Abstract
Gametocyte proteins are being explored as potential vaccine candidates against Eimeria sp. in chicken since they are the components of the resilient oocyst wall. The aim of this study was to investigate the immunoprophylactic efficacy of recombinant Eimeria tenella gametocyte antigen 22 (EtGam22) in chickens against homologous oocyst challenge. Broiler chicks were subcutaneously immunized individually with 100 μg of recombinant EtGam22 adjuvanted with Montanide ISA 71 VG at 7 days of age and boosted 2 weeks later. The immunized chickens were challenged individually with 1 × 104 sporulated oocysts of E. tenella 1 week post-booster immunization. The anti-EtGam22 IgY and serum cytokine response was measured post-immunization. The results showed that the anti-EtGam22 IgY antibody, serum IFN-γ, IL-2, TGF-β, and IL-4 levels in chickens vaccinated with recombinant protein were significantly increased post-immunization as compared to unimmunized challenged controls (P < 0.05). The peripheral blood lymphocyte proliferation activity was also found significantly higher in EtGam22-immunized group on day 28, i.e., pre-challenge (P < 0.05). Upon homologous oocyst challenge, chickens immunized with rEtGam22 exhibited a significant drop in the total oocyst output per bird (246.78 ± 36.9 × 106, 45.23% reduction) and a significantly higher weight gain (497.7 ± 19.2 g) as compared to unimmunized challenged controls. Taken together, these data indicate that EtGam22 is a potent immunogen for use as a subunit vaccine against cecal coccidiosis in chickens as it induces a diverse and robust immune response involving multiple cytokines and strong antibody titers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poultry coccidiosis, caused by protozoan parasites of the genus Eimeria, is associated with global economic losses in excess of 2 billion Euros (Peek and Landman 2011). Among seven recognized species of Eimeria in chickens, Eimeria tenella is arguably the most pathogenic and prevalent species causing mortality, malabsorption, inefficient feed utilization, and impaired growth rate in broilers. These protozoan parasites are ubiquitous, having asexual (sporozoites and merozoites) and sexual (macrogametocyte/macrogamete and microgametocyte/microgamete) stages of development in the intestinal epithelium, subsequently forming the oocysts, which are excreted by the host. The oocyst has a rigid bilayered wall, forming a resilient structure that protects it from the adverse environmental conditions. The contents of two specific organelles of macrogametes, types 1 and 2 wall-forming bodies (WFBI and WFBII), are the precursors of the oocyst wall (Belli et al. 2006). Hence, if the oocyst wall formation is manipulated by immunization, thereby deterring the survival of oocyst in the environment, it would be possible to stop this vicious cycle of ingestion and excretion of oocysts.
Among the plethora of gametocyte proteins involved in oocyst wall formation, only a few genes encoding gametocyte proteins have been cloned and sequenced from avian Eimeria species, such as EmGam56 (Belli et al. 2002), EmGam82 (Belli et al. 2003), and EmGam230 (Fried et al. 1992) of E. maxima; EtGam56 (EtGam56 tmp 1), EtGam59 (EtGam56 tmp 2), and EtGam22 of E. tenella (Krücken et al. 2008, Belli et al. 2009); EaGam56 of E. acervulina (Belli et al. 2009); and EnGam22 of E. necatrix (Liu et al. 2014). Potential use of gametocyte antigens involved in the formation of the oocyst wall has been described for Eimeria maxima (Wallach 2002; Belli et al. 2004). A commercial vaccine “CoxAbic®” based on native gametocyte antigens of E. maxima, Gam56 and Gam82, was launched in the year 2002. In laboratory and floor pen studies, CoxAbic® reduced oocyst shedding of the three major species of Eimeria (E. maxima, E. tenella, and E. acervulina) in broiler chickens by 50–80% (Wallach 2002). Since the production of this vaccine relied on affinity purification of the native gametocyte antigens from parasites, it is expensive, time-consuming, and laborious. Recombinant Gam56 and Gam82 of E. maxima were recognized by protective chicken serum raised against affinity purified gametocyte antigen and could elicit a dose-dependent antibody response in chickens, suggesting that the recombinant antigens maintain the antigenic and immunogenic properties of the native proteins (Belli et al. 2004).
Genomic studies have established EtGam22 as the first multi-copy gene for Eimeria species having extraordinarily high copy number and extremely conserved sequences between copies. Thus, EtGam22 has emerged as an important oocyst wall protein which may have an important role in oocyst wall formation. EtGam22, like EmGam56, is transported to the WFBII and participates in the formation of the inner oocyst wall and/or the Stieda body (Krücken et al. 2008). In view of the interest in exploiting gametocyte antigens of E. tenella and a dearth of reports of immunoprotective efficacy of recombinant gametocyte antigens of E. tenella, the present study was undertaken to evaluate the immunoprotective properties of recombinant EtGam22 (rEtGam22) against homologous oocyst challenge in broiler chicken.
Materials and method
Experimental birds
One-day-old CariBro Vishal broiler chickens were obtained from the ICAR-Central Avian Research Institute, Izatnagar, Uttar Pradesh, India. The chickens were reared in sterilized steel cages with raised wire flooring housed inside a well-ventilated room of experimental animal shed of the Division of Parasitology, ICAR-IVRI. Chickens were fed standard broiler ration ad libitum without any coccidiostat. The fecal droppings of the birds were periodically screened for coccidiosis-free status (Kumar et al. 2014).
Parasites
The clonal line of Indian isolate of E. tenella maintained in the Division of Parasitology, ICAR-IVRI, was used (Kundu et al. 2015). Sporulated oocysts were separated by salt floatation and cleaned by 2.5% sodium hypochlorite treatment, washed three times with PBS, and enumerated using a McMaster chamber prior to the infection.
RNA extraction and amplification of the Eimeria tenella Gam22 gene
The total RNA was isolated from cecal scrapings of E. tenella–infected chickens collected after 120 h of infection using RNeasy mini kit (Qiagen, Germany) following the manufacturer’s protocol. The cDNA was synthesized from the total RNA using oligo-dT primer following the standard protocol described in AccuScript high-fidelity first-strand cDNA synthesis kit (Stratagene). The E. tenella Gam22 (EtGam22) coding sequence was amplified by PCR using a blend of Pfu DNA polymerase and Dream Taq polymerase, in the ratio of 1 U:30 U with the expression primer sequences (EtGam22F primer: ATGGATCCCCGTTTACTGAGGCTACAAG and EtGam22R primer: GCAAGCTTTCCCGACCAATAGCTTAGTT) containing BamHI and HindIII restriction enzyme site (underlined). PCR reaction was carried out at an initial denaturation of 95 °C for 3 min followed by 40 cycles of denaturation at 95 °C for 30 s, an annealing at 58 °C for 1 min, an extension at 72 °C for 1 min, and a final extension at 72 °C for 7 min. The PCR products were analyzed by 1.5% agarose gel electrophoresis. Amplicons were cloned into the pET32a (+) plasmid vector and transformed into Escherichia coli (Nova Blue strain) using a Transform Aid bacterial transformation kit (Thermo Scientific, USA). The cloned plasmids were verified by sequence analysis and used to transform competent BL21pLysS E. coli cells.
Bioinformatics analysis
Nucleotide sequences obtained after custom sequencing were searched for similarity using the BLASTn program (nucleotide blast) through the National Center for Biotechnology Information website (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and aligned using the Megalign program in DNA Star (Laser gene Suite 6.0) software. The protein-encoding nucleotide sequences were translated in silico using the Edit Sequence program of DNA Star (Laser gene Suite 6.0) and BLASTp (protein–protein BLAST) was performed. The sequences generated here were compared to the reference sequences available in the public domain. The EtGam22 sequence generated in the present study was submitted to GenBank and the accession number (KY887585) was obtained.
Recombinant protein expression and purification
rEtGam22 protein expression from E. coli BL21 cells was achieved following the induction of bacterial culture with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). The induced bacterial cells were incubated for 6 h and the recombinant protein was purified under denaturing conditions as per QIAexpressionist™ manual (Qiagen, Germany), extensively dialyzed against decreasing concentrations of urea and finally PBS. The purity of the recombinant protein was checked by electrophoresis on 12% SDS-PAGE gel following Coomassie brilliant blue staining. The concentration of the recombinant protein was determined by the Bradford protein assay kit (Amresco) following the manufacturer’s protocol and kept at − 80 °C until further use.
Expression and purification of recombinant thioredoxin
Thioredoxin, a protein encoded in the pET32 vector backbone, is co-expressed in the recombinant protein when the recombinant vector is induced with IPTG. Hence, it was expressed and purified for subsequent use in mock immunization as described previously (Kundu et al. 2017).
Immunoblot analysis of rEtGam22
A purified rEtGam22 protein initially resolved on 12% SDS-PAGE gel was transferred on to the nitrocellulose membrane, with a pore size of 0.45 μm (Thermo Scientific, USA) using a Bio-Rad mini trans-blot system, with a constant power supply of 100 V for 1 h. Expression of the recombinant protein was confirmed by probing the blotted proteins with a 1:1000 dilution of Ni-NTA HRP conjugate (Qiagen), as per the protocol provided by the manufacturer. The specificity of rEtGam22 protein was confirmed by immunoblot using anti-E. tenella convalescent serum (Kundu et al. 2017) and negative serum at 1:20 dilutions.
Experimental design
Prior to immunization, chicks (n = 75) were reared in two large cages up to 7 days of age. Thereafter, they were separated into five groups of 15 chicks each viz. Gp. I (rEtGam22 immunized), Gp. II (oocyst-immunized), Gp. III (thioredoxin (rTrx) immunized), Gp. IV (unimmunized and challenged control), and Gp. V (unimmunized and unchallenged control). Chickens of Gp. I and III were subcutaneously immunized on 7 and 21 days of age individually with 100 μg of rEtGam22 and rTrx, respectively, along with Montanide ISA 71 VG adjuvant, while those of Gp. II were orally gavaged individually with 1000 E. tenella sporulated oocysts on the same days. Chickens of Gp. I to IV were orally challenged individually with 10,000 sporulated E. tenella oocysts on 28 days of age.
Collection of blood and serum
Blood was collected from brachial vein from six birds of each group under aseptic conditions and serum was harvested on day 7 (prior to primary immunization, 0 DPI), day 21 (prior to booster immunization, 14 DPI), day 28 (pre-challenge, 7DPB), and day 35 (7 days post-challenge, 7DPC) of the experiment. Blood collected on 7DPB and 7DPC was also used for the separation of peripheral blood lymphocytes for the use in a lymphocyte proliferation assay.
Evaluation of protective efficacy
Body weight of chickens in each group (n = 8) was measured on the day of challenge (28 days of age) and on the 10th day post-challenge (38 days of age). The body weight gain was determined by subtracting the body weight of birds on the 10th day post-challenge from the body weight at the time of challenge. Relative weight gain (%) was calculated using the formula: weight gain in chickens of the immunized group ∕ weight gain in chickens of the unchallenged control group × 100%. Lesion scores were determined at 6 days post-challenge on a scale of 0 (none) to 4 (high) in a blinded fashion by two independent observers as described (Johnson and Reid 1970). Fecal droppings from each group were collected daily between the 6th and 11th days post-challenge on a plastic sheet placed under the cage. All the fecal droppings were scraped from the plastic sheet, thoroughly homogenized, and weighed. Three random samples (technical replicates) were taken from homogenized feces, and oocysts per gram of droppings (OPG) were estimated by McMaster counting technique (Rafiqi et al. 2018). Total oocyst output per bird for each group was calculated by multiplying the OPG with the total weight of feces in each group and dividing it by the number of birds in each group. Any mortality following the challenge was also recorded.
Anti-Gam22 IgY response
Circulating anti-Gam22 IgY was estimated by indirect ELISA after laboratory standardization of antigen, test serum, and conjugate concentration by chequerboard titration. Microtiter plates were coated overnight with purified rEtGam22 protein (0.5 μg/ml) diluted in carbonate–bicarbonate buffer (pH 9.6), washed with PBS containing 0.05% Tween-20, and blocked with 5% skimmed milk (Sigma, USA) in PBS. Diluted test sera (1:100) were added, incubated for 2 h at 37 °C, washed, and bound antibody detected with 1:10000 goat anti-chicken IgG-HRP conjugate (Bethyl, USA). Optical density was recorded with a microplate reader at 492 nm (OD492) (Bio-Rad 680, USA).
Determination of serum cytokine concentrations
Serum was harvested from blood collected from six chickens of each group and then pooled into three aliquots of two chickens for each group. Biological triplicates (n = 3) were used for each group in the study for the estimation of serum cytokine levels. The concentration of cytokines viz. IFN-γ, IL-2, IL-4, and TGF-β was quantified by sandwich enzyme-linked immunosorbent assay (sandwich ELISA) using commercially available kits (Biospes Co., Ltd. China) as per manufacturer’s protocol.
Lymphocyte proliferation assay
Density gradient centrifugation using lymphocyte separation medium (HiSep LSM 1084, Himedia) was performed for isolation of lymphocytes from blood collected on 7DPB and 7DPC. Further lymphocyte proliferation assay was performed as described previously (Rafiqi et al. 2018). Antigen was used at the concentration of 5 μg/ml in the assay. At the end of incubation, 10 μl freshly prepared 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) in PBS (5 mg/ml) was added to each well and incubated further for 4 h. DMSO at 100 μl/well was added finally to dissolve formazan crystals and the wells were read at A570 optical density (OD) to calculate stimulation index.
Data analysis
The results obtained in the present study were analyzed using a one-way ANOVA Tukey’s HSD post hoc test (SPSS for Windows 20 software). In the analyses, P ≤ 0.05 was considered to be significant.
Results
Cloning and expression of EtGam22 recombinant protein
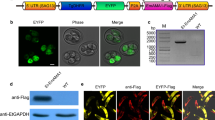
PCR amplification of the EtGam22 coding sequence from the Indian E. tenella isolate resulted in a 644-bp product (Fig. 1a). Amplicons were cloned into the pET32a (+) plasmid vector and sequenced (GenBank accession number KY887585). Sequence similarity searches in BLASTn revealed that the EtGam22 gene of Indian isolate was differing by three nucleotide substitutions from E. tenella gametocyte gene sequence CS000361, leading to change in only one amino acid at position 109 from S (Serine) to N (asparagine). Further, E. tenella gametocyte 22 protein of Indian isolate showed two amino acid substitutions at positions 103 (H to Q) and 194 (P to L) from E. tenella hypothetical protein sequence (XM013379496). The Indian isolate also showed 92% similarity with E. necatrix 22 kDa gametocyte protein gene sequence (KF649255) in BLASTn.
Cloning and expression of EtGam22 recombinant protein. a Lane 1, PCR-amplified EtGam22 DNA coding sequence. Lane M, DNA ladder. b Lane 1, His-tagged rEtGam22 protein. Lane M, protein marker. c Western blot analysis of purified rEtGam22 protein. Lane 1, rEtGam22 probed with Ni-NTA HRP conjugate. Lane 2, rEtGam22 probed with convalescent E. tenella–infected chicken serum. Lane M, protein marker
The Gam22 cDNA containing an NH2-terminal His6 epitope tag was expressed in E. coli, and the encoded protein was purified by Ni2+ chelate affinity chromatography. A protein band of approximately 44 kDa was observed on SDS-PAGE analysis (Fig. 1b). A similar band profile was detected by Western blotting using a monoclonal antibody against the His epitope tag as well as convalescent E. tenella–infected chicken sera (Fig. 1c).
Protective efficacy of rEtGam22 in immunized chickens following E. tenella challenge
EtGam22-immunized chickens exhibited a significantly increased (P < 0.05) average weight gain (497.7 ± 19.2 g) as compared to unimmunized challenged control (416.4 ± 40.3 g) chickens, while it was insignificantly higher (P > 0.05) than oocyst-immunized (473.6 ± 74.4 g) chickens (Supplementary Table 1). The relative body weight gain was also highest in group I, i.e., rEtGam22-immunized group (94.4%) followed by group II, i.e., oocyst-immunized group (89.8%). The mean lesion score of rEtGam22-immunized chickens (1.83 ± 0.75) was lower than the unimmunized challenged chickens (2.5 ± 0.54); the difference was statistically insignificant (P > 0.05). Mean cecal lesion score in oocyst-immunized chickens was significantly lower (P < 0.05) than that in unimmunized challenge controls. Chicken immunized with rEtGam22 exhibited significantly reduced OPG of feces (33.28 ± 6.23 × 104 amounting to 68.5% reduction in OPG) as well as the total output per bird (246.78 ± 36.9 × 106 amounting to 45.23% reduction in total oocyst output) as compared to unimmunized challenged (OPG 105.67 ± 8.06 × 104, total oocyst output per bird 450.55 ± 50.08 × 106) chickens (Supplementary Table 1).
Antibody (IgY) response to rEtGam22 vaccination
The anti-Gam22 IgY antibody titers in group I were significantly higher (P < 0.05) than the rTrx-immunized and unimmunized control groups throughout the experiment (Fig. 2). The anti-Gam22 IgY titers were also elevated significantly in group II (OI) as compared to unimmunized and rTrx-immunized groups on 7DPC.
Anti-EtGam22 IgY response in immunized and challenged chickens. rEtGam22 + Montanide ISA 71 VG adjuvant immunized (Gam22), oocyst-immunized (OI), thioredoxin + Montanide ISA 71 VG adjuvant mock immunized (adjuvant), unimmunized challenged (UNC), unimmunized unchallenged (UNUC). Chickens of Gam22, OI, and adjuvant control groups were immunized on 7 and 21 days of age. Chickens of all the groups, except UNUC, were challenged with 10,000 sporulated oocysts of E. tenella on 7DPB (28 days of age). Anti-EtGam22 antibodies were detected by antigen-specific indirect ELISA. Data points represent mean ± SE values (n = 6)
Lymphocyte proliferation assay
The cell-mediated lymphocyte proliferation patterns (in different groups of immunized and unimmunized chickens), as indicated by MTT, are depicted in Fig. 3 and Supplementary Table 2. Compared with the oocyst-immunized, mock immunized, or unimmunized controls, birds immunized with rEtGam22 showed a markedly increased (P < 0.05) lymphocyte proliferation activity in peripheral blood lymphocytes at day 7 post-booster immunization (7DPB), i.e., pre-challenge. The lymphocyte proliferation activity was maintained on 7DPC, with rEtGam22-immunized birds showing significantly increased stimulation index compared to unimmunized challenged birds (P < 0.05).
Recombinant EtGam22 antigen-specific lymphoproliferative response in chickens. (For abbreviations, immunization, and challenge schedule, see Fig. 2.) Lymphocytes were separated from blood just prior to challenge (pre-challenge) and on 7 days post-challenge. Lymphocyte proliferation in response to stimulation with 5 μg/ml of rEtGam22 was measured by taking OD at A570 after labeling with MTT. Stimulation index was determined after dividing the OD of stimulated culture by OD of unstimulated culture. Each bar represents mean ± SE values (n = 6)
Serum cytokine response
Following the immunization with rEtGam22, high levels of serum IFN-γ levels were recorded from 14 DPI onwards and reached a peak on 7DPC (216.23 ± 4.49 pg/ml). Similarly, in the oocyst-immunized group, there was a significant increase in IFN-γ concentration from 14DPI onwards (Fig. 4a).
Serum cytokine response in chickens following immunization and Eimeria tenella challenge. (For abbreviations, immunization, and challenge schedule, see Fig. 2.) Serum from six chickens of each group was pooled into three aliquots of two chickens for each group. Biological triplicates were used for estimation of INFγ, IL-2, IL-4, and TGF-β concentrations in serum of chickens of each group at specified time intervals by sandwich ELISA using commercially available kits. Data points represent mean ± SE values (n = 3)
Serum IL-2 levels were significantly elevated in the rEtGam22 group (477.33 ± 7.41 pg/ml) on 7DPB as compared to all other groups (Fig. 4b). However, chickens of the oocyst-immunized group were having the highest IL-2 levels on 7DPC (543.46 ± 4.29 pg/ml).
A significant increase in the level of IL-4 was observed in the rEtGam22-immunized and oocyst-immunized birds from 14DPI as compared to mock immunized and unimmunized challenge control birds. After an initial significant increase in IL-4 levels on 14DPI, the levels regressed on 7DPB in the rEtGam22-immunized group. However, the IL4 level again increased on 7DPC, although it did not exceed the 14DPI levels. In the oocyst-immunized group, the highest concentration of serum IL-4 was also observed on 14DPI. In unimmunized challenged group significant change in IL-4 was observed on 7DPC, albeit the values were still lower than rEtGam22 and oocyst-immunized groups (Fig. 4c).
A significant TGF-β response was observed in birds of the rEtGam22-immunized group wherein a sharp rise in the concentration of TGF-β was evident from 14DPI which peaked on 7DPB. There was no significant difference in levels of serum TGF-β between oocyst-immunized and rEtGam22 groups on 7DPB immunization; however, after the challenge, rEtGam22-immunized chicken displayed increased serum TGF-β levels (Fig. 4d).
Discussion
Since decades, coccidiosis in poultry has been controlled either by use of anticoccidials or by vaccination with live or attenuated oocysts. There have been general limitations to these approaches, viz. residues in meat, decreased feed conversion ratios (Crouch et al. 2003), and an everlasting risk of introducing virulent species into the gene pool. Moreover, antigenic diversity and strain variations limit the use of these live vaccines in a particular geographical area (Martin et al. 1997). Hence, the past few decades witnessed an escalation towards the development of subunit vaccines for control of coccidiosis. A number of candidate antigens expressed in different expression systems have been tested for their efficacy against coccidiosis (Kundu et al. 2017; Rafiqi et al. 2018; Jang et al. 2010; Lin et al. 2015). Initially, much importance was given to the antigens derived from asexual stages of the parasite, while the role of sexual stage antigens in eliciting an immune response was considered ambiguous (Rose and Hesketh 1976). Wallach and coworkers (Wallach et al. 1989, 1992) reported that gametocyte antigens were recognized by serum IgY collected from chickens that had recovered from E. maxima infection, and in breeding hens, these protective antibodies were transferred to the developing embryo via the egg yolk, providing partial immunity to chicks upon hatching. Since then, several proteins of molecular weight 14, 22, 30, 56, 82, and 230 kDa associated with E. maxima, E. tenella, and E. necatrix gametocytes have been identified as potential vaccine targets for inducing transmission-blocking immunity (Fried et al. 1992; Belli et al. 2002, 2003, 2009; Krücken et al. 2008). The success of commercially available vaccine CoxAbic® is also based on affinity purified gametocyte antigens. However, considering the cost and labor involved in the purification of native gametocyte antigens, a recombinant gametocyte antigen–based vaccine is warranted. Eimeria tenella gametocyte antigen 22 (EtGam22) is a multi-copy intronless gene and like EmGam56, it is transported to the WFBII and participates in the formation of the inner oocyst wall and/or the Stieda body (Krücken et al. 2008). In view of the limited information available on immunoprophylactic efficacy of EtGam22, the EtGam22 gene from an Indian isolate was cloned and expressed into a prokaryotic expression system to evaluate its vaccine potential against E. tenella infection in chickens. In the present study, minor amino acid substitutions between E. tenella Gam22 of Indian isolate and other E. tenella Gam22 sequences (CS000361.1, XM_0133746) available in GenBank were recorded, which substantiated the previous observations that the gene is highly conserved (Krücken et al. 2008).
The effect of vaccination using recombinant rEtGam22 protein was evaluated by oocyst reduction, weight gain, and lesion scoring. Immunization with rEtGam22 protein and subsequent challenge of chickens with live oocysts resulted into a drop in total oocyst output per bird (45.23%) as compared to unimmunized controls. Xu et al. 2013 reported 25.2–53.7% reduction in oocyst output among birds immunized with pcDNA-Gam56 in different dosages (25–100 μg). The reduced number of oocysts in the environment further elicit an active immune response following re-infection in chickens, thereby reducing the severity of disease (Wallach et al. 1995). One of the major drawbacks with the use of live vaccines is the decrease in weight gain which is unacceptable in the broiler industry (Crouch et al. 2003). Remarkably, in the present study, 94.4% relative weight gain was recorded in the rEtGam22-immunized group which was significantly higher than that in oocyst-immunized birds. In an earlier study, no significant weight gain in recombinant E. maxima Gam82-immunized birds was reported (Jang et al. 2010) and around 71–87% increase in relative weight gain was reported in pcDNA-Gam56-immunized birds (Xu et al. 2013).
The lesion score in EtGam22-immunized birds (1.83 ± 0.75) was lower than unimmunized birds, but the difference was considered insignificant. At times, lesion score is not of much significance and rather than the extent of lesions, the presence of parasitic stages and body weight loss are of utmost importance (Williams and Andrews 2001). In such cases, the lesions may be due to the localized or generalized immune response, and thus, lesion score may not be a sole indicator for immune protection (Chapman et al. 2005).
Although cell-mediated immunity is considered to play a central role in immune response against coccidiosis, it is apparent that birds produce parasite-specific antibodies in both circulations and across mucosal surfaces in response to primary infection (Lillehoj and Trout 1996). The protective role of antibodies in conferring immunity to coccidial infections has been widely studied (Lee et al. 2009). Wallach et al. 2003 reported that in addition to high antibody titers in the hens immunized with gametocyte antigen, reduced fecal oocyst shedding was observed on challenge with E. tenella in the progeny as well. In the present study, we found anti-Gam22 IgY titers in recombinant protein-immunized group were significantly increased on 14DPI which reached a peak on 7DPC.
Chickens infected with Eimeria produce IL-15 which stimulates the proliferation of antigen-specific T lymphocytes and NK cells (Lillehoj et al. 2001). An in vitro study of antigen-specific lymphocyte proliferation was performed by MTT assay on the day of challenge and on day 7 post-challenge. The proliferation of lymphocytes in response to rEtGam22 antigen was significantly higher in comparison to the unimmunized challenged and unchallenged group (P < 0.05). Several workers have evaluated lymphocyte proliferation in response to an antigen as an indicator of cell-mediated immunity and have reported significantly higher lymphocyte proliferation in the recombinant protein-immunized groups as compared to control groups (Rafiqi et al. 2018; Xu et al. 2013).
Extensive experimental evidence supports the concept that immunity mediated by lymphocytes and their secreted products, such as cytokines, mediate antigen-specific protection against challenge infection with Eimeria (Lillehoj et al. 2004). It has been reported that IFN-γ and IL-2 transcript levels of chickens immunized with different subunit vaccines are significantly increased and result in higher oocyst reduction (Kundu et al. 2017; Rafiqi et al. 2018; Lin et al. 2015). In a similar line, the present study also revealed that the IFN-γ levels of rEtGam22-immunized chicken was significantly (P < 0.05) higher than mock immunized and unimmunized unchallenged control birds throughout the experiment. Interleukin-2 (IL-2), one of the first T cell growth factors identified, is important in enhancing vaccine-induced immune responses against coccidiosis (Lillehoj et al. 2000). In the present study, consistently higher levels of IL-2 were recorded in rEtGam22-immunized group as compared to the controls. Jang et al. (2010) have also reported the upregulated expression of IL-2 in chickens following Gam82 immunization.
Interleukin-4 is an essential cytokine for B cell differentiation and proliferation and, accordingly, production of antibodies (Hofman et al. 1988), and thus, IL-4 can be expected to increase antibody production and enhance the anticoccidial immune response in chickens. In the present study, serum IL-4 was significantly increased in rEtGam22-immunized chickens post-primary immunization, but it decreased post-booster immunization and challenge. The possible reason for the same could be the activation of the cell-mediated immune response as evidenced by a sharp rise in serum IFN-γ levels post-booster immunization and challenge in rEtGam22-immunized chickens in the present study.
TGF-β is an important regulator of inflammation, exhibiting pro-inflammatory properties at low concentration and anti-inflammatory effects at high concentrations, and stimulates the repair of damaged mucosal epithelial integrity following injury (Omer et al. 2000). The results of our study are in concordance, as the concentration of TGF-β was found to be significantly higher in chickens following immunization with rEtGam22 and post-challenge. In previous vaccination trials against coccidiosis, significantly higher levels of TGF-β have been reported in immunized birds (Rafiqi et al. 2018; Song et al. 2010).
The findings of the present study indicate that the recombinant EtGam22 significantly elicited both Th1 and Th2 cytokine-mediated immune responses which resulted in an increased live weight gain, reduced cecal lesions, and reduced oocyst output in immunized chickens following challenge with E. tenella. It would be interesting to study the immunoprotective efficacy of rEtGam22 in chickens using different adjuvants, along with other gametocyte antigens (as subunit cocktail vaccine) and different routes of vaccination.
References
Belli SI, Witcombe D, Wallach MG, Smith NC (2002) Functional genomics of gam56: characterisation of the role of a 56 kilodalton sexual stage antigen in oocyst wall formation in Eimeria maxima. J Parasitol 32:1727–1737
Belli SI, Wallach MG, Smith NC (2003) Cloning and characterization of the 82 kDa tyrosine-rich sexual stage glycoprotein, GAM82, and its role in oocyst wall formation in the apicomplexan parasite, Eimeria maxima. Gene 307:201–212
Belli SI, Mai K, Skene CD, Gleeson MT, Witcombe DM, Katrib M, Finger A, Wallach MG, Smith NC (2004) Characterisation of the antigenic and immunogenic properties of bacterially expressed, sexual stage antigens of the coccidian parasite, Eimeria maxima. Vaccine 22:4316–4325
Belli SI, Smith NC, Ferguson DJ (2006) The coccidian oocyst: a tough nut to crack! Trend Parasitol 22:416–423
Belli SI, David JP, Ferguson MK, Iveta S, Kelly MJS, Flowers SA, Miska KB, Tomley FM, Shirley MW, Wallach MJ, Smith NC (2009) Conservation of proteins involved in oocyst wall formation in Eimeria maxima, Eimeria tenella and Eimeria acervulina. Int J Parasitol 39:1063–1070
Chapman HD, Roberts B, Shirley MW, Williams RB (2005) Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol 34:279–290
Crouch CF, Andrews SJ, Ward RG, Francis MJ (2003) Protective efficacy of a live attenuated anticoccidial vaccine administered to 1-day-old chickens. Avian Pathol 32:297–304
Fried M, Mencher D, Sar-Shalom O, Wallach M (1992) Developmental gene expression of a 230-kilodalton macrogamete-specific protein of the avian coccidial parasite, Eimeria maxima. Mol Biochem Parasitol 51:251–262
Hofman FM, Brock M, Taylor CR, Lyons B (1988) IL-4 regulates differentiation and proliferation of human precursor B cells. J Immunol 141:1185–1190
Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Cha SR, Lillehoj EP, Subramanian BM, Sriraman R, Srinivasan VA (2010) Eimeria maxima recombinant Gam82 gametocyte antigen vaccine protects against coccidiosis and augments humoral and cell-mediated immunity. Vaccine 28:2980–2985
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36
Krücken J, Hosse RJ, Mouafo AN, Entzeroth R, Bierbaum S, Marinovski P, Hain K, Greif G, Wunderlich F (2008) Excystation of Eimeria tenella sporozoites impaired by antibody recognizing gametocyte/oocyst antigens GAM22 and GAM56. Eukaryot Cell 7:202–211
Kumar S, Garg R, Moftah A, Clarke E, MacDonald SE, Chaudhary AS, Sparagano O, Banerjee PS, Kundu K, Tomley FM, Blake DP (2014) An optimized protocol for molecular identification of Eimeria from chickens. Vet Parasitol 199:24–31
Kundu K, Banerjee PS, Garg R, Kumar S, Mandal M, Tomley F, Blake D (2015) Cloning and sequencing of beta-tubulin and internal transcribed spacer-2 (ITS-2) of Eimeria tenella isolate from India. J Parasit Dis 39:539–544
Kundu K, Garg R, Kumar S, Mandal M, Tomley FM, Blake DP, Banerjee PS (2017) Humoral and cytokine response elicited during immunisation with recombinant immune mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet Parasitol 244:44–53
Lee SH, Lillehoj HS, Park DW, Jang SI, Morales A, García D, Lucio E, Larios R, Victoria G, Marrufo D, Lillehoj EP (2009) Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet Parasitol 163:123–126
Lillehoj HS, Trout JM (1996) Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev 9:349–360
Lillehoj HS, Choi KD, Jenkins MC, Vakharia VN, Song KD, Han JY, Lillehoj EP (2000) A recombinant Eimeria protein inducing interferon-γ production: comparison of different gene expression systems and immunization strategies for vaccination against coccidiosis. Avian Dis 44:379–389
Lillehoj HS, Min W, Choi KD, Babu US, Burnside J, Miyamoto T, Rosenthal BM, Lillehoj EP (2001) Molecular, cellular, and functional characterization of chicken cytokines homologous to mammalian IL-15 and IL-2. Vet Immunol Immunopathol 82:229–244
Lillehoj HS, Min W, Dalloul RA (2004) Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poult Sci 83:611–623
Lin Z, Shi Y, Deng B, Mao X, Yu D, Li W (2015) Protective immunity against Eimeria tenella infection in chickens following oral immunization with Bacillus subtilis expressing Eimeria tenella 3-1E protein. Parasitol Res 114:3229–3236
Liu D, Cao L, Zhu Y, Deng C, Su S, Xu J, Jin W, Li J, Wu L, Tao J (2014) Cloning and characterization of an Eimeria necatrix gene encoding a gametocyte protein and associated with oocyst wall formation. Parasit Vectors 7:27
Martin AG, Danforth HD, Barta JR, Fernando MA (1997) Analysis of immunological cross protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of E. maxima. Int J Parasitol 5:527–533
Omer FM, Kurtzhals JA, Riley EM (2000) Maintaining the immunological balance in parasitic infections: a role for TGF-beta. Parasitol Today 16:18–23
Peek HW, Landman WJM (2011) Coccidiosis in poultry: anticoccidial products, vaccines and their prevention strategies. Vet Qtly 31:143–161
Rafiqi SI, Garg R, Reena KK, Ram H, Singh M, Banerjee PS (2018) Immune response and protective efficacy of Eimeria tenella recombinant refractile body protein, EtSO7, in chickens. Vet Parasitol 258:108–113
Rose ME, Hesketh P (1976) Immunity to coccidiosis: stages of the life cycle of E. maxima which induce, and are affected by, the response of the host. Parasitology 73:25–37
Song H, Song X, Xu L, Yan R, Shah MA, Li X (2010) Changes of cytokines and IgG antibody in chickens vaccinated with DNA vaccines encoding Eimeria acervulina lactate dehydrogenase. Vet Parasitol 173:219–227
Wallach M (2002) The development of CoxAbic® a novel vaccine against coccidiosis. World Poult 18:24–26
Wallach M, Mencher D, Yarus S, Pillemer G, Halabi A, Pugatsch T (1989) Eimeria maxima: identification of gametocyte protein antigens and their possible role in protective immunity. Exp Parasitol 68:49–56
Wallach M, Halabi A, Pillemer G, Sar-Shalom O, Mencher D, Gilad M, Bendheim U, Danforth HD, Augustine PC (1992) Maternal immunization with gametocyte antigens as a means of providing protective immunity against Eimeria maxima in chickens. Infect Immun 60:2036–2039
Wallach M, Smith NC, Petracca M, Miller CMD, Eckert J, Braun R (1995) Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine 13:347–354
Wallach MG, Ashash U, Michael A, Smith NC (2003) Field application of a subunit vaccine against an enteric protozoan disease. PLoS One 12:e3948
Williams RB, Andrews SJ (2001) The origins and biological significance of the coccidial lesions that occur in chickens vaccinated with a live attenuated anticoccidial vaccine. Avian Pathol 30:215–220
Xu J, Zhang Y, Tao J (2013) Efficacy of a DNA vaccine carrying Eimeria maxima gam56 antigen gene against coccidiosis in chickens. Korean J Parasitol 2:147–154
Acknowledgements
The authors express sincere thanks to the Director, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, India, for providing the necessary facilities to conduct the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal studies were carried out in the Experimental Animal Shed of the Division of Parasitology, ICAR-Indian Veterinary Research Institute, Izatnagar, India. Prior approval for experimental trials and procedures to be used on chickens was obtained from the Institutional Animal Ethics Committee, IVRI (registered with the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Environment, Forest and Climate Change, Government of India). Permission for the use of recombinant proteins in the experiment was also obtained from the Institutional Biosafety Committee, IVRI, as per the norms of Department of Biotechnology, Government of India.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: David S. Lindsay
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
(DOCX 13 kb)
Supplementary Table 2
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Rafiqi, S.I., Garg, R., Ram, H. et al. Immunoprophylactic evaluation of recombinant gametocyte 22 antigen of Eimeria tenella in broiler chickens. Parasitol Res 118, 945–953 (2019). https://doi.org/10.1007/s00436-018-06198-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-06198-2