venatorum and B. divergens means that B. divergens antigen has proved useful for the detection of seroreactivity in patients infected with B. venatorum [10, 14]. For B. microti, titers from 1:32 to 1:160 were reported to be both diagnostic and specific, with positive predictive values of 69–100 % and negative predictive values of 96–99 % [113]. For IFA based on B. divergens antigen, such data are lacking due to the small number of clinical human samples with confirmed B. divergens infection and the fact that most cases present before the onset of a serologic response.
In immunocompetent individuals with B. microti infection, specific antibodies are usually detectable at the time of disease manifestation. Whereas specific IgG antibodies can be found in patients with acute and chronic infections, the detection of IgM antibodies may indicate acute infection even in the absence of demonstrable parasitemia. The detection of Babesia-specific IgM, however, is known to be less specific than testing for IgG antibodies [3, 5, 44]. In immunocompromised patients, prolonged prepatent periods with a significant delay of antibody production may occur [85]. After primary infection, elevated antibody titers may persist from 13 months to 6 years [105].
Limitations of serologic testing
In acute B. divergens infection, IFA is not helpful because specific antibodies usually do not become detectable until 1 week after the onset of fever and hemoglobinuria. Therefore, seronegative results at the onset of clinical symptoms are common and may delay the initiation of appropriate antimicrobial treatment. While false negative test results can be observed early in the course of disease caused by both B. microti and B. venatorum infection [4, 6, 14], false-positive IFA results have also been described in connective tissue disorders such as systemic lupus erythematosus and rheumatoid arthritis [3, 43, 44, 113].
Non-specific cross reactions
Some babesial proteins can cross-react with non-specific antibodies occurring in patients with connective tissue diseases, autoimmune disorders, or with various parasitic, bacterial, and viral infections. Cross-reactive antibodies may also be observed in individuals with infectious disorders caused by closely related members of the Apicomplexa such as Plasmodium spp. and Toxoplasma [44, 115]. In this context, the evaluation of serologic tests in the local setting is very important to guarantee both epidemiological and diagnostic reliability of such assays by determination of appropriate cut-off titers [44, 116].
ELISA, immunoblot, and immunochromatography
The diagnostic interpretation of ELISA or immunochromatographic tests, which have been mainly developed in the veterinary sector [109], is much less subjective than that of conventional IFA and can be automated to process larger numbers of samples in a timely and cost-effective manner. However, problems may arise because greater amounts of antigen are required compared with traditional IFA testing and test specificity is mainly dependent on optimized blocking conditions and purification procedures of the antigens [99]. Many of the antigens that are currently used for diagnostic testing or that are being considered for vaccine production have been identified as merozoite surface proteins or rhoptry-associated proteins, which are involved in immunoevasion or erythrocyte invasion [117–119]. ELISA testing is also useful to confirm IFA results and to identify babesia-positive carriers. Currently, enzyme-linked immunosorbent assays (ELISA) and immunoblot tests are under development and peptide-based ELISAs have been described using peptides derived from secreted antigens of babesia parasites [120]. However, to date ELISAs and blots are not sufficiently standardised for routine use in diagnostic laboratories for the detection of specific antibodies in individuals with suspected babesiosis [7, 47, 49].
Treatment options
Current knowledge of the clinical course and treatment of human babesiosis is mostly derived from clinical data on B. microti and B. divergens-infected patients. For cases caused by other Babesia spp., published accounts of treatment are extremely limited and mainly based on case reports. In general, atovaquone, azithromycin, clindamycin, and quinine represent the drugs of choice for treatment of human babesiosis. These drugs show both proven activity against babesia in animal model assays and favorable outcomes in human cases and clinical treatment trials. Due to issues of resistance development the two major antimicrobial regimens consist of a combination of quinine plus clindamycin or atovaquone plus azithromycin [6, 49, 121, 122]. These regimens are usually administered orally for 7–10 days. Tables 2 and 3 provide an overview of effective drugs, dosing regimens and commonly used drug combinations for the treatment of human babesiosis [85, 109, 121–123]. Typical adverse effects associated with atovaquone plus azithromycin include diarrhea and rash (8 percent each). Adverse effects associated with quinine plus clindamycin include diarrhea and other symptoms of cinchonism (e.g., tinnitus, decreased hearing, and vertigo) [122, 124]. These effects can be so severe that the regimen has to be discontinued or the dosage decreased in one-third of cases. Chloroquine, another anti-malarial, has been used for the treatment of babesiosis but is regarded as relatively ineffective [3, 99]. Other antimalarial and antiprotozoal drugs have been largely unsuccessful, including primaquine, quinacrine, pyrimethamine, pyrimethamine-sulfadoxine, artesunate, sulfadiazine, tetracycline, minocycline, pentamidine, and trimethoprim-sulfamethoxazole [87, 99, 122, 125, 126].
B. divergens infections
Individuals with B. divergens infection—especially those with splenectomy—are usually regarded as medical emergencies and require immediate treatment to arrest hemolysis and prevent renal failure. The combination of quinine and clindamycin for 7–10 days (see Tables 2 and 3) dramatically improves disease outcome [5, 122, 127–129]. The in vivo effectiveness of quinine, however, has always been questioned and drug-related toxicity for quinine is significant [130]. Quinine can be exchanged for quinidine and administered intravenously along with clindamycin, if necessary (see Tables 2 and 3) [7, 122, 123]. It is noteworthy, however, that in recent years a more favorable outcome has been increasingly reported in B. divergens-infected patients with severe complications, even though they were not treated with a full course of quinine and clindamycin, mainly because of quinine side effects [122, 131, 132]. These findings underscore the impact of improved adjunctive measures provided by modern intensive care medicine, including exchange transfusion, which is usually reserved for extremely ill individuals, i.e., those with severe hemolysis, asplenia, immunosuppression and parasitemia of more than 10 % [84, 99]. Clindamycin monotherapy has been proposed in conjunction with such adjunctive measures (see Table 3) [122, 130–132].
Imidocarb, probably the best anti-babesial for use in animals is also highly effective in vitro and has been used successfully to treat two Irish patients infected with B. divergens [99], but is not licensed for use in humans. However, the anti-malarial atovaquone alone proved more effective than imidocarb in an experimental B. divergens/gerbil model [133], and perhaps should be considered for general emergency treatment of babesia infections, especially those caused by Babesia sensu stricto spp.
Exchange transfusion has been recommended for all emergency cases involving B. divergens [6, 7, 122, 134]. This measure is particularly helpful because as far as is known Babesia spp. in humans have no exo-erythrocytic stages. Therefore, the removal of parasitized erythrocytes is potentially curative. In addition, anemia is corrected by this procedure and toxic and harmful metabolites are removed.
B. microti infections
For treatment of B. microti infections, animal studies showed that azithromycin in combination with quinine [135], azithromycin with atovaquone [125], and atovaquone with clindamycin [136], were all effective (see Tables 2 and 3). The mortality rate for clinically apparent B. microti infections is ~5 %. However, most infections take a benign course and subclinical or mild infections mostly resolve on their own [137]. Chemotherapy, is thus indicated only in moderately to severely ill cases and in asymptomatic individuals with prolonged parasitemia ≥ 3 months [122, 138]. More recently, randomized trials in humans infected with B. microti showed that atovaquone plus azithromycin therapy was as effective as the standard quinine/clindamycin combination and there were fewer side effects (15 versus 72 %) [124]. In view of the low risk of side effects associated with atovaquone/azithromycin, it has been argued that all patients diagnosed with B. microti infection should be treated with this drug combination. In severe cases, similar adjunctive measures to those used for B. divergens infections may be necessary (see Table 3).
Newly recognized Babesia spp.
Little is yet known about the exact in vitro susceptibilities to potential anti-Babesia drugs of the newly recognized Babesia spp. such as B. duncani, B. venatorum or the B. divergens-like organisms from the United States and Europe [6, 7, 122, 139]. The currently available information on antimicrobial susceptibility data from case reports, and clinical investigations published to date [3, 10, 14, 122, 123], suggest that there is no convincing scientific evidence for any clinically relevant differences in the susceptibilities of different pathogenic Babesia spp. to the therapeutic agents commonly used to treat human disease. However, it is known that in animal models B. microti-like infections are more difficult to treat than those caused by Babesia spp. such as B. divergens [136]. Note that as yet, it has not proved possible to cultivate B. microti in vitro, which somewhat restricts the development of drugs against this parasite. At present the clindamycin and quinine combination, however, appears to be the regimen of choice for human cases (see Tables 2 and 3), despite problems with side effects and the requirement for aggressive adjunctive procedures in rapidly fulminating infections. Most of the recent cases of human babesiosis caused by previously unknown Babesia spp. have responded well to this drug combination [10–12, 14, 15, 25, 121, 122]. In case of obvious side effects the atovaquone/azithromycin combination may serve as an alternative and was successfully applied in a European case of a B. venatorum infection [14]. However, problems with speed of response to therapy and parasite persistence remain [14, 93], emphasizing the importance of closely monitoring the course of parasitemia, the necessity for long-term follow-up in such patients, and the need for further anti-Babesia drug research.
Immunocompromised patients
In HIV patients and in otherwise immunocompromised individuals substantially higher dosage regimens and longer treatment schedules may be required to clear the infection (see Table 2) [42, 87]. Moreover, it is important to emphasize that clinical resistance to atovaquone has been observed and application of currently available anti-babesials cannot guarantee the elimination of parasites so the possibility of parasite persistence and occasional recrudescence has to be taken into account [138].
Monitoring of treatment and response to therapy
Hematocrit, platelet count, parasitemia, renal function, and hepatic function should be monitored daily or every other day until symptoms abate and parasitemia is < 5 percent. Usually, symptoms should begin to improve within 48 h of antimicrobial therapy, although fatigue and malaise may persist somewhat longer. Full clinical resolution, however, should appear within 3 months after initiation of therapy [6, 49, 121, 122]. Symptoms that persist for ≥ 3 months should prompt repeat blood smear and/or PCR. If parasites are detected by microscopy or babesial DNA is detected by PCR, a second course of antimicrobial therapy should be administered [121, 122]. The second course may consist of the same regimen but should be administered for ≥ 6 weeks, including 2 weeks after parasites are no longer detected by PCR [14, 122, 138]. Persistent and relapsing babesiosis typically occurs in patients who are immunocompromised by the following conditions: malignancy, asplenia, immunosuppressive therapy, or HIV/AIDS [6, 14, 121, 122]. In a retrospective case control study comparing 14 patients with persistent parasitemia and babesial illness (despite repeated courses of antimicrobial therapy) to 46 control patients (who cleared parasites and resolved symptoms within 1 month following a single course of standard antimicrobial therapy), the case patients required treatment for ≥ 6 weeks to achieve cure, including 2 weeks after parasites are no longer detected on blood smear [5, 42, 122]. PCR should be considered as a more sensitive diagnostic alternative in the monitoring of treatment success in such cases [14, 122, 138].
Babesia and blood products
Blood donors without clinical symptoms or with prolonged parasitemias, often harboring circulating parasites for up to 27 months [93], can cause cases of transfusion-transmitted babesiosis (TTB), so far almost exclusively concerning B. microti infections in the United States. Circulating parasites are potentially transmittable via blood products and episodes of immunosuppression can lead to recrudescence of infection with severe complications [140]. Theoretically a single parasite is capable of transmitting infection and in 2009 the Transfusion-Transmitted Diseases Committee of AAEB (formerly, the American Association of Blood Banks) categorized babesiosis, (along with variant Creutzfeldt-Jakob disease and dengue virus) as the highest risk level for blood safety to be prioritized for intervention [141]. The incubation period is 4–9 weeks [142] compared to 1–4 weeks for disease resulting from a the tick-transmitted infection [137]. Johnson et al. proved that trophozoites of Babesia spp. remain viable for 42 days at 4 °C, which is the routine period and temperature for storage of red blood cell concentrates [106]. Reported cases of TTB have implicated cryopreserved red cell units [143, 144]. Extracellular parasites have been reported sporadically [89, 145], even though continued growth of these forms and/or replication at 4 °C has not been published so far. Cases of transfusion-transmitted B. microti infections in the US began to become a public health concern in the early 1980s. Within the last three decades a dramatic rise in numbers of reported transfusion-associated cases has been documented, with at least 12 fatalities [47]. Outside America only two other cases of transfusion-transmitted babesiosis have been reported so far, one from Japan that involved a B. microti-like parasite [146] and one from Germany caused by B. microti [25]. In Europe, reports of low-grade parasitemia [24, 35] are a cause of concern and information on the prevalence of asymptomatic carriers and the possible risk of transfusion-associated babesiosis is urgently required to evaluate the importance of babesia parasites for blood products and their recipients.
Prevention
In order to detect, monitor and prevent transfusion and tick-borne babesiosis, the disease has been made a nationally notifiable in the US. So far, seven states in the Northeast and the upper Midwest are considered to be endemic areas for B. microti [4]. In Europe, however, data on the regional distribution and possible risk areas for acquiring babesiosis via tick infestation are not available. It is important to note that babesiosis is a “moving target” so that endemic areas can shift [112]. Additionally, migratory birds can theoretically contribute to the dispersal of infected ticks [70–73], in which case the pathogen may arise in regions that were previously non-endemic. In view of the additional zoonotic diseases that the vectors of human babesiosis transmit, general measures to prevent tick bites are the most appropriate. Total avoidance of tick habitats by the public may not be practical, but increasing public awareness of the threat posed by ticks and of personal protection measures, such as the wearing of appropriate clothing, application of repellents, and prompt removal of attached ticks, probably represent the most effective preventive measures currently available [147]. At present the only intervention to mitigate the risk of transfusion-transmitted babesiosis in some parts of Europe is a question on standardised donor-health questionnaires asking whether they have a history of babesiosis [148]. Unfortunately, this approach proved to be largely ineffective in the United States [47] and such a question is likely to be even more ineffective in Europe, where awareness of the disease is lower.
Conclusion
Human babesiosis is attracting increasing attention as a worldwide emerging zoonosis. The most important modes of transmission are the bite of an infected ixodid tick and the transfusion of contaminated blood products. Human infection results in a variable but potentially very severe host-mediated pathology, and the frequent delay in diagnosis and treatment can lead to organ failure and death. Increased medical awareness, improved information on the specific epidemiology, risk factors of babesiosis in Europe, and enhanced diagnostic and preventive measures are urgently needed to provide better clinical knowledge and management of this rare but potentially life-threatening zoonotic disease. More information on the risks of transfusion-transmitted babesiosis in Europe is required before a reasonable balance can be reached between disease prevention and excessive exclusion of blood donors.
References
Telford SR 3rd, Spielman A. Reservoir competence of white-footed mice for Babesia microti. J Med Entomol. 1993;30:223–7.
Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9:11–6.
Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–69.
Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. doi:10.1059/0003-4819-155-8-201110180-00362.
Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407. doi:10.1056/NEJMra1202018.
Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37. doi:10.1016/j.ijpara.2008.03.001.
Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. doi:10.1016/j.ttbdis.2009.11.003.
Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–8.
Cho SH, Kim TS, Lee HW, Tsuji M, Ishihara C, Kim JT, Wee SH, Lee CG. Identification of newly isolated Babesia parasites from cattle in Korea by using the Bo-RBC-SCID mice. Korean J Parasitol. 2002;40:33–40.
Herwaldt BL, Cacció S, Gherlinzoni F, Aspöck H, Slemenda SB, Piccaluga P, Martinelli G, Edelhofer R, Hollenstein U, Poletti G, Pampiglione S, Löschenberger K, Tura S, Pieniazek NJ. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9:942–8.
Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, Fritsche TR, Persing DH, Limaye AP. Babesia divergens-like infection, Washington State. Emerg Infect Dis. 2004;10:622–9.
Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, Quick R, Telford SR 3rd, Herwaldt BL. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36:779–89. doi:10.1016/j.ijpara.2006.03.008.
Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet Parasitol. 2006;138:103–11. doi:10.1016/j.vetpar.2006.01.044.
Häselbarth K, Tenter AM, Brade V, Krieger G, Hunfeld KP. First case of human babesiosis in Germany - Clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol. 2007;297:197–204. doi:10.1016/j.ijmm.2007.01.002.
Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, Chung GT, Ju JW, Cheun HI, Lee HW, Lee YH, Kim TS. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol. 2007;45:2084–7. doi:10.1128/JCM.01334-06.
Michael SA, Morsy TA, Montasser MF. A case of human babesiosis (preliminary case report in Egypt). J Egypt Soc Parasitol. 1987;17:409–10.
Bush JB, Isaäcson M, Mohamed AS, Potgieter FT, de Waal DT. Human babesiosis-a preliminary report of 2 suspected cases in South Africa. S Afr Med J. 1990;78:699.
Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–4.
Ríos L, Alvarez G, Blair S. Serological and parasitological study and report of the first case of human babesiosis in Colombia. Rev Soc Bras Med Trop. 2003;36:493–8.
Marathe A, Tripathi J, Handa V, Date V. Human babesiosis-a case report. Indian J Med Microbiol. 2005;23:267–9.
El-Bahnasawy MM, Morsy TA. Egyptian human babesiosis and general review. J Egypt Soc Parasitol. 2008;38:265–72.
Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, Wang Z, Yang D, Wang S, Chai T. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res. 2011;109:241–5. doi:10.1007/s00436-011-2382-8.
Senanayake SN, Paparini A, Latimer M, Andriolo K, Dasilva AJ, Wilson H, Xayavong MV, Collignon PJ, Jeans P, Irwin PJ. First report of human babesiosis in Australia. Med J Aust. 2012;196:350–2.
Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, Jouglin M, Chauvin A, De Briel D. Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis. 2011;17:114–6. doi:10.3201/eid1701.100737.
Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, Kiehntopf M, Fricke HJ, Straube E. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26:595–601. doi:10.1007/s10096-007-0333-1.
Baumann D, Pusterla N, Péter O, Grimm F, Fournier PE, Schar G, Bossart W, Lutz H, Weber R. Fever after a tick bite: clinical manifestations and diagnosis of acute tick bite-associated infections in northeastern Switzerland. Dtsch Med Wochenschr. 2003;128:1042–7. doi:10.1055/s-2003-39103.
Nohýnková E, Kubek J, Mĕst’ánková O, Chalupa P, Hubálek Z. A case of Babesia microti imported into the Czech Republic from the USA. Cas Lek Cesk. 2003;142:377–81.
Ramharter M, Walochnik J, Lagler H, Winkler S, Wernsdorfer WH, Stoiser B, Graninger W. Clinical and molecular characterization of a near fatal case of human babesiosis in Austria. J Travel Med. 2010;17:416–8. doi:10.1111/j.1708-8305.2010.00446.x.
Poisnel E, Ebbo M, Berda-Haddad Y, Faucher B, Bernit E, Carcy B, Piarroux R, Harlé JR, Schleinitz N. Babesia microti: an unusual travel-related disease. BMC Infect Dis. 2013;13:99. doi:10.1186/1471-2334-13-99.
Berens-Riha N, Zechmeister M, Hirzmann J, Draenert R, Bogner J, Löscher T. Babesiose bei einem splenektonierten Reisenden aus den USA - Nach Deutschland importierte Infektion durch Zecken. Flugmedizin Tropenmedizin Reisemedizin - FTR. 2012;19:113–5.
Jablonska J, Zarnowska-Prymek H, Stanczak J, Kozlowska J, Wiercinska-Drapalo A (2013) Symptomatic co-infection with Babesia microti and Borrelia burgdorferi in Polish immuncompetent patient. in review process.
Haapasalo K, Suomalainen P, Sukura A, Siikamaki H, Jokiranta TS. Fatal babesiosis in man, Finland, 2004. Emerg Infect Dis. 2010;16:1116–8. doi:10.3201/eid1607.091905.
Andrić B, Golubović M, Terzic D, Dupanovic B, Icevic M. First diagnostic cases of human babesiosis in Montenegro. Braz J Infect Dis. 2012;16:498–9. doi:10.1016/j.bjid.2012.04.001.
Centeno-Lima S, do Rosário V, Parreira R, Maia AJ, Freudenthal AM, Nijhof AM, Jongejan F. A fatal case of human babesiosis in Portugal: molecular and phylogenetic analysis. Trop Med Int Health. 2003;8:760–4.
Welc-Falęciak R, Hildebrandt A, Siński E. Co-infection with Borrelia species and other tick-borne pathogens in humans: two cases from Poland. Ann Agric Environ Med. 2010;17:309–13.
Zahler M, Rinder H, Gothe R. Genotypic status of Babesia microti within the piroplasms. Parasitol Res. 2000;86:642–6.
Humiczewska M, Kuźna-Grygiel W. A case of imported human babesiosis in Poland. Wiad Parazytol. 1997;43:227–9.
Meer-Scherrer L, Adelson M, Mordechai E, Lottaz B, Tilton R. Babesia microti infection in Europe. Curr Microbiol. 2004;48:435–7. doi:10.1007/s00284-003-4238-7.
Moreno Giménez JC, Jiménez Puya R, Galàn Gutiérrez M, Ortega Salas R, Dueñas Jurado JM. Erythema figuratum in septic babesiosis. J Eur Acad Dermatol Venereol. 2006;20:726–8. doi:10.1111/j.1468-3083.2006.01492.x.
Falagas ME, Klempner MS. Babesiosis in patients with AIDS: a chronic infection presenting as fever of unknown origin. Clin Infect Dis. 1996;22:809–12.
Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin Infect Dis. 2007;45:1208–13. doi:10.1086/522181.
Krause PJ, Gewurz BE, Hill D, Marty FM, Vannier E, Foppa IM, Furman RR, Neuhaus E, Skowron G, Gupta S, McCalla C, Pesanti EL, Young M, Heiman D, Hsue G, Gelfand JA, Wormser GP, Dickason J, Bia FJ, Hartman B, Telford SR 3rd, Christianson D, Dardick K, Coleman M, Girotto JE, Spielman A. Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis. 2008;46:370–6. doi:10.1086/525852.
Hunfeld KP, Allwinn R, Peters S, Kraiczy P, Brade V. Serologic evidence for tick-borne pathogens other than Borrelia burgdorferi (TOBB) in Lyme borreliosis patients from midwestern Germany. Wien Klin Wochenschr. 1998;110:901–8.
Hunfeld KP, Lambert A, Kampen H, Albert S, Epe C, Brade V, Tenter AM. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J Clin Microbiol. 2002;40:2431–6.
Chmielewska-Badora J, Moniuszko A, Żukiewicz-Sobczak W, Zwoliński J, Piątek J, Pancewicz S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann Agric Environ Med. 2012;19:271–4.
Foppa IM, Krause PJ, Spielman A, Goethert H, Gern L, Brand B, Telford SR 3rd. Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg Infect Dis. 2002;8:722–6.
Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi:10.1128/CMR.00022-10.
Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22:469–88, viii–ix. doi:10.1016/j.idc.2008.03.010.
Hildebrandt A, Tenter AM, Straube E, Hunfeld KP. Human babesiosis in Germany: Just overlooked or truly new? Int J Med Microbiol 2008;298:336–46.
Wójcik-Fatla A, Bartosik K, Buczek A, Dutkiewicz J. Babesia microti in adult Dermacentor reticulatus ticks from eastern Poland. Vector Borne Zoonotic Dis. 2012;12:841–3. doi:10.1089/vbz.2011.0904.
Walter G. Transmission of Babesia microti by nymphs of Dermacentor marginatus, D. reticulatus, Haemaphysalis punctata, Rhipicephalus sanguineus and Ixodes hexagonus. Z Parasitenkd. 1982;66:353–4.
Skotarczak B, Cichocka A. Isolation and amplification by polymerase chain reaction DNA of Babesia microti and Babesia divergens in ticks in Poland. Ann Agric Environ Med. 2001;8:187–9.
Hartelt K, Oehme R, Frank H, Brockmann SO, Hassler D, Kimmig P. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int J Med Microbiol. 2004;293:86–92.
Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis HJ, Vayssier-Taussat M. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87. doi:10.1051/vetres:2004052.
Casati S, Sager H, Gern L, Piffaretti JC. Presence of potentially pathogenic Babesia sp. for human in Ixodes ricinus in Switzerland. Ann Agric Environ Med. 2006;13:65–70.
Piccolin G, Benedetti G, Doglioni C, Lorenzato C, Mancuso S, Papa N, Pitton L, Ramon MC, Zasio C, Bertiato G. A study of the presence of B. burgdorferi, Anaplasma (previously Ehrlichia) phagocytophilum, Rickettsia, and Babesia in Ixodes ricinus collected within the territory of Belluno, Italy. Vector Borne Zoonotic Dis. 2006;6:24–31. doi:10.1089/vbz.2006.6.24.
Wielinga PR, Fonville M, Sprong H, Gaasenbeek C, Borgsteede F, van der Giessen JW. Persistent detection of Babesia EU1 and Babesia microti in Ixodes ricinus in the Netherlands during a 5-year surveillance: 2003–2007. Vector Borne Zoonotic Dis. 2009;9:119–22. doi:10.1089/vbz.2008.0047.
Reye AL, Hübschen JM, Sausy A, Muller CP. Prevalence and seasonality of tick-borne pathogens in questing Ixodes ricinus ticks from Luxembourg. Appl Environ Microbiol. 2010;76:2923–31. doi:10.1128/AEM.03061-09.
Cassini R, Bonoli C, Montarsi F, Tessarin C, Marcer F, Galuppi R. Detection of Babesia EU1 in Ixodes ricinus ticks in northern Italy. Vet Parasitol. 2010;171:151–4. doi:10.1016/j.vetpar.2010.03.009.
Hildebrandt A, Franke J, Schmoock G, Pauliks K, Kramer A, Straube E. Diversity and coexistence of tick-borne pathogens in central Germany. J Med Entomol. 2011;48:651–5.
Franke J, Hildebrandt A, Meier F, Straube E, Dorn W. Prevalence of Lyme disease agents and several emerging pathogens in questing ticks from the German Baltic coast. J Med Entomol. 2011;48:441–4.
Gigandet L, Stauffer E, Douet V, Rais O, Moret J, Gern L. Prevalence of three zoonotic Babesia species in Ixodes ricinus (Linne, 1758) nymphs in a suburban forest in Switzerland. Vector Borne Zoonotic Dis. 2011;11:363–6. doi:10.1089/vbz.2010.0195.
Schorn S, Pfister K, Reulen H, Mahling M, Silaghi C. Occurrence of Babesia spp., Rickettsia spp. and Bartonella spp. in Ixodes ricinus in Bavarian public parks, Germany. Parasit Vectors. 2011;4:135. doi:10.1186/1756-3305-4-135.
Lempereur L, De Cat A, Caron Y, Madder M, Claerebout E, Saegerman C, Losson B. First molecular evidence of potentially zoonotic Babesia microti and Babesia sp. EU1 in Ixodes ricinus ticks in Belgium. Vector Borne Zoonotic Dis. 2011;11:125–30. doi:10.1089/vbz.2009.0189.
Reis C, Cote M, Paul RE, Bonnet S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 2011;11:907–16. doi:10.1089/vbz.2010.0103.
Egyed L, Elő P, Sréter-Lancz Z, Széll Z, Balogh Z, Sréter T. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick Borne Dis. 2012;3:90–4. doi:10.1016/j.ttbdis.2012.01.002.
Lommano E, Bertaiola L, Dupasquier C, Gern L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl Environ Microbiol. 2012;78:4606–12. doi:10.1128/AEM.07961-11.
Øines Ø, Radzijevskaja J, Paulauskas A, Rosef O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit Vectors. 2012;5:156. doi:10.1186/1756-3305-5-156.
Blaschitz M, Narodoslavsky-Gföller M, Kanzler M, Stanek G, Walochnik J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol. 2008;74:4841–6. doi:10.1128/AEM.00035-08.
Franke J, Fritzsch J, Tomaso H, Straube E, Dorn W, Hildebrandt A. Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in Central Germany. Appl Environ Microbiol. 2010;76:6829–36. doi:10.1128/AEM.01630-10.
Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E. The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis. 2010;1:105–7. doi:10.1016/j.ttbdis.2009.12.003.
Hasle G, Leinaas HP, Røed KH, Øines Ø. Transport of Babesia venatorum-infected Ixodes ricinus to Norway by northward migrating passerine birds. Acta Vet Scand. 2011;53:41. doi:10.1186/1751-0147-53-41.
Movila A, Reye AL, Dubinina HV, Tolstenkov OO, Toderas I, Hübschen JM, Muller CP, Alekseev AN. Detection of Babesia sp. EU1 and members of spotted fever group rickettsiae in ticks collected from migratory birds at Curonian Spit, North-Western Russia. Vector Borne Zoonotic Dis. 2011;11:89–91. doi:10.1089/vbz.2010.0043.
Skotarczak B, Rymaszewska A, Wodecka B, Sawczuk M. Molecular evidence of coinfection of Borrelia burgdorferi sensu lato, human granulocytic ehrlichiosis agent, and Babesia microti in ticks from northwestern Poland. J Parasitol. 2003;89:194–6. doi:10.1645/0022-3395(2003)089[0194:MEOCOB]2.0.CO;2.
Wójcik-Fatla A, Szymańska J, Wdowiak L, Buczek A, Dutkiewicz J. Coincidence of three pathogens (Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Babesia microti) in Ixodes ricinus ticks in the Lublin macroregion. Ann Agric Environ Med. 2009;16:151–8.
Hildebrandt A, Fritzsch J, Franke J, Sachse S, Dorn W, Straube E. Co-circulation of emerging tick-borne pathogens in Middle Germany. Vector Borne Zoonotic Dis. 2011;11:533–7. doi:10.1089/vbz.2010.0048.
Sytykiewicz H, Karbowiak G, Hapunik J, Szpechciński A, Supergan-Marwicz M, Golawska S, Sprawka I, Czerniewicz P. Molecular evidence of Anaplasma phagocytophilum and Babesia microti co-infections in Ixodes ricinus ticks in central-eastern region of Poland. Ann Agric Environ Med. 2012;19:45–9.
Hunfeld KP, Brade V. Zoonotic Babesia: possibly emerging pathogens to be considered for tick-infested humans in Central Europe. Int J Med Microbiol. 2004;293:93–103.
Esernio-Jenssen D, Scimeca PG, Benach JL, Tenenbaum MJ. Transplacental/perinatal babesiosis. J Pediatr. 1987;110:570–2.
New DL, Quinn JB, Qureshi MZ, Sigler SJ. Vertically transmitted babesiosis. J Pediatr. 1997;131:163–4.
Sethi S, Alcid D, Kesarwala H, Tolan RW Jr. Probable congenital babesiosis in infant, new jersey, USA. Emerg Infect Dis. 2009;15:788–91. doi:10.3201/eid1505.070808.
Aderinboye O, Syed SS. Congenital babesiosis in a four-week-old female infant. Pediatr Infect Dis J. 2010;29:188. doi:10.1097/INF.0b013e3181c3c971.
Joseph JT, Purtill K, Wong SJ, Munoz J, Teal A, Madison-Antenucci S, Horowitz HW, Aguero-Rosenfeld ME, Moore JM, Abramowsky C, Wormser GP. Vertical transmission of Babesia microti, United States. Emerg Infect Dis. 2012;18:1318–21. doi:10.3201/eid1808.110988.
Mylonakis E. When to suspect and how to monitor babesiosis. Am Fam Physician. 2001;63:1969–74.
Telford SR, 3rd, Maguire JH. Babesiosis. In: Guerrant RL, Walker DH, Weller PF editors. Tropical infectious diseases: principles, pathogens, and practice. Oxford: Churchill Livingstone. 2006. p. 1063–71.
Benach JL, Habicht GS, Hamburger MI. Immunoresponsiveness in acute babesiosis in humans. J Infect Dis. 1982;146:369–80.
Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30:1323–37.
Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117–25. doi:10.1086/319742.
Pantanowitz L, Cannon ME. Extracellular Babesia microti parasites. Transfusion. 2001;41:440.
Gubernot DM, Nakhasi HL, Mied PA, Asher DM, Epstein JS, Kumar S. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion. 2009;49:2759–71. doi:10.1111/j.1537-2995.2009.02429.x.
Krause PJ, Telford SR 3rd, Spielman A, Sikand V, Ryan R, Christianson D, Burke G, Brassard P, Pollack R, Peck J, Persing DH. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–60.
Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from ixodes ticks. Clin Microbiol Rev. 2006;19:708–27. doi:10.1128/CMR.00011-06.
Krause PJ, Spielman A, Telford SR 3rd, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–5. doi:10.1056/NEJM199807163390304.
Leiby DA. Babesiosis and blood transfusion: flying under the radar. Vox Sang. 2006;90:157–65. doi:10.1111/j.1423-0410.2006.00740.x.
Gray JS, Weiss LM. Babesia microti. In: Khan NA, editor. Emerging Protozoan Pathogens. Abingdon: Taylor and Francis. 2008. p. 303–49.
Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol. 2000;38:4511–6.
Vannier E, Krause PJ. Update on babesiosis. Interdiscip Perspect Infect Dis. 2009;2009:984568. doi:10.1155/2009/984568.
Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters TP. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501.
Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–36.
Bruckner DA, Garcia LS, Shimizu RY, Goldstein EJ, Murray PM, Lazar GS. Babesiosis: problems in diagnosis using autoanalyzers. Am J Clin Pathol. 1985;83:520–1.
Krause PJ, Telford SR 3rd. Babesiosis. In: Gilles HM, editor. Protozoal Diseases. Arnold: London; 1999. p. 236–48.
Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50:903–8. doi:10.1128/JCM.05848-11.
Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–103.
Thomford JW, Conrad PA, Telford SR 3rd, Mathiesen D, Bowman BH, Spielman A, Eberhard ML, Herwaldt BL, Quick RE, Persing DH. Cultivation and phylogenetic characterization of a newly recognized human pathogenic protozoan. J Infect Dis. 1994;169:1050–6.
Persing DH, Conrad PA. Babesiosis: new insights from phylogenetic analysis. Infect Agents Dis. 1995;4:182–95.
Johnson ST, Cable RG, Leiby DA. Lookback investigations of Babesia microti-seropositive blood donors: seven-year experience in a Babesia-endemic area. Transfusion. 2012;52:1509–16. doi:10.1111/j.1537-2995.2011.03345.x.
Bloch EM, Lee TH, Krause PJ, Telford SR 3rd, Montalvo L, Chafets D, Usmani-Brown S, Lepore TJ, Busch MP. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013;. doi:10.1111/trf.12098.
Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JD, Wengenack NL, Rosenblatt JE, Cockerill FR 3rd, Smith TF. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi:10.1128/CMR.19.1.165-256.2006.
Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GJ. Current advances in detection and treatment of babesiosis. Curr Med Chem. 2012;19:1504–18.
Johnson N, Voller K, Phipps LP, Mansfield K, Fooks AR. Rapid molecular detection methods for arboviruses of livestock of importance to northern Europe. J Biomed Biotechnol. 2012;2012:719402. doi:10.1155/2012/719402.
Krause PJ, Telford S 3rd, Spielman A, Ryan R, Magera J, Rajan TV, Christianson D, Alberghini TV, Bow L, Persing D. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34:2791–4.
Herwaldt BL, McGovern PC, Gerwel MP, Easton RM, MacGregor RR. Endemic babesiosis in another eastern state: New Jersey. Emerg Infect Dis. 2003;9:184–8.
Krause PJ, Telford SR 3rd, Ryan R, Conrad PA, Wilson M, Thomford JW, Spielman A. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–6.
Gelfand JA. Babesia. In: Mandell GL, Douglas RG, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 5th ed. Churchill Livingstone: New York; 2000. p. 2899–902.
Gabrielli S, Galuppi R, Marcer F, Marini C, Tampieri MP, Moretti A, Pietrobelli M, Cancrini G. Development of culture-based serological assays to diagnose Babesia divergens infections. Vector Borne Zoonotic Dis. 2012;12:106–10. doi:10.1089/vbz.2011.0706.
WHO. World Health Organisation Veterinary Public Health Unit: WHO Workshop on Lyme Borreliosis Diagnosis and Surveillance. Document No. WHO/CDS/VPH/95.141. Geneva: World Health Organization 1995.
Palmer GH, McElwain TF. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–53.
Musoke AJ, Palmer GH, McElwain TF, Nene V, McKeever D. Prospects for subunit vaccines against tick-borne diseases. Br Vet J. 1996;152:621–39.
Priest JW, Moss DM, Won K, Todd CW, Henderson L, Jones CC, Wilson M. Multiplex assay detection of immunoglobulin G antibodies that recognize Babesia microti antigens. Clin Vaccine Immunol. 2012;19:1539–48. doi:10.1128/CVI.00313-12.
Homer MJ, Lodes MJ, Reynolds LD, Zhang Y, Douglass JF, McNeill PD, Houghton RL, Persing DH. Identification and characterization of putative secreted antigens from Babesia microti. J Clin Microbiol. 2003;41:723–9.
Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi:10.1086/508667.
Gelfand JA Vannier E. Clinical manifestations, diagnosis, treatment, and prevention of babesiosis. http://wwwuptodatecom/contents/clinical-manifestations-diagnosis-treatment-and-prevention-of-babesiosis#H1. Accessed online 11 Sept 2013.
Vial HJ, Gorenflot A. Chemotherapy against babesiosis. Vet Parasitol. 2006;138:147–60. doi:10.1016/j.vetpar.2006.01.048.
Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR 3rd, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–8. doi:10.1056/NEJM200011163432004.
Wittner M, Lederman J, Tanowitz HB, Rosenbaum GS, Weiss LM. Atovaquone in the treatment of Babesia microti infections in hamsters. Am J Trop Med Hyg. 1996;55:219–22.
Marley SE, Eberhard ML, Steurer FJ, Ellis WL, McGreevy PB, Ruebush TK 2nd. Evaluation of selected antiprotozoal drugs in the Babesia microti-hamster model. Antimicrob Agents Chemother. 1997;41:91–4.
Rowin KS, Tanowitz HB, Wittner M. Therapy of experimental babesiosis. Ann Intern Med. 1982;97:556–8.
Wittner M, Rowin KS, Tanowitz HB, Hobbs JF, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy GR. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–4.
Weiss LM. Babesiosis in humans: a treatment review. Expert Opin Pharmacother. 2002;3:1109–15. doi:10.1517/14656566.3.8.1109.
Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet JJ. Quinine in the treatment of Babesia divergens infections in humans. Eur J Clin Microbiol Infect Dis. 1996;15:840–1.
Denes E, Rogez JP, Dardé ML, Weinbreck P. Management of Babesia divergens babesiosis without a complete course of quinine treatment. Eur J Clin Microbiol Infect Dis. 1999;18:672–3.
Corpelet C, Vacher P, Coudore F, Laurichesse H, Conort N, Souweine B. Role of quinine in life-threatening Babesia divergens infection successfully treated with clindamycin. Eur J Clin Microbiol Infect Dis. 2005;24:74–5. doi:10.1007/s10096-004-1270-x.
Pudney M, Gray JS. Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J Parasitol. 1997;83:307–10.
Gorenflot A, Bazin C, Ambroise-Thomas P. Human babesiosis. Treatment of severe forms. Presse Med. 1987;16:1099.
Weiss LM, Wittner M, Wasserman S, Oz HS, Retsema J, Tanowitz HB. Efficacy of azithromycin for treating Babesia microti infection in the hamster model. J Infect Dis. 1993;168:1289–92.
Gray JS, Pudney M. Activity of atovaquone against Babesia microti in the Mongolian gerbil, Meriones unguiculatus. J Parasitol. 1999;85:723–8.
Meldrum SC, Birkhead GS, White DJ, Benach JL, Morse DL. Human babesiosis in New York State: an epidemiological description of 136 cases. Clin Infect Dis. 1992;15:1019–23.
Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, Mittleman A, Aguero-Rosenfeld M, Topal J, Krause PJ. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis. 2010;50:381–6. doi:10.1086/649859.
Spencer AM, Goethert HK, Telford SR 3rd. Holman PJ. In vitro host erythrocyte specificity and differential morphology of Babesia divergens and a zoonotic Babesia sp. from eastern cottontail rabbits (Sylvilagus floridanus). J Parasitol. 2006;92:333–40. doi:10.1645/GE-662R.1.
Kogut SJ, Thill CD, Prusinski MA, Lee JH, Backerson PB, Coleman JL, Anand M, White DJ. Babesia microti, upstate New York. Emerg Infect Dis. 2005;11:476–8.
Stramer SL, Hollinger FB, Katz LM, Kleinman S, Metzel PS, Gregory KR, Dodd RY. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49:1S–29S. doi:10.1111/j.1537-2995.2009.02279.x.
Pantanowitz L, Aufranc S 3rd, Monahan-Earley R, Dvorak A, Telford SR 3rd. Transfusion medicine illustrated. Morphologic hallmarks of Babesia. Transfusion. 2002;42:1389.
Grabowski EF, Giardina PJ, Goldberg D, Masur H, Read SE, Hirsch RL, Benach JL. Babesiosis transmitted by a transfusion of frozen-thawed blood. Ann Intern Med. 1982;96:466–7.
Zhao Y, Love KR, Hall SW, Beardell FV. A fatal case of transfusion-transmitted babesiosis in the State of Delaware. Transfusion. 2009;49:2583–7. doi:10.1111/j.1537-2995.2009.02454.x.
Setty S, Khalil Z, Schori P, Azar M, Ferrieri P. Babesiosis. Two atypical cases from Minnesota and a review. Am J Clin Pathol. 2003;120:554–9. doi:10.1309/N3DP-9MFP-NUJD-4XJY.
Matsui T, Inoue R, Kajimoto K, Tamekane A, Okamura A, Katayama Y, Shimoyama M, Chihara K, Saito-Ito A, Tsuji M. First documentation of transfusion-associated babesiosis in Japan. Rinsho Ketsueki. 2000;41:628–34.
Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol. 2008;53:323–43. doi:10.1146/annurev.ento.53.103106.093429.
Siński E, Welc-Falęciak R, Pogłód R. Babesia spp. infections transmitted through blood transfusion. Wiad Parazytol. 2011;57:77–81.
White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL. Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–54.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jeremy S. Gray, Klaus-Peter Hunfeld are members of the ESCMID Study Group for Lyme Borreliosis (ESGBOR).
Rights and permissions
About this article
Cite this article
Hildebrandt, A., Gray, J.S. & Hunfeld, KP. Human Babesiosis in Europe: what clinicians need to know. Infection 41, 1057–1072 (2013). https://doi.org/10.1007/s15010-013-0526-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-013-0526-8