Abstract
The present study aimed to investigate the in vitro antileishmanial activity of strychnobiflavone flavonoid against Leishmania infantum, as well as its mechanism of action, and evaluate the ex vivo biodistribution profile of the flavonoid in naive BALB/c mice. The antileishmanial activity (IC50 value) of strychnobiflavone against stationary promastigote and amastigote-like stages of the parasites was of 5.4 and 18.9 μM, respectively; with a 50% cytotoxic concentration (CC50) value of 125.0 μM on murine macrophages, resulting in selectivity index (SI) of 23.2 and 6.6, respectively. Amphotericin B, used as a positive control, presented SI values of 7.6 and 3.3 for promastigote and amastigote-like stages of L. infantum, respectively. The strychnobiflavone was also effective in reducing in significant levels the percentage of infected macrophages, as well as the number of amastigotes per macrophage, after the treatment of infected macrophages using the flavonoid. By using different fluorescent probes, we investigated the bioenergetics metabolism of L. infantum promastigotes and demonstrated that the flavonoid caused the depolarization of the mitochondrial membrane potential, without affecting the production of reactive oxygen species. In addition, using SYTOX® green as a fluorescent probe, the strychnobiflavone demonstrated no interference in plasma membrane permeability. For the ex vivo biodistribution assays, the flavonoid was labeled with technetium-99m and studied in a mouse model by intraperitoneal route. After a single dose administration, the scintigraphic images demonstrated a highest uptake by the liver and spleen of the animals within 60 min, resulting in low concentrations after 24 h. The present study therefore demonstrated, for the first time, the antileishmanial activity of the strychnobiflavone against L. infantum, and suggests that the mitochondria of the parasites may be the possible target organelle. The preferential distribution of this compound into the liver and spleen of the animals could warrant its employ in the treatment of visceral leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is considered an important infectious disease in the world, with a high incidence and ability to produce deformities, as well as cause death. The disease affects approximately 12 million people in 98 countries and territories worldwide (WHO, 2010; Alvar et al., 2012). Visceral leishmaniasis (VL) caused by Leishmania donovani and Leishmania infantum/Leishmania chagasi is an extremely serious disease, leading to nearly 500,000 new cases and 50,000 deaths annually (Minodier and Parola 2007).
The parenteral administration of pentavalent antimony compounds continues to be the first choice to treat leishmaniasis; however, the occurrence of side effects, such as anorexy, myalgias, arthralgias, chemical pancreatitis, leucopenia, and cardiotoxicity are observed in patients (Grevelink & Lerner, 1996; Croft & Coombs, 2003). Amphotericin B, a second-line drug, is a highly hydrophobic antifungal product with an effective antileishmanial activity; however, its clinical use is also limited by its high toxicity (Annaloro et al., 2009; Ribeiro et al., 2014). To improve the therapeutic index of AmpB in an attempt to reduce its cytotoxicity, lipid-based formulations have been developed, such as Ambisome®, AmphocilH®, and Abelcet® (Bern et al., 2006). The World Health Organization has recommended the use of liposomal AmpB (L-AmpB) based on its high efficacy and safety (WHO, 2010). Despite improvements in therapeutic indexes for these lipid formulations, their use still remains limited due mainly to their high cost (Egger et al. 2010).
Miltefosine has been also employed in the treatment for VL as part of the Kalazar elimination program, launched to tackle the widespread antimony-resistance in the Indian subcontinent, aiming to reduce the number of cases of the disease in the next years (Mondal et al., 2009). Since miltefosine has a long elimination half-life and requires a long treatment regimen, the development of drug resistance has been reported (Dorlo et al., 2012; Rijal et al., 2013). Also, miltefosine therapy has been linked to teratogenicity and should not be prescribed to pregnant women or to those of childbearing age (Bhattacharya et al., 2004). In addition, leishmaniasis has emerged as an opportunistic infection in human immunodeficiency virus-infected patients (Alvar et al., 1997; Cota et al., 2014; Singh, 2015). Therefore, the development of new strategies to treat leishmaniasis has become a priority (Goto, 2012).
Natural products have traditionally played an important role in drug discovery and were the basis of most early medicines (Butler, 2005). It has been shown that the number of natural product-derived drugs present in the total amount of drug launchings in the market from 1981 to 2002 represented a significant source of new compounds (Newman et al., 2003). In recent years, considerable attention has been given to secondary plant-purified products, in an attempt to search for new antileishmanial drugs (Tiuman et al., 2005; Khaliq et al., 2009; Vendrametto et al., 2010). The Strychnos genus includes approximately 200 plant species, many of which are known for their potential medicinal secondary metabolites (Thongphasuk et al., 2003; Philippe et al., 2004). Strychnos pseudoquina St. Hil. is a native cinchona-like tree of the Brazilian Savanna, popularly known as “quina,” which is used in folk medicine to treat hepatic and stomach diseases (Correa, 1952), as well as malaria (Andrade-Neto et al., 2003).
Recently, a study performed by the present study’s research group using an ethyl acetate extract derived from S. pseudoquina stem bark isolated two flavonoids, quercetin 3-O-methyl ether and strychnobiflavone, which presented an effective antileishmanial activity against the L. amazonensis species (Lage et al., 2013). In this study, it was shown that both flavonoids presented an effective activity against in vitro stationary promastigotes and amastigotes of L. amazonensis, as well as a low toxicity in murine macrophages, in addition to a null hemolytic activity in human red blood cells. Moreover, strychnobiflavone proved to be effective in inhibiting macrophage infections caused by the parasites that had been pre-incubated with the cells, as well as in reducing the parasite burden in macrophages that had previously been infected with L. amazonensis, and that were later treated with the flavonoid.
In this context, in the present study, the antileishmanial activity and mechanism of action of the strychnobiflavone was evaluated against the L. infantum species. Aimed at performing future in vivo studies employing this flavonoid in the treatment of VL, an ex vivo biodistribution study of strychnobiflavone was also performed in BALB/c mice.
Materials and methods
Mice
Murine peritoneal macrophages were obtained from female BALB/c mice (8 weeks old), which were purchased from Institute of Biological Sciences from Federal University of Minas Gerais (UFMG). Experiments were performed in compliance with the National Guidelines of the Institutional Animal Care and Use Committee for the Ethical Handling of Research Animals (CEUA/UFMG), which approved this study under protocol number 136/2012.
Parasite
Leishmania infantum (MHOM/BR/1970/BH46) strain was used. Parasites were grown at 24°C in Schneider’s medium (Sigma, St. Louis, MO, USA), which was supplemented with 20% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, USA) and 20 mM L-glutamine, at pH 7.4. Stationary-phase promastigotes were cultured as described (Coelho et al., 2003). The amastigote-like stage of L. infantum was prepared following a modified technical protocol (Doyle et al., 1991). Briefly, 1 x 109 stationary promastigotes were washed in sterile phosphate buffer saline (PBS). Then, parasites were incubated in 5 mL of FBS for 48 h at 37°C. After, parasites were washed two times in sterile PBS, and visualized in an optical light microscopy. The cellular density was estimated by counting in a Newbauer chamber, and their morphology was evaluated after staining by Giemsa (Valadares et al., 2011).
In vitro antileishmanial activity
The inhibition of Leishmania spp. growth was assessed by in vitro cultivating stationary-phase promastigotes of L. infantum (1 × 106 cells) in the presence of strychnobiflavone (0.4 to 16.0 μM), in 96-well culture plates (Nunc, Nunclon, Roskilde, Denmark), for 48 h at 24°C. A previous titration curve was performed to determine the best time of inhibition of L. infantum growth incubating the evaluated product, and the used concentrations were derived from Lage et al. (2013). Cell viability was assessed by measuring the cleavage of 5 mg/mL of MTT [3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide] (Sigma). Absorbances were measured by using a multiwell scanning spectrophotometer (Molecular Devices, Spectra Max Plus, Canada), at 570 nm. Amphotericin B (1 \( \mu \)M) was used as a control. The concentration of the flavonoid needed to inhibit 50% of the Leishmania spp. viability (IC50) was determined by applying a sigmoidal regression of the dose-response curve, using different concentrations of the compound. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Cytotoxicity assay
The inhibition of 50% of the macrophages’ viability (CC50) was calculated by cultivating macrophages (5 × 105 cells) with different concentrations of strychnobiflavone (0.4 to 16.0 μM), in 96-well plates for 48 h at 37°C. A previous titration curve was performed to determine the best time of inhibition of macrophages’ viability incubating with the evaluated product. The cellular viability was assessed by the MTT assay, and AmpB was used as a control. The selectivity index (SI) was calculated by determining the ratio between the CC50 and IC50 values. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Treatment of infected macrophages
Murine macrophages were collected from BALB/c mice and seeded on round glass coverslips within 24-wells plate, at a concentration of 5 x 105 cells in RPMI 1640 medium, which was supplemented with 20% FBS and 20 mM L-glutamine, at pH 7.4. A recent promastigote in vitro culture passage (1–3) was used to enrich the number of metacyclic promastigotes. After 24 h of incubation of the macrophages at 37°C in 5% CO2, stationary promastigotes of L. infantum (5 x 106) were added to the wells (in a ratio of 10 parasites per one macrophage), and the cultures were incubated for 24 h at 37°C, 5% CO2. Free parasites were removed by extensive washing with RPMI 1640 medium, and infected macrophages were lately treated with the strychnobiflavone (40, 80 and 160 μM) for 24, 48 and 72 h at 24°C, in 5% CO2. Amphotericin B (1, 10 and 50 \( \mu \)M) was used as a positive control. After fixation with 4% paraformaldehyde, cells were stained by Giemsa and observed in a light microscope to determine the percentage of infected macrophages, as well as the number of intra-macrophage amastigotes out of 200 macrophages (Valadares et al., 2011). Data shown in this study represent the average ± standard deviation of three independent experiments, performed in triplicate.
Evaluation of reactive oxygen species production
Stationary-phase promastigotes of L. infantum (2 × 106 cells per well) were washed in Hanks’ balanced salt solution (HBSS, Sigma-Aldrich, USA) medium, and parasites were incubated for 60 min with strychnobiflavone, using its IC99 value. Then, H2DCF-DA (5 μM) was added, and the cells were incubated for 15 min at 24°C. The fluorescence intensity was detected using a fluorimetric microplate reader (FilterMax F5 Multi-Mode Microplate Reader, Molecular Devices), at 485 and 520 nm for excitation and emission, respectively. Sodium azide (10 mM) was used as a positive control (Mesquita et al., 2013). The following internal controls were used in the present investigation: (i) possible strychnobiflavone fluorescence at 485 nm for excitation and 520 nm for emission and (ii) interference of the DMSO diluent in the parasites. Non-treated promastigotes and medium without cells were used as negative controls and blanks, respectively. The samples were examined in triplicate.
Evaluation of cellular membrane permeability
Stationary-phase promastigotes of L. infantum (2 × 106 cells per well) were washed in PBS 1× and incubated with 1 μM SYTOX® green, for 15 min at 24°C (Pinto et al., 2013). Then, strychnobiflavone was incubated with parasites using its IC99 value, and the fluorescence intensity was measured every 20 min, for a total of 120 min. The maximum permeabilization was obtained with 0.1% Triton X-100. Fluorescence intensity was determined using a fluorimetric microplate reader, with excitation and emission wavelengths of 485 and 520 nm, respectively. The internal controls were following like described to the evaluation of the reactive oxygen species (ROS) production.
Activity on mitochondrial membrane potential
Stationary-phase promastigotes of L. infantum (2 × 106 cells per well) were washed twice in HBSS medium, seeded, and incubated with strychnobiflavone at the IC99 value, for 60 and 120 min. Rhodamine 123 (0.3 μg/mL) was added, and the cells were incubated for 10 min in the dark. The cells were washed twice in HBSS, and the fluorescence intensity was measured using a fluorimetric microplate reader, with excitation and emission wavelengths of 485 and 520 nm, respectively (Coimbra et al., 2002). Also, the internal controls were the same of the evaluation of ROS production and of the cellular membrane permeability.
Strychnobiflavone radiolabeling and radiochemical purity
For strychnobiflavone radiolabeling, the flavonoid was dissolved in a solution composed of ethanol and SnCl2∙2H2O, which was prepared in 0.25 N HCl. A solution comprised of 500 μL of strychnobiflavone (1 mg/mL), 20 μL of SnCl2∙2H2O (1 mg/mL), and 200 μL of PBS 1×, at pH 7.6, was mixed in a 10-mL glass vial, and 100 μL of sodium 99mTc-pertechnetate solution (Na99mTcO4 −/63 MBq; IPEN, CNEN, São Paulo, Brazil) was added. The mixture was kept at room temperature for 30 min, and incubated for 15 min with 200 mg of silica, followed by centrifugation. The supernatant was removed and the radiochemical purity (RP) of 99mTc-strychnobiflavone was determined by thin-layer chromatography on silica strips (TLC-SG, Merck, Darmstadt, Germany). Acetone was used to quantify the hydrolyzed technetium (99mTcO2), and PBS 1× was used to determine the amount of free technetium (99mTcO4 −). The 99mTc-strychnobiflavone usually remained immobile on silica strips when PBS 1× was used, while the radiolabeled compound migrated to the top of the strip when acetone was used as a solvent. Radioactivity was measured using a gamma counter (Wallac 1470 Wizard Gamma Counter, Perkin Elmer, Turku, Finland). The RP was determined from the following equation:
where: cpm = counts per minute
Ex vivo biodistribution studies
For the ex vivo biodistribution studies, BALB/c mice (n = 3 per group) were used. For this, aliquots containing 3.2 MBq of 99mTc-strychnobiflavone were administered into the tail vein of the animals. At 1, 3, 6, and 24 h after injection, animals were anesthetized with a solution containing ketamine (80 mg/kg) and xylazine (15 mg/kg), and then euthanized. Blood samples, heart, lungs, spleen, liver, stomach, and kidneys were harvested for analysis. Each organ or tissue was weighed, and the radioactivity was determined using an automatic gamma counter (Wizard, Finland). An aliquot of 99mTc-strychnobiflavone containing the same injected dose was counted simultaneously in a separate tube, which was defined as 100% radioactivity. The experiments were repeated three times and presented similar results. The results were expressed as the percentage of the injected dose per gram of tissue (% ID/g), according to the following equation:
Scintigraphic images
For scintigraphic images, BALB/c mice (n = 3 per group) were used. Aliquots containing 3.2 MBq of 99mTc-strychnobiflavone were administered into the tail vein of the animals. At 1, 3, 6, and 24 h after administration, mice were anesthetized with a solution containing ketamine (80 mg/kg) and xylazine (15 mg/kg), and were placed in a supine position under a gamma camera (Mediso, Budapest, Hungary), using a low-energy high-resolution collimator. Images were acquired with a 256×256×16 matrix size, with a 20% energy window set at 140 keV for a period of 10 min. Experiments were repeated three times and presented similar results.
Statistical analysis
The results were evaluated in Microsoft Excel (version 10.0) and analyzed using GraphPad PrismTM (version 6.0 for Windows). The IC50, CC50, and IC99 values were calculated from the mean percentage reduction of the stationary-phase promastigotes and amastigote-like (IC50) or macrophages (CC50), respectively, compared to that in the non-treated controls. The curves were determined by applying sigmoidal regression to the logarithm concentration/response data. In the assays where the mitochondrial function was evaluated, the differences among the groups were statistically evaluated by the two-tailed unpaired Student´s t test, and ANOVA test was used to test its significance (P < 0.05). In the biodistribution studies, one-way ANOVA followed by Bonferroni’s post-test was used to compare differences between different time points, and in each organ. Differences were considered significant when P < 0.05.
Results
Antileishmanial activity, cytotoxicity, and treatment of infected macrophages
The inhibition of L. infantum viability using the strychnobiflavone flavonoid was evaluated against the stationary promastigote and amastigote-like stages of the parasites. In the results, it could be observed that the flavonoid was effective against both L. infantum stages, presenting IC50 values of 5.4 and 18.9 \( \mu \)M for the promastigotes and amastigotes-like, respectively; whereas its CC50 value was of 125 \( \mu \)M (Table 1). Calculating the selectivity index for both stages of the parasites, the values found were of 23.2 and 6.6 for the promastigote and amastigote-like forms, respectively. Amphotericin B was used as a positive control, and it presented IC50 values of 1.0 and 2.3 \( \mu \)M for the promastigote and amastigote-like stages of L. infantum, respectively; and a CC50 value of 7.6 \( \mu \)M, and SI values of 7.6 and 3.3, respectively (Table 1). To assess the capacity of the strychnobiflavone in treating macrophages previously infected with L. infantum, cells were pre-infected with stationary-phase promastigotes (in a ratio of 10 parasites per 1 macrophage), and lately treated with 40, 80 or 160 \( \mu \)M of the flavonoid (Table 2). Amphotericin B (AmpB) was used as a positive control (1, 5 or 10 \( \mu \)M). It was possible to observe that when the cells were previously infected and lately treated with strychnobiflavone (at a concentration of 160 \( \mu \)M) during 48 or 72 hours, they presented reductions in the infection degree about 50% and 78%, respectively, when compared to untreated controls. AmpB (10 \( \mu \)M) was also effective in reducing the percentage of infected macrophages after the treatment for 48 and 72 hours, with percentage reduction in the infection degree by 94% and 96%, respectively, when compared to untreated controls (Table 2). However, when the number of amastigotes per cell was evaluated after treatments, macrophages first infected and lately treated for 48 or 72 hours with the strychnobiflavone (160 \( \mu \)M) presented an average of 1.2 and 0.9 amastigotes per macrophage, whereas cells treated with AmpB (10 \( \mu \)M) showed an average of 2.8 and 2.2 amastigotes per macrophage, respectively (Table 3). In this context, although AmpB had been able to induce a more pronounced reduction in the percentage of infected macrophages, the strychnobiflavone was more effective in reducing the number of recovered amastigotes in the infected and treated cells.
Evaluation of the lethal action of strychnobiflavone on L. infantum promastigotes
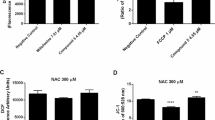
Strychnobiflavone-treated promastigotes were incubated with rhodamine 123 for the evaluation of mitochondrial alterations. Our results showed a significant increase in the fluorescence intensity in the order of 42%, when compared to untreated cells, after 60 min of incubation (Fig. 1a). Sodium azide was used as a positive control, and resulted in a reduced potential when compared to untreated cells. To evaluate the ROS production, strychnobiflavone was incubated with stationary promastigotes of L. infantum for 60 min, and this production was evaluated using the fluorescent probe H2DCF-DA. The fluorescence intensity levels of the flavonoid-treated parasites proved not to be different from the intensity levels of untreated cells, demonstrating no capacity to upregulate the ROS production (Fig. 1b). Sodium azide was used as a positive control, and it was effective to induce the ROS production in the parasites. The possible alteration in the permeability of the plasma membrane of L. infantum promastigotes was also evaluated in the presence of the fluorescent probe SYTOX® green. The results showed that the strychnobiflavone did not induce any change in permeability, when compared to untreated cells (Fig. 1c). The Tritox X-100 was used as a positive control, and it induced 100% effect on plasma membrane permeation.
Detection of reactive oxygen species, plasma membrane permeability, and evaluation of the mitochondrial membrane potential. The permeability of the L. infantum membrane incubated with strychnobiflavone was analyzed using a vital dye SYTOX® green. Stationary-phase promastigotes of the parasites were pre-treated with the Triton X-100 for 100% permeabilization, as a positive control. An untreated group was also included (a). The production of reactive oxygen species (ROS) in L. infantum promastigotes incubated with strychnobiflavone was determined. A fluorescent probe (H2DCf-DA) was incubated with the cells, and sodium azide was used as a positive control. An untreated group was also included (b). The evaluation of the L. infantum mitochondrial membrane potential incubated with strychnobiflavone was determined. Sodium azide was used as a positive control (c). The results are expressed as medium ± standard deviation from the experimental groups. Abbreviations: N.D. not detected, SPEA-2 strychnobiflavone. Statistically significant differences between the groups were observed (***P < 0.001)
Ex vivo biodistribution studies and scintigraphic images
The quality control of the 99mTc-strychnobiflavone demonstrated a radiochemical purity in the order of 90.2% ± 1.0%, allowing for its use in the ex vivo biodistribution assay. The data demonstrated that the 99mTc-strychnobiflavone had a high uptake by the animals’ liver and spleen. Although the levels of this compound had been higher in the liver of the animals, after 6 h, this concentration diminished in this organ, whereas in the spleen, the levels were maintained stable during the evaluated period of time (Fig. 2a). This evaluation was also observed in the scintigraphic images, demonstrating a high uptake of 99mTc-strychnobiflavone in the liver and spleen of the evaluated animals. Also, the results showed a decrease in the radioactivity in the animals’ abdominal region after 24 h of administration (Fig. 2b).
Ex vivo biodistribution profile of 99mTc-strychnobiflavone. The biodistribution profile of the 99mTc-strychnobiflavone in the blood and some organs was evaluated at different periods of time (1, 3, 6, and 24 h) after its administration in naive BALB/c mice (a). The scintigraphic images were obtained after the intravenous administration of radiolabeled strychnobiflavone, which were evaluated at different periods of time (1, 3, 6, and 24 h, b). Abbreviation: %ID/g percentage of the injected dose per gram of tissue. * indicates statistically significant difference in relation to the other groups (P < 0.05)
Discussion
Natural compounds are an important source of new and selective drug prototypes for the treatment of tropical diseases caused by protozoans, such as leishmaniasis (Delorenzi & Attias, 2001; Valadares et al., 2011). The antileishmanial activity observed in total extracts prepared from these materials has been attributed to compounds belonging to diverse chemical groups, such as isoquinoline alkaloids, indole alkaloids, quinones, terpenes, steroids, carbohydrates, lignans, proteins, and flavonoids (Rocha et al., 2005; Marín et al., 2009; Valadares et al., 2011). Among these plant-derived products, flavonoids represent a large family of polyphenolic compounds found in vegetables and fruits. As humans consume large amounts of flavonoids every day, it is generally accepted that flavonoids are safe and not toxic (Wong et al., 2012). Flavonoids have shown antiparasitic activity against a number of organisms. They are a promising new class of immune modulators for Leishmania spp. and have proven to bind to the nucleotide-binding site of MDR proteins with a concomitant increase in intracellular drug accumulation (Pérez-Victoria et al., 1999). Additionally, flavones potentiate the antibiotics of berbine and norfloxacin in Staphylococcus aureus, as well as artemisinin in Plasmodium falciparum (Liu et al., 1992), indicating their potential combined use within a chemotherapeutic regimen (Mead & McNair, 2006).
This research group has previously reported that strychnobiflavone was effective against L. amazonensis and has proven to be active in inhibiting the infection of phagocytic cells, as well as in reducing the parasite burden in previously infected macrophages. In addition, this flavonoid presented a low toxicity in murine macrophages, as well as a low hemolytic activity in human red blood cells (Lage et al., 2013). Hence, the antileishmanial activity and the mechanism of action of the strychnobiflavone were evaluated in L. infantum, as well as the ex vivo biodistribution profile in a known murine model. The results showed that strychnobiflavone was highly effective against L. infantum promastigotes, as detected by the absence of mitochondrial oxidation of MTT. Considering previous reports (Lage et al., 2013), this compound demonstrated to be 1.7-fold more effective against the L. amazonensis than against the L. infantum promastigotes presented in this study. Strychnobiflavone also showed a low toxicity for murine macrophages, with an SI value of 23.1, indicating a satisfactory selectivity of this substance (Osorio et al., 2007).
Previous reports have shown that the quercetin flavonoid, which presents a similar chemical structure to strychnobiflavone, has a wide range of reported biological effects, such as antioxidant, anti-hypertensive, anti-inflammatory, antimicrobial, and antiprotozoan activities (Mamani-Matsuda et al., 2004; Bischoff, 2008; Fonseca-Silva et al., 2011). The intracellular amastigote stage has been logically designated as the more relevant target for primary screening against Leishmania spp. (De Muylder et al., 2011). Compounds active against axenic parasites might be unable to reach the intracellular amastigotes, because of their inability to cross the host cell membranes, or maintain stability under low pH, whereas other compounds may need to be metabolized by macrophages to gain activity (Vermeersch et al., 2009). This is in accordance with previous studies, which found that only 4% of their hits identified in a promastigote primary screening were actually active in an intracellular context (Siqueira-Neto et al., 2010). Nonetheless, the present study’s results showed that macrophages infected and later treated with the flavonoid presented significant reductions in the parasite burden in the order of 52% of the infection rate, indicating that strychnobiflavone is active against intracellular amastigotes.
Studies evaluating the mechanism of action of drugs in parasites could provide important information about the development of new compounds (Fumarola et al., 2004). Mitochondria are essential cellular organelles that play a central role in energy metabolism, and are considered critical for the survival of any cell (Fidalgo & Gille, 2011). Several studies have demonstrated changes in the mitochondria morphology of some Leishmania spp. previously treated with antileishmanial agents (Delorenzi et al., 2001; Santa-Rita et al., 2004; Ueda-Nakamura et al., 2006; Rodrigues et al., 2007; Santos et al. 2008). These studies reported that significant alterations in the mitochondria led to the loss of cell viability and confirmed the importance of this organelle in the viability of L. infantum (Fonseca-Silva et al., 2011). To elucidate the possible mechanism of action induced by strychnobiflavone in L. infantum, the mitochondrial potential was investigated, due to the fact that previous studies have shown that the single mitochondria of the kinetoplastid parasites can well be considered good indicators of cellular dysfunction (Luque-Ortega et al., 2001; Mehta & Shaha, 2006; Menna-Barreto et al., 2009).
The maintenance of mitochondrial membrane potential (ΔΨ m) is vital for the metabolic process, as well as for cellular survival (Mehta & Shaha, 2006; Souza et al., 2009). Studies have shown that variations in ΔΨ m induced by drugs are associated with survival in Trypanosoma cruzi (Mukherjee et al., 2009; Menna-Barreto et al., 2009), L. donovani (Mehta & Shaha, 2006), and L. amazonensis (Rodrigues et al., 2007). In the present study, the evaluation of mitochondrial membrane potential was performed using fluorescent rhodamine 123. The mitochondrial damage was confirmed by an increase in the rhodamine 123 fluorescence, indicating hyperpolarization and, consequently, an alteration of ΔΨ m. This may well have decreased the ATP synthesis, and resulted in the parasites’ death. A previous study showed that the epigallocatechin-3-gallate (EGCG) flavonoid promoted alterations of ΔΨm in L. amazonensis, suggesting that EGCG exerts its antileishmanial effect on L. amazonensis promastigotes by affecting the parasites’ mitochondrial function (Inacio et al., 2012). Therefore, it can be concluded that strychnobiflavone may well be exerting its antileishmanial activity on L. infantum by affecting the parasites’ mitochondrial function.
To investigate the possible cause of the mitochondrial dysfunction induced by strychnobiflavone in L. infantum, the production of ROS was also evaluated in parasites, using the cell-permeant probe H2DCF-DA, which is a chemically reduced form of fluorescein used as a ROS indicator in cells (Mesquita et al., 2013). The ROS products are mainly produced in the electron transport chain of mitochondria (mainly in complex III) as a superoxide (O2−), and further converted to H2O2 (Carvalho et al., 2010). This study’s data clearly showed that strychnobiflavone had no influence in either the upregulation of ROS or its detoxification system, since strychnobiflavone-treated Leishmania spp. resulted in a non-altered production of ROS. Damage to the plasma membranes of Leishmania can rapidly change the cellular homeostasis, resulting in cell damage, including a mitochondrial dysfunction (Diaz-Achirica et al., 1998). Therefore, this study investigated the plasma membrane permeability of strychnobiflavone-treated parasites, using the fluorescent probe SYTOX® green. The resulting data demonstrated that strychnobiflavone had no effect on the plasma membrane permeability of L. infantum.
Visceral leishmaniasis (VL) is a systemic form of leishmaniasis, and following a bite by an infected sand fly, parasites disseminate through the lymphatic and vascular systems and they are taken by macrophages of the reticulum-endothelial system in the liver, spleen, bone marrow, lymph nodes, and other organs (Chappuis et al., 2007). The liver appears to serve as an indicator of the multiplication of parasites in the acute phase of the infection (Oliveira et al., 2012), and granuloma formation has been associated with a self-limiting hepatic infection, whereas these same granuloma fail to take form in the spleen, where parasites more commonly persist (Murray, 2001). Together, these observations suggest a causal association between granuloma formation and host resistance to visceralizing species of Leishmania spp., such as L. infantum (Moore et al., 2013). The ex vivo biodistribution studies and scintigraphic images performed here showed a high radioactivity uptake of 99mTc-strychnobiflavone by the animals’ liver and spleen. Taking this into account, strychnobiflavone presents a high potential to be used in future in vivo studies aimed at treating L. infantum infection.
Several geographical regions in the world are endemic for multiple Leishmania spp., which is the case of South America, where leishmaniasis is caused by at least eight different species of parasites, each with its own different determining factors of virulence and pathogenesis, although many display common areas of transmission (Lainson, 1983; Ashford, 2000; Lainson & Shaw, 2010). Taking this into account, it would be desirable to develop active compounds against diverse Leishmania spp.; however, the in vitro evidences of inter-species differences in the susceptibility of parasites to antileishmanial drugs have also been reported (Escobar et al., 2002; Obonaga et al., 2014). In recent years, considerable attention has been given to new compounds in an attempt to search for new antileishmanial drugs (Croft & Coombs, 2003; Khaliq et al., 2009; Seifert et al., 2010; Vendrametto et al., 2010). Despite the efficacy in our in vitro studies, it is important to consider that the use of other Leishmania spp. species and host cells can modify the current efficacy and IC50 values demonstrated in our study. Seifert et al., 2010, demonstrated this variability when different host cells were used for efficacy studies; including peritoneal murine macrophages, mouse bone marrow-derived macrophages, human peripheral blood monocyte-derived macrophages (THP-1 cells). This fact can have direct impact the in vitro activity of compounds, and also in the evaluation of drug susceptibility of clinical isolates.
In conclusion, our study demonstrates the antileishmanial activity of strychnobiflavone against L. infantum, and suggests that its mechanism of action may well be associated with alterations in the parasites’ mitochondrial membrane potential. Moreover, the higher uptake of this compound in the animals’ liver and spleen, organs highly parasitized by L. infantum; could benefit the targetability of this flavonoid, which could be further explored as a potential candidate for the treatment of VL.
References
Alvar J, Cañavate C, Gutiérrez-Solar B, Jiménez M, Laguna F, López-Vélez R, Molina R, Moreno J (1997) Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin Microbiol Rev 10:298–319
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:0035671. doi:10.1371/journal.pone.0035671
Andrade-Neto VF, Brandão MGL, Stehmann JR, Oliveira LA, Krettli AU (2003) Antimalarial activity of Cinchona-like plants used to treat fever and malaria in Brazil. J Ethnopharmacol 87:253–256. doi:10.1016/S0378-8741(03)00141-7
Annaloro C, Olivares C, Usardi P, Onida F, Della Volpe A, Tagliaferri E, Deliliers GL (2009) Retrospective evaluation of amphotericin B deoxycholate toxicity in a single centre series of haematopoietic stem cell transplantation recipients. J Antimicrob Chemother 63:625–626. doi:10.1093/jac/dkn549
Ashford RW (2000) The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol 30:1269–1281. doi:10.1016/S0020-7519(00)00136-3
Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, Davidson RN, Figueras C, Gradoni L, Kafetzis DA, Ritmeijer K, Rosenthal E, Royce C, Russo R, Sundar S, Alvar J (2006) Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis 43:917–924. doi:10.1086/507530
Bhattacharya SK, Jha TK, Sundar S, Thakur CP, Engel J, Sindermann H, Junge K, Karbwang J, Bryceson AD, Berman JD (2004) Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin Infect Dis 38:217–221. doi:10.1086/380638
Bischoff SC (2008) Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 11:733–740. doi:10.1097/MCO.0b013e32831394b8
Butler MS (2005) Natural products to drugs: natural product derived compounds in clinical trials. Nat Prod Rep 22:162–195. doi:10.1039/b402985m
Carvalho L, Luque-Ortega JR, Manzano JI, Castanys S, Rivas L, Gamarro F (2010) Tafenoquine, an antiplasmodial 8-aminoquinoline, targets Leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother 54:5344–5351. doi:10.1128/AAC.00790-10
Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M (2007) Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5:873–882. doi:10.1038/nrmicro1748
Coelho EAF, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994. doi:10.1128/IAI.71.7.3988-3994.2003
Coimbra ES, Gonçalves-da-Costa SC, Corte-Real S, De Freitas FG, Durão AC, Souza CS, Silva-Santos MI, Vasconcelos EG (2002) Characterization and cytochemical localization of an ATP diphosphohydrolase from Leishmania amazonensis promastigotes. Parasitology 124:137–143. doi:10.1017/S0031182001001056
Correa MP (1952) Dicionário das plantas úteis do Brasil e exóticas cultivadas, 3rd edn. Rio de Janeiro
Cota GF, de Sousa MR, de Mendonça ALP, Patrocinio A, Assunção LS, de Faria SR, Rabello A (2014) Leishmania-HIV Co-infection: clinical presentation and outcomes in an urban area in Brazil. PLoS Negl Trop Dis 8:2–8. doi:10.1371/journal.pntd.0002816
Croft SL, Coombs GH (2003) Leishmaniasis: current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 19:502–508
De Muylder G, Ang KKH, Chen S, Arkin MR, Engel JC, McKerrow JH (2011) A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis. doi: 10.1371/journal.pntd.0001253
De Souza W, Attias M, Rodrigues JCF (2009) Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida). Int J Biochem Cell Biol 41:2069–2080. doi:10.1016/j.biocel.2009.04.007
Delorenzi J, Attias M (2001) Antileishmanial activity of an indole alkaloid Frompeschiera australis. Antimicrob Agents 45:1349–1354. doi:10.1128/AAC.45.5.1349
Diaz-Achirica P, Ubach J, Guinea A, Andreu D, Rivas L (1998) The plasma membrane of Leishmania donovani promastigotes in the main target for CA (1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem J 330:453–460
Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ (2012) Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi:10.1093/jac/dks275
Doyle PS, Engel JC, Pimenta PF, da Silva PP, Dwyer DM (1991) Leishmania donovani: long-term culture of axenic amastigotes at 37oC. Exp Parasitol 73:326-334. doi:10.1016/0014-4894(91)90104-5
Egger SS, Meier S, Leu C, Christen S, Gratwohl A, Krähenbühl S, Haschke M (2010) Drug interactions and adverse events associated with antimycotic drugs used for invasive aspergillosis in hematopoietic SCT. Bone Marrow Transplant 45:1197–1203. doi:10.1038/bmt.2009.325
Escobar P, Matu S, Marques CNH, Croft SL (2002) Sensitivities of Leishmania species to (edelfosine) and amphotericin B. Acta Trop 81:151–157. doi:10.1016/S0001-706X(01)00197-8
Fidalgo LM, Gille L (2011) Mitochondria and trypanosomatids: targets and drugs. Pharm Res 28:2758–2770. doi:10.1007/s11095-011-0586-3
Fonseca-Silva F, Inacio JDF, Canto-Cavalheiro MM, Almeida-Amaral EE (2011) Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One. doi: 10.1371/journal.pone.0014666
Fumarola L, Spinelli R, Brandonisio O (2004) In vitro assays for evaluation of drug activity against Leishmania spp. Res Microbiol 155:224–230. doi:10.1016/j.resmic.2004.01.001
Goto H (2012) Review of the current treatments for leishmaniases. Res Rep Trop Med 69. doi: 10.2147/RRTM.S24764
Grevelink SA, Lerner EA (1996) Leishmaniasis. J Am Acad Dermatol 34:257–272
Inacio JDF, Canto-Cavalheiro MM, Rubem RF, Almeida-Amaral EE (2012) Mitochondrial damage contribute to epigallocatechin-3-gallate induced death in Leishmania amazonensis. Exp Parasitol 132:151–155. doi:10.1016/j.exppara.2012.06.008
Khaliq T, Misra P, Gupta S, Reddy KP, Kant R, Maulik PR, Dube A, Narender T (2009) Peganine hydrochloride dihydrate an orally active antileishmanial agent. Bioorganic Med Chem Lett 19:2585–2586. doi:10.1016/j.bmcl.2009.03.039
Lage PS, De Andrade PHR, Lopes ADS, Chávez Fumagalli MA, Valadares DG, Duarte MC, Pagliara Lage D, Costa LE, Martins VT, Ribeiro TG, Filho JD, Tavares CA, de Pádua RM, Leite JP, Coelho EA (2013) Strychnos pseudoquina and its purified compounds present an effective in vitro antileishmanial activity. Evidence-based Complement Altern Med 1–9. doi: 10.1155/2013/304354
Lainson R (1983) The American leishmaniases: some observations epidemiology and epidemiology. Trans R Soc Trop Med Hyg 77:569–596
Lainson R, Shaw JJ (2010) New World leishmaniasis. Topley and Wilson’s Microbiology and Microbial Infections.
Liu KCSC, Yang SL, Roberts MF, Elford BC, Phillipson JD (1992) Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep 11:637–640. doi:10.1007/BF00236389
Luque-Ortega JR, Rivero-Lezcano OM, Croft SL, Rivas L (2001) In vivo monitoring of intracellular atp levels in Leishmania donovani promastigotes as a rapid method to screen drugs targeting bioenergetic metabolism. Antimicrob Agents Chemother 45:1121–1125. doi:10.1128/AAC.45.4.1121
Mamani-Matsuda M, Malvy D, Thiolat D, Lejoly-Boisseau H, Daulouède S, Thiolat D, Coves S, Courtois P, Vincendeau P, Mossalayi MD (2004) Quercetin induces apoptosis of Trypanosoma brucei gambiense and decreases the proinflammatory response of human macrophages. Antimicrob Agents Chemother 48:924–929. doi:10.1128/AAC.48.3.924
Marín C, Boutaleb-Charki S, Díaz JG, Huertas O, Rosales MJ, Pérez-Cordon G, Guitierrez-Sánchez R, Sánchez-Moreno M (2009) Antileishmaniasis activity of flavonoids from Consolida oliveriana. J Nat Prod 72:1069–1074. doi:10.1021/np8008122
Mead JR, McNair N (2006) Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol Lett 259:153–157. doi:10.1111/j.1574-6968.2006.00263.x
Mehta A, Shaha C (2006) Mechanism of metalloid-induced death in Leishmania spp.: role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic Biol Med 40:1857–1868. doi:10.1016/j.freeradbiomed.2006.01.024
Menna-Barreto RFS, Goncalves RLS, Costa EM, Silva RS, Pinto AV, Oliveira MF, de Castro SL (2009) The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic Biol Med 47:644–653. doi:10.1016/j.freeradbiomed.2009.06.004
Mesquita JT, Pinto EG, Taniwaki NN, Galisteo AJ Jr, Tempone AG (2013) Lethal action of the nitrothiazolyl-salicylamide derivative nitazoxanide via induction of oxidative stress in Leishmania (L.) infantum. Acta Trop 128:666–673. doi:10.1016/j.actatropica.2013.09.018
Minodier P, Parola P (2007) Cutaneous leishmaniasis treatment. Travel Med Infect Dis 5:150–158. doi:10.1016/j.tmaid.2006.09.004
Mondal D, Singh SP, Kumar N, Joshi A, Sundar S, Das P, Siddhivinayak H, Kroeger A, Boelaert M (2009) Visceral leishmaniasis elimination programme in India, Bangladesh, and Nepal: reshaping the case finding/case management strategy. PLoS Negl Trop Dis 3:e355. doi:10.1371/journal.pntd.0000355
Moore JWJ, Moyo D, Beattie L, Andrews PS, Timmis J, Kaye PM (2013) Functional complexity of the Leishmania granuloma and the potential of in silico modeling. Front Immunol 4:1–7. doi:10.3389/fimmu.2013.00035
Mukherjee P, Majee SB, Ghosh S, Hazra B (2009) Apoptosis-like death in Leishmania donovani promastigotes induced by diospyrin and its ethanolamine derivative. Int J Antimicrob Agents 34:596–601. doi:10.1016/j.ijantimicag.2009.08.007
Murray HW (2001) Tissue granuloma structure-function in experimental visceral leishmaniasis. Int J Exp Pathol 82:249–267. doi:10.1046/j.1365-2613.2001.00199.x
Newman D, Cragg G, Snader K (2003) Natural products as sources of new drugs over the period 1981–2002. J Nat Prod 66:1022–1037. doi:10.1021/np030096l
Obonaga R, Fernández OL, Valderrama L, Rubiano LC, Castro Mdel M, Barrera MC, Gomez MA, Gore Saravia N (2014) Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus viannia species. Antimicrob Agents Chemother 58:144–152. doi:10.1128/AAC.01023-13
Oliveira DM, Costa MAF, Chavez-Fumagalli MA, Valadares DG, Duarte MC, Costa LE, Martins VT, Gomes RF, Melo MN, Soto M, Tavares CA, Coelho EA (2012) Evaluation of parasitological and immunological parameters of Leishmania chagasi infection in BALB/c mice using different doses and routes of inoculation of parasites. Parasitol Res 110:1277–1285. doi:10.1007/s00436-011-2628-5
Osorio E, Arango GJ, Jiménez N, Alzate F, Ruiz G, Gutiérrez D, Paco MA, Giménez A, Robledo S (2007) Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J Ethnopharmacol 111:630–635. doi:10.1016/j.jep.2007.01.015
Pérez-Victoria JM, Chiquero MJ, Conseil G, Dayan G, Di Pietro A, Barron D, Castanys S, Gamarro F (1999) Correlation between the affinity of flavonoids binding to the cytosolic site of Leishmania tropica multidrug transporter and their efficiency to revert parasite resistance to daunomycin. Biochemistry 38:1736–1743. doi:10.1021/bi982455v
Philippe G, Angenot L, Tits M, Frédérich M (2004) About the toxicity of some Strychnos species and their alkaloids. Toxicon 44:405–416. doi:10.1016/j.toxicon.2004.05.006
Pinto EG, Pimenta DC, Antoniazzi MM, Jared C, Tempone AG (2013) Antimicrobial peptides isolated from Phyllomedusa nordestina (Amphibia) alter the permeability of plasma membrane of Leishmania and Trypanosoma cruzi. Exp Parasitol 135:655–660. doi:10.1016/j.exppara.2013.09.016
Ribeiro TG, Chávez-Fumagall MA, Valadares DG, França JR, Rodrigues LB, Duarte MC, Lage PS, Andrade PH, Lage DP, Arruda LV, Abánades DR, Costa LE, Martins VT, Tavares CA, Castilho RO, Coelho EA, Faraco AA (2014) Novel targeting using nanoparticles: an approach to the development of an effective anti-leishmanial drug-delivery system. Int J Nanomedicine 9:877–890. doi:10.2147/IJN.S55678
Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC (2013) Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis 56:1530–1538. doi:10.1093/cid/cit102
Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM (2005) A review of natural products with antileishmanial activity. Phytomedicine 12:514–535. doi:10.1016/j.phymed.2003.10.006
Rodrigues JCF, Bernardes CF, Visbal G, Urbina JA, Vercesi AE, de Souza W (2007) Sterol methenyl transferase inhibitors alter the ultrastructure and function of the Leishmania amazonensis mitochondrion leading to potent growth inhibition. Protist 158:447–456. doi:10.1016/j.protis.2007.05.004
Santa-Rita RM, Henriques-Pons A, Barbosa HS, de Castro SL (2004) Effect of the lysophospholipid analogues edelfosine, ilmofosine and miltefosine against Leishmania amazonensis. J Antimicrob Chemother 54:704–710. doi:10.1093/jac/dkh380
Santos DO, Coutinho CER, Madeira MF, Bottino CG, Vieira RT, Nascimento SB, Bernardino A, Bourguignon SC, Corte-Real S, Pinho RT, Rodrigues CR, Castro HC (2008) Leishmaniasis treatment: a challenge that remains: a review. Parasitol Res 103:1–10. doi:10.1007/s00436-008-0943-2
Seifert K, Escobar P, Croft SL (2010) In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother 65:508–511. doi:10.1093/jac/dkp500
Singh S (2015) Changing trends in the epidemiology, clinical presentation, and diagnosis of Leishmania–HIV co-infection in India. Int J Infect Dis 29:103–112. doi:10.1016/j.ijid.2014.07.011
Siqueira-Neto JL, Song OR, Oh H, Sohn JH, Yang G, Nam J, Jang J, Cechetto J, Lee CB, Moon S, Genovesio A, Chatelain E, Christophe T, Freitas-Junior LH (2010) Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl Trop Dis 4:1–8. doi:10.1371/journal.pntd.0000675
Thongphasuk P, Suttisri R, Bavovada R, Verpoorte R (2003) Alkaloids and a pimarane diterpenoid from Strychnos vanprukii. Phytochemistry 64:897–901. doi:10.1016/S0031-9422(03)00508-9
Tiuman TS, Ueda-nakamura T, Prado B, Filho D (2005) Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob Agents 49:176–182. doi:10.1128/AAC.49.11.176
Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, Korehisa Maza P, Prado Dias Filho B, Aparício Garcia Cortez D, Alviano DS, Rosa Mdo S, Lopes AH, Alviano CS, Nakamura CV (2006) Antileishmanial activity of eugenol-rich essential oil from Ocimum gratissimum. Parasitol Int 55:99–105. doi:10.1016/j.parint.2005.10.006
Valadares DG, Duarte MC, Oliveira JS, Chávez-Fumagalli MA, Martins VT, Costa LE, Leite JP, Santoro MM, Régis WC, Tavares CA, Coelho EA (2011) Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol Int 60:357–363. doi:10.1016/j.parint.2011.06.001
Vendrametto MC, Santos AO, Nakamura CV, Dias Filho BP, Cortez DA, Ueda-Nakamura T (2010) Evaluation of antileishmanial activity of eupomatenoid-5, a compound isolated from leaves of Piper regnellii var. pallescens. Parasitol Int 59:154–158. doi:10.1016/j.parint.2009.12.009
Vermeersch M, Da Luz RI, Toté K, Timmermans JP, Cos P, Maes L (2009) In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob Agents Chemother 53:3855–3859. doi:10.1128/AAC.00548-09
Wong ILK, Chan KF, Chan TH, Chow LMC (2012) Flavonoid dimers as novel, potent antileishmanial agents. J Med Chem 55:8891–8902. doi:10.1021/jm301172v
World Health Organization (2010) Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. World Health Organization Tech Rep Ser 949. WHO, Geneva
Acknowledgments
We would like to thank to Mariana C. Duarte, Vívian T. Martins, Lourena E. Costa, and Daniela P. Lage for their technical assistance. This work was supported by grants from Instituto Nacional de Ciência e Tecnologia em Nanobiofarmacêutica (INCT-Nanobiofar), FAPEMIG (CBB-APQ-00819-12), CNPq (APQ-472090/2011-9, APQ-482976/2012-8, and APQ-488237/2013-0) and São Paulo State Research Fundation (FAPESP 2012/18756-1). MACF is a grant recipient of FAPEMIG/CAPES. EAFC, VNC and AGT are grant recipient of CNPq.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lage, P.S., Chávez-Fumagalli, M.A., Mesquita, J.T. et al. Antileishmanial activity and evaluation of the mechanism of action of strychnobiflavone flavonoid isolated from Strychnos pseudoquina against Leishmania infantum . Parasitol Res 114, 4625–4635 (2015). https://doi.org/10.1007/s00436-015-4708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4708-4