Abstract
The current experiment was conducted to construct recombinant Bacillus subtilis WB600 expressing Eimeria tenella 3-1E protein to investigate the oral immunization protective effects against E. tenella. The merozoite surface antigen 3-1E gene of E. tenella was introduced into the pBS-H1 expression vector with a novel signal peptide sequence. After the electro-transformation, the expression of objective protein in B. subtilis WB600 was detected by Western blot. The results showed that the recombinant B. subtilis strain with the ability of high-level secretion of 3-1E was constructed successfully. Seven-day-old broiler chickens were orally vaccinated with B. subtilis WB600 harboring 3-1E (B.S-pBS-H1-3-1E) or B. subtilis WB600 with empty plasmid (B.S-pBS-H1) 10 days prior to challenge with sporulated E. tenella oocysts. The results showed the recombinant B. subtilis strain with the ability of high-level secretion of 3-1E was constructed successfully. Vaccination with B.S-pBS-H1-3-1E strain significantly increased the anti-coccidial index and reduced cecal lesion scores compared with the positive control group (chickens were challenged with sporulated E. tenella oocysts without oral administration of B.S-pBS-H1-3-1E strain) and B.S-pBS-H1 group. Ceca mucosal sIgA, secretion, and IL-2, IL-12, IFN-γ, and IL-10 level after challenge were greater in the B.S-pBS-H1-3-1E group than in the positive control group. Taken together, these results indicated that B. subtilis WB600 harboring 3-1E protein induces protective immunity against E. tenella.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis is a widespread intestinal tract infectious disease caused by at least seven Eimeria species in poultry industry (Jang et al. 2010). Eimeria tenella is one of the most pathogenic and serious, in terms of the distribution, outbreak frequency, and economic losses (McDougald and Reid 1991; Abi-Ghanem et al. 2008). Currently, the main methods to control the disease are drugs and live vaccines. However, because of increasing problems with prolonged drug usage and high cost of vaccines, there is an urgent need for more effective and safer alternative strategies for control of coccidiosis (Dalloul et al. 2006; Williams 2006; Lee et al. 2007). Many secreted or membrane-bound proteins of Eimeria involved in the interaction with the host immune system are considered as candidates for immunological interventions (Ma et al. 2013). 3-1E antigen is a 20-kDa protein of Eimeria that is highly immunogenic and conserved, inducing partial protection against avian coccidiosis (Lillehoj and Lillehoj 2000). Therefore, it can be used as vaccine candidate.
Genetically engineering live vector vaccine expressing different target antigens via mucosal routes has successfully induced protective immunity against a variety of infectious disease including avian coccidiosis. However, due to transit of strain through the mammalian gastrointestinal tract and low antigen loads particularly after deliver via mucosal routes, the limited immunogenicity of both spores and cells has been questioned (Song et al. 2000; Min et al. 2001; Lee et al. 2010a). Recently, several heterologous antigens have been successfully adapted to Bacillus subtilis as vectors eliciting both systemic and secreted antibody, as well as cellular immune response to the passenger antigens following oral or parenteral administration to organism (Paccez et al. 2006, 2007; Fu et al. 2008; Luiz et al. 2008; Lee et al. 2010b).
B. subtilis has been explored as a tool for expression and delivery of antigen proteins due to the superior capacity of secreting proteins, lacking pathogenicity, propagating quickly, forming spores, and stimulating the immune system (Fu et al. 2008, 2011; Tseng et al. 2009).
A study has been designed to construct a recombinant B. subtilis expression vector with a processing-efficient signal peptide sequence to achieve high-level secretion of 3-1E in B. subtilis. Subsequently, another trial was taken to evaluate immunogenicity and protective effects of this recombinant B. subtilis strain against E. tenella in broiler chickens after oral vaccination.
Materials and methods
Bacterial strains and growth conditions
B. subtilis WB600 (△nprE △aprE △epr △bpf △mpr △nprB) was used in all experiments. WB600 was routinely grown in Luria-Bertani (LB) medium (1 % tryptone, 0.5 % yeast extract, and 1 % NaCl, pH 7.5). To express the protein, wild-type and recombinant B. subtilis strains were grown in LB containing kanamycin(10 μlg/ml) in Erlenmeyer flasks. Sporulation of the B. subtilis strain was induced in Difco sporulation media (DSM) using the exhaustion method as preciously described (Nicholson and Setlow 1990).
Recombinant plasmids construction
About 0.1 g (×107) sporulated oocysts of E. tenella were grinded to sporozoites in mortar on ice. Oocyst purification, sporulation, and excystation were done as Chapman and Shirley (2003) described. Total RNA was extracted from sporulated oocysts of E. tenella using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s protocol.
The recombinant plasmid pBS-H1-3-1E was constructed according to the procedure of previously constructed B. subtilis expression systems in our Lab (Fu et al. 2008). The complete E. tenella 3-1E open reading frame (GenBank accession number EF069437) was amplified with the following primers: 5P31E-BS(5′-ggcCTGCAGCGATGGGTGAAGAGGCTGATACT-3′), 3P31E-BS(5′-ggcAAGCTTAGTGATGGTGATGGTGATGGAAGCCGCCCTGGTACAGGT-3′) (the restriction sites are in italics and the six-His-tag codons are underlined; the specific primers used for PCR amplification were synthesized by Invitrogen Biotechnology Co., Ltd.), and the fragment was inserted into the pBS-H1 vector which was constructed as described in Fu et al. (2008) to generate recombinant plasmid pBS-H1-3-1E.
Competent cells of B. subtilis WB600 were transformed by electroporation (Xue et al. 1999) with the pBS-H1-3-1E and an empty vector pBS-H1 (control). After restriction analysis and nucleotide sequencing, the positive plasmids were verified, and the single colonies of transformants were incubated in Luria-Bertani (LB) broth at 37 °C for 8, 12, 16, 20, and 24 h to investigate the optimum expression time.
Detection of the expression of 3-1E protein in B. subtilis WB600
The recombinant B. subtilis WB600 strain harboring B.S-pBS-H1 and B.S-pBS-H1-3-1E plasmid were harvested and centrifuged at 5000 × g and 4 °C for 10 min. The supernatant was resuspended in SDS-PAGE loading buffer and analyzed on 15 % SDS-polyacrylamide gels. The sorted proteins were transferred to nitrocellulose membranes, which were incubated with an anti-His-tag antibody. The amount of secreted 3-1E protein was estimated with the densitometry analysis software BandScan (Glyko, Novato, CA, USA). To further confirm this protein, the distinct band of specific protein was cut from gel and the mass spectrometric analysis was performed. The sample was soaked with sinapinic acid matrix (saturated in 50 % acetonitrile, 0.1 % formic acid). The protein mass spectra were obtained using a Voyager DE-STR matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF) (Applied Biosystems, Foster City, CA, USA).
Preparation of live vaccines
Sporulation of the B. subtilis was induced in Difco sporulation media (DSM) using the exhaustion method as preciously described (Nicholson and Setlow 1990). The spore suspension was titrated immediately for colony forming units (CFU) per milliliter before freezing at −20 °C. The recombinant stains were made into powder at a final concentration of 1 × 108 CFU/g, then mixed with the basal diet at 1 × 108 CFU/kg formulated feed.
Chickens and parasites
A total of 300 1-day-old male broiler chickens (Avian) were purchased from Haining’s Hatchery, Chia Tai Broiler Development Center, Hangzhou, Zhejiang, China. The chickens were randomly divided into four groups with five replicates in each (n = 15) group and reared in an electrically heated battery and fed with coccidiostat-free, non-medicated broiler ration, and water ad libitum. The basal diet (Table 1) is formulated according to the nutrient requirements for broilers as recommended by the NRC (1994). All experiments related to animals were conducted according to guidelines of Animal Care Committee of Animal Science College, Zhejiang University.
The wild-type strains of E. tenella oocysts isolated in Haining’s Hatchery, Chia Tai Broiler chicken farm, Zhejiang, China, were used in the present study. Oocysts were resuspended in phosphate buffered saline (PBS pH 7.2) separated using centrifugation (5000 g, 10 min) and washed three times with PBS. Then, oocysts were incubated in 2.5 % potassium dichromate at 28 °C. Sporulated oocysts were stored in 2.5 % potassium dichromate at 4 °C, and oocysts numbers were calculated using a hemocytometer prior to experimental infections.
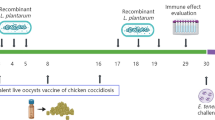
Experimental design and immunizing procedure
The unchallenged unimmunized control group (CK1, negative control) and challenged unimmunized control group (CK2, positive control) were fed with basal diet, After acclimatization, 7-day-old chickens of the B.S-pBS-H1 and B.S-pBS-H1-3-1E groups were vaccinated by feeding with the same diet supplemented with B. subtilis WB600 harboring the empty plasmid (1 × 108 CFU/kg of feed) and B. subtilis WB600 expressing 3-1E protein for 10 days. With the exception of CK1 group, all chickens at 17 days of age were inoculated orally with 5.0 × 104 sporulated E. tenella oocysts. The challenge dose was determined according to the results of preliminary trial in our lab. The CK1 group received an equal amount of PBS. Feces of the chickens were observed everyday to record the presence of oocysts and survival rate.
Evaluation of animal protection
To evaluate efficacy of the immunization, average body weight gain, survival rates, oocyst decrease ratio, oocysts per gram (OPG), mean lesion scores, and anti-coccidial index (ACI) were calculated.
The average weight gain at day (d) 7 (pre-immunization), d 17 (d 1 of infection, prior to challenge), and d 24 (the end of the experiment) was calculated. Percentage increase in weight gain was calculated using the following equation: Wg = (Wb/Wa) × 100 %, where Wg is the percent increase in weight gain, Wb is the average body weight gain of chickens in immunized group and in challenged unimmunized group, and Wa is the average body weight gain of chickens in the unchallenged unimmunized group.
Feces from each group were collected separately at d 5 and d 8 post-challenge. The lesion scores of chickens from each group were investigated as Johnson and Reid (1970) described. Additionally, the fecal samples from each group were collected separately and the oocyst shedding of 1 g of feces was microscopically counted using McMaster Egg Slide Counting Chamber (Lillehoj and Ruff 1987). Oocyst decrease ratio was calculated as follows: (the number of oocysts from challenge control chickens − immunized chickens)/(challenged control chickens) × 100 %. The ACI is a synthetic criterion for assessing the protective effect of a medicine or vaccine and calculated as follows: (The survival rate + relative weight gain rate) − (the scores of cecal lesions + the number of oocysts). The mucosa of cecum was collected by scraping with a microscalpel, and then transferred to separate sterilized tubes. All samples were preserved at −80 °C for further analyses.
Determination of cecal mucosa cytokines by ELISA
The cecal mucosa cytokines of interleukin IL-2, IL-4, IL-6, IL-10, IL-12, and interferon γ (IFN-γ) were analyzed by enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instruction (Komabiotech Ltd., Seoul, South Korea). Briefly, polyclonal goat anti-chicken IL-2, IL-4, IL-6, IL-10, IL-12, and IFN-γ were applied as capturing antibodies, and biotinylated polyclonal rabbit anti-chicken IL-2, IL-4, IL-6, IL-10, IL-12, and IFN-γ antibodies as detecting antibodies. Streptavidin-RP and TMBS were used as the color indicator. OD values at 450 nm were measured with an automated microplate reader right after color reaction was stopped with acid.
Cecal mucosa secretory IgA levels
The mucosa samples were diluted 1:2 in PBS containing 0.05 trypsin inhibitory units per milliliter (Sigma, St. Louis, Missouri) and centrifuged at 2500 g for 20 min. The cecal mucosa secretory IgA (sIgA) were measured according to manufacturer’s instructions (ADL, Atlantic Diagnostic Laboratories, Bensalem, PA, USA) by an indirect double antibody sandwich ELISA. Briefly, 96-well microtiter plate was coated overnight with 100 μl/well (10 μg/ml) recombinant coccidial antigen 3-1E in carbonate buffer (pH 9.6) at 4 °C. The plates were washed with PBS containing 0.05 % Tween-20 (PBST), wells blocked with blocking buffer for 1 h at 37 °C, and washed again. Samples (100 μl) were added and incubated for 1 h at 37 °C with continuous gentle shaking. After four washes with PBST, plates were incubated for 2 h at 37 °C with 100 μl/well of HRP-conjugated rabbit anti-chicken IgA (Sigma). After washing, the substrate solution was added (100 µL/well). After incubation for 20 min at 37 °C, the reaction was stopped by addition of 0.5 N sulfuric acid. An automated microtiter plate reader monitored reaction at 450 nm.
Statistical analysis
Data analysis was carried out using SPSS 15.0 for windows (SPSS Inc., Chicago, IL, USA) and expressed as mean ± SD. Means were compared using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range tests. Statistical significance between two groups was calculated at P < 0.05.
Results
High-level expression and secretion of 3-1E protein in B. subtilis WB600
The presence of E. tenella 3-1E in B. subtilis was analyzed. As shown in Fig. 1a, There was obviously a specific band at the position equivalent to a molecular mass of 20 kDa by SDS-PAGE. A similar band of the expected size, 20 kDa (Fig. 1b), was observed from the cell-free supernatant of the recombinant B. subtilis (B.S-pBS-H1-3-1E) by Western blot. On extrapolation of BandScan, the amount of 3-1E protein accumulated by B. subtilis cell reached a maximum level (c. 623 mg/l) in crude culture supernatant after 24 h of cultivation and remained at a relatively stable level for further incubation (data not shown). MALDI-TOF MS and MS/MS analysis showed the distinct band contained sequences that matched E. tenella 3-1E protein (protein MW 18,512, protein score C. I. % 98.628, Table S1).
SDS-PAGE and western blot analysis of secreted 3-1E in the culture supernatant of recombinant B. subtilis WB600 strain. a SDS-PAGE analysis, lane M, molecular mass marker, lanes 1–2, B.S-pBS-H1 supernatant cultures at 24 h (negative control); lanes 3–4, B.S-pBS-H1-3-1E supernatant cultures at 24 h. b Western blot analysis, lanes 1–5, B. subtilis WB600 (pBS-H1-3-1E) supernatant cultures at 8, 12, 16, 20, and 24 h, respectively. Lane 6, B. subtilis WB600 (pBS-H1) supernatant cultures at 24 h (negative control). Twenty microliters of supernatant cultures loaded per lane
Protective effects of B.S-pBS-H1-3-1E spore against E. tenella challenge
No dead chickens were found in any group after E. tenella challenge. Average body weight gain, relative body weight gain (%), oocyst decrease ratio, oocysts per gram (OPG), mean lesion scores, and anti-coccidial index (ACI) were described in Table 2. Chickens in the infected unimmunized group had significantly lower average body weight gain and ACI, higher OPG, mean lesion scores than those in the uninfected unimmunized and B.S-pBS-H1-3-1E group (P < 0.05). Significantly OPG and oocyst decrease ratio were observed in the B.S-pBS-H1 group compared with infected unimmunized group. No significant difference in ACI between infected unimmunized and B.S-pBS-H1 groups was recorded. Chickens orally immunized with B.S-pBS-H1-3-1E spores showed significantly higher average weight gains and the ACI (189.2), higher OPG, and mean lesion scores than those of infected chickens that were non-immunized or immunized with B.S-pBS-H1 (P < 0.05).
Cytokine levels in cecal mucosa
As shown in Fig. 2, at 17 days, immunized with B. subtilis WB600 with empty plasmid (B.S-pBS-H1) had significantly elevated IL-2, Th2 key cytokines IL-4 regulatory cytokines, anti-inflammatory IL-10, and the pro-inflammatory cytokine IL-6 levels compared with any other groups (P < 0.05). Th1 signature cytokine IFN-γ level in B.S-pBS-H1-3-1E and B.S-pBS-H1 strains were significantly lower than in the CK1 (P < 0.05). Interestingly, B.S-pBS-H1-3-1E strain significantly decreased the anti-inflammatory cytokines IL-4 level and increased the production of IL-12 (P < 0.05).
Cytokines level in cecal mucosa of IL-2, IL-4, IL-6, IL-10, IL-12, and INF-γ were calculated at 17 and 24 days. Each bar represents the mean ± SD value (N = 15). Significant difference (P < 0.05) between numbers with different letters. No significant difference (P > 0.05) between numbers with the same letter
As shown in Fig. 2, in response to B. subtilis WB600 harboring 3-1E protein, the higher IL-2 level in chickens was observed at 24 days post infection (P < 0.05). B.S-pBS-H1-3-1E significantly increased IL-10 and IL-12 levels compared with the CK2 and B.S-pBS-H1 group. Moreover, B.S-pBS-H1-3-1E strain could stimulate IFN-γ secretion compared with the CK2 group (P < 0.05).
Effect of oral administration of B.S-pBS-H1-3-1E strain on cecal mucosa secretory IgA responses
As indicated in Fig. 3, before infection, the 3-1E-specific sIgA concentration was significantly increased (P < 0.05) in the B.S-pBS-H1 and B.S-pBS-H1-3-1E group compared with the CK1 group. By contrast, B.S-pBS-H1 strain was more effective in enhancing 3-1E-reactive sIgA levels compared with B.S-pBS-H1-3-1E strain (P < 0.05).
Following infection with E. tenella, immunized with B. subtilis WB600 harboring 3-1E protein increased sIgA in cecal mucosa levels compared with other groups (P < 0.05; Fig. 3). However, there was no difference between group immunized with B.S-pBS-H1 and infected unimmunized group.
Discussion
3-1E antigen was presented on the outer surface of both sporozoites and merozoites in Eimeria life cycle. Vaccination with the recombinant Eimeria 3-1E protein could significantly affect both the cellular-mediated and mucosa-associated immune responses to live Eimeria species (Jang et al. 2011). E. tenella sporozoite/merozoites antigen was originally expressed in Escherichia coli (Subramanian et al. 2008; Jang et al. 2010; Zhao et al. 2011), but use of B. subtilis to express 3-1E protein has not been reported before. A potential advantage of B. subtilis spores as vaccine vehicles is that bacterial spores are naturally resistant to different environmental stresses, such as extremely heat and pH. In addition, B. subtilis has an immense capacity for secreting proteins and is also readily adaptable to genetic manipulation (Ferreira et al. 2005). Secretory form of lipase (Heydari et al. 2013), envelope protein VP28, the most abundant exposed protein in the WSSV envelope (Fu et al. 2008) were successfully expressed in B. subtilis. Paccez et al. (2007) have reported that B. subtilis vaccine strains encoding the B subunit of the heat-labile toxin (LTB) could elicit both systemic IgG and secreted IgA response to LTB in mice (Paccez et al. 2007). In this experiment, the recombinant B. subtilis expressing 3-1E gene was constructed successfully. Western blot and MALDI-TOF mass spectrometer result confirmed the 3-1E protein. The mass matches the theoretical value (Fig. 1b, Table S1).
Challenge at a dose of 5.0 × 104 sporulated E. tenella oocysts could cause apparent intestinal damage and induce growth depression, which is consistent with the observations made by Ma et al. (2013). The findings of the present study showed that oral immunization with B.S-pBS-H1-3-1E strain offered more protection against homologous infection, as demonstrated by increased average body weight gain, reduced oocyst decrease ratio (63.70 %), higher ACI, and lower average lesion scores. These results could be explained by the fact that secretion of the encoded antigen did result in significant induction of 3-1E-specific immune responses, which could effectively block the invasion of sporozoites or merozoites into the intestinal epithelial cells to reduce the damage of chicken gut tissue (Ma et al. 2011). However, the protective efficacy against E. tenella challenge recorded in chickens immunized with oral vaccine B.S-pBS-H1-3-1E was lower than previous reported vaccines based on 3-1E antigen including DNA vaccine (Min et al. 2001) and plant vaccine (Sathish et al. 2012). A possible explanation for this investigation might be that partial degradation of the expressed protein may result in reduced immunogenicity before reaching the intestine following oral immunization. Future studies on the improvements in the performance of B. subtilis-based vaccine vehicle involving immunogenicity should be pursued.
The antigen load of a vaccine vehicle, especially live carrier, is a key factor affecting immunogenicity particularly following delivery via the oral route. As robust life forms, B. subtilis spores could resist to the gastrointestinal environment, colonize, and conduct the entire life cycle within the animal gastrointestinal tract before being excreted (Fu et al. 2008). Mucosal vaccination via the oral or ocular routes normally favors the generation of neutralizing sIgA antibodies and protective cell-mediated immune responses (Wang et al. 2004). In this study, our results demonstrated that before infection, chickens in B.S-pBS-H1 group also showed higher sIgA level. B. subtilis WB600 as live carrier possibly exert their probiotic action to enhance immune protection. Post infection, we observed that orally vaccination with the recombinant B. subtilis WB600 harboring 3-1E protein strain induced significantly cecal mucosa sIgA response compared to any other group. Increased level of sIgA or IgY antibodies in the gut was also seen in chickens immunized with a profiling/adjuvant complex (Lillehoj et al. 2004; Lee et al. 2009; Jang et al. 2011). These results strongly suggested that secretion of the encoded antigen did result in significant induction of 3-1E-specific humoral immune responses of chickens.
Previous studies showed that E. tenella stimulated CD4+ T cells, macrophage response, and an increase in the IL-2, IL-4, IL-8, IL-10, IL-18, and INF-γ response in the cecum (Cornelissen et al. 2009; Zhang et al. 2012a). A protective immune response can be stimulated as either a humoral-mediated (Th2) response or a cell-mediated (Th1) response, depending on the combination of cytokines generated (Mosmann and Sad 1996). IL-2 might be relevant to the enhanced CD4+ frequencies as an inducer for T-cell proliferation found in the cecum of an E. tenella infection at d 6 (Cornelissen et al. 2009). Jang et al. (2010) reported that subcutaneous injection of the recombinant 3-1E protein increased levels of gene transcripts encoding IFN-γ and enhanced protective immunity to E. acervulina challenge. The current study showed that immunization with B. subtilis WB600 harboring 3-1E protein stimulated the IL-2 cytokine and IFN-γ secretion compared with infected unimmunized group at 7 days post infection. These cytokines are belonging to the cellular set of the immune response (Th1) (Jang et al. 2010). IFN-γ expression is a common marker of cellular immunity against avian coccidiosis mediated by CD4+ and CD8+ effector lymphocytes (Choi et al. 1999; Yun et al. 2000; Cornelissen et al. 2009). These finding indicates that local cell-mediated immunity was activated. Before infection, we also observed an early cellular response manifested by the higher IL-2, IL-4, IL-6, and IL-10 cytokines ratios in ceca mucosal treated with B. subtilis WB600 harboring empty vector as compared to other groups. It might be attributed to local immunostimulation caused by feeding the probiotic bacteria even before the challenge (Dalloul et al. 2003) and activated humoral immune response resulting from the oral administration of B. subtilis as an adjuvant. It is noted that B. subtilis WB600 harboring 3-1E protein increased IL-10 and IL-12 production. Possibly Th2-type cytokines, IL-10 by reducing the level of Th1-type cytokines in the gut perform a regulatory role to induce immune-mediated protection (Inagaki-Ohara et al. 2006), while IL-12 also plays a critical role during Eimeria infection by promoting the early production of IFN-γ (Lillehoj and Lillehoj 2000). These studies showed that cytokines produced via the innate immune system could regulate host protective immunity (major in cell-mediated immunity) against E. tenella infection (Zhang et al. 2012b).
B. subtilis-based vaccine vehicles carrying antigen of 3-1E induce humoral and local cell-mediated immunity responses against E. tenella. Nonetheless, future studies are needed to further shed light on the mechanism of this action before this efficient antigen expression systems could be effectively used in the development of vaccine vehicles for other poultry diseases.
Conclusion
In conclusion, 3-1E protein was successfully expressed in B. subtilis WB600 at a high secretion level. Orally administration of the recombinant B. subtilis WB600 strain carrying the 3-1E protein to the chickens could offer partial protection against homologous challenge.
References
Abi-Ghanem D, Waghela SD, Caldwell DJ, Danforth HD, Berghman LR (2008) Phage display selection and characterization of single-chain recombinant antibodies against Eimeria tenella sporozoites. Vet Immunol Immunopathol 121:58–67
Chapman HD, Shirley MW (2003) The Houghton strain of Eimeria tenella: a review of the type strain selected for genome sequencing. Avian Pathol 32:115–127
Choi KD, Lillehoj HS, Zalenga DS (1999) Changes in local IFN-gamma and TGF-beta4 mRNA expression and intraepithelial lymphocytes following Eimeria acervulina infection. Vet Immunol Immunopathol 71(3–4):263–275
Cornelissen JB, Swinkels WJ, Boersma WA, Rebel JM (2009) Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet Parasitol 162(1–2):58–66
Dalloul RA, Lillehoj HS (2006) Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines 5(1):143–163
Dalloul RA, Lillehoj HS, Shellem TA, Doerr JA (2003) Enhanced mucosal immunity against Eimeria acervulina in broilers fed a Lactobacillus-based probiotic. Poultry Sci 82(1):62–66
Ferreira LC, Ferreira RC, Schumann W (2005) Bacillus subtilis as a tool for vaccine development: from antigen factories to delivery vectors. Ann Acad Bras Cienc 77:113–124
Fu LL, Li WF, Du HH, Dai W, Xu ZR (2008) Oral vaccination with envelope protein VP28 against white spot syndrome virus in Procambarus clarkii using Bacillus subtilis as delivery vehicles. Lett Appl Microbial 46(5):581–586
Fu LL, Wang Y, Wu ZC, Li WF (2011) In vivo assessment for oral delivery of Bacillus subtilis harboring a viral protein (VP28) against white spot syndrome virus in Litopenaeus vannamei. Aquaculture 322–323:33–38
Heydari A, Moosazadeh Moghaddam M, Aghamollaei H, Yakhchali M, Bambaee B, Latifi A (2013) Cloning and expression of the Bacillus pumilus F3 lipase gene into Bacillus subtilis and determining of comparative expression level between native and recombinant enzyme. New cell Mol Biotech J 3(9):67–73
Inagaki-Ohara K, Dewi FN, Hisaeda H, Smith A, Jimi F, Miyahira M, Abdel-Aleem AS, Horii Y, Nawa Y (2006) Intestinal intraepithelial lymphocytes sustain the epithelial barrier function against Eimeria vermiformis infection. Infect Immun 74(9):5292–5301
Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Bauchan GR, Lillehoj EP, Bertrand F, Dupuis L, Deville S (2010) Immunoenhancing effects of Montanide™ ISA oil-based adjuvants on recombinant coccidia antigen vaccination against Eimeria acervulina infection. Vet Parasitol 172(3–4):221–228
Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Bertrand F, Dupuis L, Deville S (2011) Mucosal immunity against Eimeria acervulina infection in broiler chickens following oral immunization with profilin in Montanide™ adjuvants. Exp Parasitol 129(1):36–41
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28(1):30–36
Lee SH, Lillehoj HS, Dalloul RA, Park DW, Hong YH, Lin JJ (2007) Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poultry Sci 86(1):63–66
Lee SH, Lillehoj HS, Park DW, Jang SI, Morales A, Garcia D, Lucio E, Larios R, Victoria G, Marrufo D, Lillehoj EP (2009) Protective effect of hyperimmune egg yolk IgY antibodies against Eimeria tenella and Eimeria maxima infections. Vet Parasitol 163:123–126
Lee S, Belitsky BR, Brinker JP, Kerstein KO, Brown DW, Clements JD, Keusch GT, Tzipori S, Sonenshein AL, Herrmann JE (2010a) Development of a Bacillus subtilis-based rotavirus vaccine. Clin Vaccine Immunol 17:1647–1655
Lee SH, Lillehoj HS, Jang SI, Lee KW, Yancey RJ, Dominowski P (2010b) The effects of a novel adjuvant complex/Eimeria profilin vaccine on the intestinal host immune response against live E. acervulina challenge infection. Vaccine 28(39):6498–6504
Lillehoj HS, Lillehoj EP (2000) Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis 44(2):408–425
Lillehoj HS, Ruff MD (1987) Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis 31(1):112–119
Lillehoj HS, Min W, Dalloul RA (2004) Recent progress on the cytokine regulation of intestinal immune responses to Eimeria. Poultry Sci 83:611–623
Luiz WB, Cavalcante RC, Paccez JD, Souza RD, Sbrogio-Almeida ME, Ferreira RC, Ferreira LC (2008) Boosting systemic and secreted antibody responses in mice orally immunized with recombinant Bacillus subtilis strains following parenteral priming with a DNA vaccine encoding the enterotoxigenic Escherichia coli (ETEC) CFA/I fimbriae B subunit. Vaccine 26(32):3998–4005
Ma D, Ma C, Pan L, Li G, Yang J, Hong J, Cai H, Ren X (2011) Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp Parasitol 127(1):208–214
Ma D, Gao M, Dalloul RA, Ge J, Ma C, Li J (2013) Protective effects of oral immunization with live Lactococcus lactis expressing Eimeria tenella 3-1E protein. Parasitol Res 112(12):4161–4167
McDougald LR, Reid WM (1991) Coccidiosis. In: Calnek BW, Barnes HJ, Beard CW, Reid WM, Yoder HW (eds) Diseases of poultry. Iowa State University Press, Ames, Iowa, pp 780–797
Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ (2001) Adjuvant effects of IL-1beta, IL-2, IL-8, IL-15, IFN-alpha, IFN-gamma TGF-beta4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine 20(1–2):267–274
Mosmann TR, Sad S (1996) The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 17(3):138–146
Nicholson WL, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM (eds) Molecular biological methods for Bacillus. Wiley Press, Chichester, UK, pp 391–450
Paccez JD, Luiz WB, Sbrogio-Almeida ME, Ferreira RC, Schumann W, Ferreira LC (2006) Stable episomal expression system under control of a stress inducible promoter enhances the immunogenicity of Bacillus subtilis as a vector for antigen delivery. Vaccine 24(15):2935–2943
Paccez JD, Nguyenb HD, Luiza WB, Ferreiraa RCC, Sbrogio-Almeidac ME, Schumanb W, Ferreiraa LCS (2007) Evaluation of different promoter sequences and antigen sorting signals on the immunogenicity of Bacillus subtilis vaccine vehicles. Vaccine 25(24):4671–4680
Sathish K, Sriraman R, Subramanian BM, Rao NH, Kasa B, Donikeni J, Narasu ML, Srinivasan VA (2012) Plant expressed coccidial antigens as potential vaccine candidates in protecting chicken against coccidiosis. Vaccine 30:4460–4464
Song KD, Lillehoj HS, Choi KD, Yun CH, Parcells MS, Huynh JT, Han JY (2000) A DNA vaccine encoding a conserved Eimeria protein induces protective immunity against live Eimeria acervulina challenge. Vaccine 19(2–3):243–252
Subramanian BM, Sriraman R, Rao NH, Raghul J, Thiagarajan D, Srinivasan VA (2008) Cloning, expression and evaluation of the efficacy of a recombinant Eimeria tenella sporozoite antigen in birds. Vaccine 26:3489–3496
Tseng DY, Ho PL, Huang SY, Cheng SC, Shiu YL, Chiu CS, Liu CH (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immun 26(2):339–344
Wang J, Thorson L, Stokes RW, Santosuosso M, Huygen K, Zganiacz A, Hitt M, Xing Z (2004) Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol 173(10):6357–6365
Williams RB (2006) Relative virulences of a drug-resistant and a drug-sensitive strain of Eimeria acervulina, a coccidium of chickens. Vet Parasitol 135(1):15–23
Xue GP, Johnson JS, Dalrymple BP (1999) High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacilluslicheniformis. J Microbiol Method 34:183–191
Yun CH, Lillehoj HS, Choi KD (2000) Eimeria tenella infection induces local gamma interferon production and intestinal lymphocyte subpopulation changes. Infect Immun 68(3):1282–1288
Zhang DF, Xu H, Sun BB, Li JQ, Zhou QJ, Zhang HL, Du AF (2012a) Adjuvant effect of ginsenoside-based nanoparticles (ginsomes) on the recombinant vaccine against Eimeria tenella in chickens. Parasitol Res 110:2445–2453
Zhang L, Liu RQ, Ma LP, Wang YW, Pan BL, Cai JP, Wang M (2012b) Eimeria tenella: expression profiling of toll-like receptors and associated cytokines in the cecum of infected day-old and three-week old SPF chickens. Exp Parasitol 130(4):442–448
Zhao YL, Wang CM, Lu YM, Amer S, Xu P, Wang JY, Lu JX, Bao YZ, Deng BL, He HX, Qin JH (2011) Prokaryotic expression and identification of 3-1E gene of merozoite surface antigen of Eimeria acervulina. Parasitol Res 109:1361–1365
Acknowledgments
The present research was funded by Science and Technology Program of Zhejiang Province (No.2011C16039) and Science and Technology Program of Hangzhou (No.20120232B26). The authors also thank Zhusuo Wang (Director of Chia Tai Broiler Development Center, Zhejiang University, China) for his kind assistance with the feeding trial.
Conflict of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhiwei Lin and Yanyun Shi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Analysis of recombinant coccidial antigen 3-1E by MALDI-TOF (PDF 475 kb)
Rights and permissions
About this article
Cite this article
Lin, Z., Shi, Y., Deng, B. et al. Protective immunity against Eimeria tenella infection in chickens following oral immunization with Bacillus subtilis expressing Eimeria tenella 3-1E protein. Parasitol Res 114, 3229–3236 (2015). https://doi.org/10.1007/s00436-015-4539-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4539-3