Abstract
Trichomoniasis is the most common curable sexually transmitted disease worldwide. Resistance to metronidazole in treating trichomoniasis is a problematic health issue. We aimed to determine the minimum lethal concentration (MLC) of metronidazole for Trichomonas vaginalis isolates detected in Mansoura, Egypt and studied the genotypic profile of these isolates. Vaginal swab specimens were obtained from 320 symptomatic and 100 asymptomatic females, for whom clinical examination, vaginal discharge wet mount, Giemsa stain, and culture in modified Diamond’s media were performed. Metronidazole susceptibility testing by an aerobic tube assay was performed. Both sensitive and resistant isolates were examined by PCR amplification followed by restriction fragment length polymorphism (RFLP). Trichomonas vaginalis was identified in 49/420 (11.7%) using either culture or PCR, while wet mount and Giemsa stain detected the parasite in 8.1 and 7.6% of participants, respectively. After 48 h incubation, most isolates were sensitive to metronidazole with a minimal lethal concentration (MLC) of 1 μg/ml. Mild resistance was observed in two isolates with MLCs of 64 μg\ml and mild to moderate resistance was observed in an additional two isolates with MLCs of 128 μg/ml. The four isolates that demonstrated low to moderate metronidazole resistance displayed a unique genotype band pattern by RFLP compared to the other 45 samples that were metronidazole sensitive. Our results highlight the presence of in vitro metronidazole tolerance in a few T. vaginalis isolates in Mansoura, Egypt that may lead to the development of drug resistance as well as the possibility of an identifying RFLP pattern in the isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The protist, Trichomonas vaginalis, is globally the most common, non-viral, and sexually transmitted pathogen (Poole and McClelland 2013). Trichomonas infection can cause vaginitis, vulval irritation, malodorous vaginal discharge, and strawberry cervix (Byun et al. 2015). Other adverse clinical manifestations like inflammatory pelvic disease (Cherpes et al. 2006) and infertility (Stark et al. 2009) are also reported.
Presently, the 5-nitroimidazole family of drugs (especially metronidazole and tinidazole) is the only oral treatment approved for trichomoniasis. As a result, drug resistance, which has been documented and is widely reported, is of concern (Paulish-Miller et al. 2014). The majority of treatment failures are produced by T. vaginalis isolates with low susceptibility to metronidazole, in both in vivo and in vitro conditions (Yarlett et al. 1987).
Classification of T. vaginalis isolates primarily considers the sensitivity of PCR and the reliability of RFLP (Conrad et al. 2012; Bradic et al. 2017). The PCR-RFLP technique combines PCR and RFLP and can reveal minor variations in a gene where a single base substitution creates or abolishes a recognition site for the restriction endonuclease enzyme. The technique has proven its effectiveness for strain typing of diverse organisms, like Chlamydia trachomatis, Treponema pallidum, and Neisseria gonorrhoeae (O’Rourke et al. 1995).
Given the growing awareness and appreciation of the serious health sequelae that are associated with T. vaginalis infection and the increasing rate of metronidazole resistance, T. vaginalis metronidazole susceptibility and its relatedness to different genotypes is still poorly understood. Thus, we chose to study phenotypic variation regarding metronidazole susceptibility of different T. vaginalis genotypes in Mansoura, Egypt as a critical consideration for both treatment strategies and epidemiologic studies.
Materials and methods
Ethics
This study was approved by the Institutional Review Board of the Faculty of Medicine, Mansoura University, Egypt (IRB reference no: 16.06.83). Prior to enrollment, informed consent was gained from each participant. Women with confirmed trichomoniasis were treated and followed up.
Study participants
The study was conducted in the period from December 2013 to February 2016. Vaginal swabs were collected from 320 female patients with symptoms consistent with vaginitis attending the Gynecology outpatient clinic at Mansoura University Hospital. In addition, another 100 asymptomatic women were recruited. Participants were of child bearing age (18–45 years); those who were pregnant or menstruating were excluded.
Specimen collection and processing
Participants were examined in accordance with a standard clinical protocol. Two speculum-assisted vaginal swab samples were obtained from the posterior fornix of the vagina of each participant. Specimens were assigned a study ID and, after linkage to a sociodemographic and clinical dataset, specimens were stripped off patient identifying data. The first swab was kept in a tube containing 3 ml sterile phosphate buffered saline (PBS) for wet mount microscopy, which was done within 10 min of sample collection and considered positive if motile T. vaginalis parasites were observed. Subsequently, PBS containing the parasite was used for Giemsa-stained smear preparations according to Radonjic et al. (2006). The second swab was transported within half an hour to the parasitology research laboratory for complete examination and incubation.
Culturing T. vaginalis isolates
The second swab was used to inoculate a pre-warmed culture tube containing modified Diamond’s medium (Fouts and Kraus 1980) with streptomycin-penicillin at 50 μg/ml and 10% heat-inactivated horse serum. Cultures were incubated at 37 °C and assessed by daily from day 2 to day 7 by collecting a drop of culture medium aseptically and examining it microscopically at 10×, positive confirmation at 40×.
In vitro drug susceptibility testing
Positive specimens were sub-cultured in modified Diamond’s medium at 48 h intervals by transferring 0.3 ml of the media to the new culture tubes till the required inoculum for drug susceptibility test was reached (Meri et al. 2000). This was done in duplicate three times a week. Trophozoites in sub-culture tubes were counted using a glass hemocytometer and Trypan blue exclusion test according to the method of Borchardt et al. (1997).
The drug susceptibility test was carried out by the aerobic tube assay method described by Kulda et al. (1982). Metronidazole (Amriya) serial dilutions at concentrations of 1, 2, 4, 8, 16, 32, 64, 128, and 256 μg/ml were prepared. The assay was run twice, using drug-free media as standard control. Hemocytometer counts were made of 48 h cultures to obtain the desired number (1 × 104 trophozoite/ml). Each culture tube was inoculated with 104 trophozoites, and the different concentrations of the drug were added to each isolate with a final volume of 2 ml per tube and incubated at 37 °C under aerobic conditions for 24 and 48 h. The assays were run in duplicate and repeated at least twice.
The MLC, which is defined as the lowest drug concentration at which no motile parasites were observed after 48 h incubation (Kulda et al. 1982) was determined. In vitro metronidazole resistance was defined as an aerobic MLC ≥ 50 μg/ml after 48 h incubation (Narcisi and Secor 1996).
DNA extraction, PCR amplification
After the drug susceptibility tests were performed, the remaining culture media was centrifuged and the pellet was washed twice in PBS at 4000 rpm for 10 min and stored at −20 °C. Genomic DNA was extracted from pellets using Thermo Scientific GeneJET Genomic DNA Purification Kit (USA) according to the manufacturer’s instruction. Quantity and purity of the extracted DNA was estimated by Nanodrop (Thermo scientific NanoDrop 2000 spectrophotometer, USA), and the quality of extracted DNA was checked by 1.5% agarose gel electrophoresis.
The Internal transcribed spacer 1 (ITS1) fragment was amplified with oligonucleotide PCR primers based on T. vaginalis ribosomal DNA (rDNA) gene (ACCESSION AF466750); TVITS F: 5′-ACA CCG CCC GTC GCT CCT AC-3′ and TVITS R: 5′ AAT TTG CAT TCA AAG ATT AAC-3′. These primers produce an amplified PCR product of the T. vaginalis ITS1. Each PCR reaction mixture contained 5 μL DNA template, 12.5 μl master mix (Tiangen Biotech, Beijing, China), and 1 μL of each of the forward and reverse primers (10 pmol) and 5.5 μL of double distilled water in a 25 μl final total volume. PCR amplification was performed according to the following procedure: initial denaturation by incubation for 5 min at 94 °C followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 62 °C for 30 s and extension at 72 °C for 1 min, with a final 10 min incubation at 72 °C. The expected single PCR product (313 bp) was confirmed by electrophoresis 2% (w/v) agarose gel, stained by ethidium bromide and visualized under a UV trans-illuminator.
Restriction fragment length polymorphism (RFLP)
After amplification, the PCR products were digested with MspI restriction enzyme (Thermo Scientific FastDigest MspI #FD0544). In brief, in each PCR tube, 17 μL of sterile D.W, 2 μL (10X) of Fast digest buffer, 10 μL of amplified PCR product, and 1 μl of the restriction endonuclease (MSp1) were added. The reaction mixture was incubated at 37 °C for 15 min followed by enzyme inactivation by heating for 5 min at 65 °C in a heat block. The restriction patterns were analyzed on a 2% agarose gel, run with 50 bp DNA ladder at 100 V for 45 min and visualized using a UV Trans-illuminator.
Statistical analysis
The data were plotted and analyzed using SPSS statistical software package, version 22. Associations between categorical variables were tested by the chi-square test. A P value < 0.05 was considered statistically significant. Fisher’s exact test was used when the assumptions of chi square were violated.
Results
The study involved 420 female patients, of whom 320 were symptomatic and 100 were asymptomatic, with a mean age ± standard deviation of 32 ± 5.6 years. The overall prevalence of T. vaginalis was 11.7% (13.8% in symptomatic patients and 5% in asymptomatic ones), primarily in the 26 to 35-year-old age group. 95.9% of participants were married, 67.3% were from rural areas, and 55.1% had a vocational level of education. Fourteen (28.6%) of the T. vaginalis positive cases were diabetic, which was significantly different compared to negative cases (P = 0.046). A burning sensation (65.9%) and pruritus vulvae (63.6%) were the most common symptoms reported by positive individuals. Of those positive for the parasite, 84.1% reported atypical vaginal discharge and 14.3% had post coital bleeding. The symptomatic characteristics of the participants are summarized in Table 1.
Culture and PCR recorded the same percentage of parasite detection among patient groups (11.7% for each), while wet mount and Giemsa stain detected fewer positives (8.1%, 7.6%, respectively). Out of 49 cases (11.7%) positive for T. vaginalis, 45 (91.8%) cases were sensitive to metronidazole and only 4 (8.2%) were found resistant. The degree of resistance was classified according to Kissinger et al. (2008) as follows: isolates with MLCs of 50–100, 101–199, 200–400, and > 400 mg/ml were considered to have mild, mild to moderate, moderate, and high resistance, respectively. Most susceptible cases had MLCs of 1 and 2 μg/ml of metronidazole after 24 and 48 h incubation, respectively, as shown in Table 2.
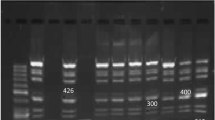
Amplification of the ITS1 fragment by PCR was applied to the 49 T. vaginalis positive samples. The amplified 313 bp product was digested by MspI restriction enzyme and electrophoresed on 2% agarose gel. The digested product revealed a 270 bp fragment in all 45 (91.8%) susceptible isolates compared to a 240 bp product in the 4 (8.1%) resistant isolates (Fig. 1, Table 2).
Two percent agarose gel electrophoresis for PCR products and after digestion with Msp1 restriction enzyme. Fifty-base-pair DNA ladder is in lane M. Lane 1, PCR amplification product (313 bp); lane 2, 3, and 5, MspI-digested PCR product (270 bp) (wild type) and lane 4, 6, and 7, MspI-digested PCR product (240 bp) (mutant type) in resistant cases
The four in vitro resistant isolates of T. vaginalis were from symptomatic women who had atypical discharge and previous history of taking metronidazole and on clinical examination, all demonstrated strawberry cervix. The isolate MLCs were 128, 128, 256, and 128 μg/ml and 64, 128, 128, and 64 μg/ml after 24 and 48 h, respectively.
We did not find significant differences between women infected with metronidazole-resistant compared to metronidazole-susceptible strains in terms of age, residence, marital status, educational level, symptoms, the use of contraception, parity, or having a previous diagnosis of trichomoniasis. Nevertheless, we found statistically significant difference between both groups regarding presence of typical discharge, post coital bleeding, lower abdominal pain, recorded partner symptoms, and history of previous treatment (Table 3). Additionally, a past history of multiple rounds of metronidazole treatment was noted in the participants infected with in vitro resistant isolates compared to the susceptible ones.
Discussion
Failure of metronidazole to cure T. vaginalis infections is of concern because metronidazole is currently the only oral drug approved for the treatment of trichomoniasis (Ali and Nozaki 2007). To our knowledge, this study is the first to investigate the in vitro metronidazole susceptibility assays of clinical T. vaginalis isolates and its relevance to T. vaginalis genotypes in Mansoura, Egypt.
The prevalence of trichomoniasis was 11.7% (13.8% in symptomatic and 5% in asymptomatic participants). This prevalence is in accordance with a study that showed a prevalence of T. vaginalis infection of 11% (Hussein et al. 2015) among symptomatic females in Benha hospital, Egypt. However, other studies demonstrated a higher prevalence of T. vaginalis infection ranging from 23 to 38.37% (Negm and el-Haleem 2004; Hegazy et al. 2012). The epidemiology of trichomoniasis is variable and depends on many factors like age, sexual activity, other infections, method of examination, and diagnostic technique (Grama et al. 2013).
Herein, 5% of asymptomatic cases were positive for T. vaginalis, a finding that reinforces the importance of conducting laboratory tests for accurate diagnosis and not only being concerned with the presence of symptoms. Among the positive cases, 28.6% (14/49) were diabetic. This information deserves attention, since diabetes is a risk factor for trichomoniasis (Younis and Elamami 2016; Kalra and Kalra 2017) and is likely due to poor glycaemic control. Hence, it is wise to follow diabetic females to ensure optimal perineal/genital and metabolic health.
Our findings showed a statistically higher frequency of discharge, post coital bleeding, lower abdominal pain, vaginal hyperemia, strawberry cervix, multiple metronidazole treatments, and increased partner symptoms in women with resistant isolates compared to those with susceptible isolates. With these findings in mind, resistance to metronidazole might be anticipated from history and examination. The prevalence of metronidazole-resistant cases (8.2%) in our locality approximates that in the study of Schwebke and Barrientes (2006) which detected 17/178 (9.6%) resistance isolates.
In our work, genotyping with PCR-RFLP using ITS1 gene revealed differences between susceptible and resistant isolates. According to Kazemi et al. (2010), the resistance may be associated with a mutation in the ITS1 gene at position 209 (C209T), in which the thymidine was replaced by cytosine. By the use of MspI enzyme, the 4 resistant isolates showed a MspI digested PCR product of 240 bp and the 45 susceptible isolates showed MspI digested PCR product of 270 bp when analyzed by agarose gel electrophoresis. Thus, our findings are in agreement with Kazemi et al. (2010).
Another study of Conrad et al. (2012) used multilocus sequence typing and microsatellite genotyping of T. vaginalis; the authors detected two genotypes and reported a correlation between metronidazole MLCs and T. vaginalis isolates using microsatellite genotyping. They found that a higher degree of metronidazole resistance was associated with the type 2 isolate. Snipes et al. (2000) used random amplified polymorphic DNA technique and found a correlation between metronidazole resistance and a point mutation in ITS1 (between 16S and 5.8S rRNA).
Conversely, other studies did not find any difference between metronidazole-susceptible and resistant T. vaginalis isolates using PCR (Rabiee et al. 2012) or HSP70 gene RFLP analysis by EcoRl-digestion (Hussien et al. 2005), possibly because they targeted a region outside the genes that code for the drug targets (Gandhi et al. 2014).
Conclusion
In essence, among symptomatic and asymptomatic females enrolled in our study, we found a 4.1% prevalence of both mild and mild to moderate metronidazole resistance. Resistant and susceptible isolates were differentiated by the use of PCR-RFLP of the ITS1 region. The outcomes of this study emphasize the need for periodic evaluations of T. vaginalis metronidazole drug susceptibility to monitor the possible emergence of resistance, empirical treatment of patients based on clinical symptoms, introduction of routine resistance testing, and pursuit of novel treatment options other than metronidazole.
References
Ali V, Nozaki T (2007) Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin Microbiol Rev 20:164–187
Borchardt KA, Zhang MZ, Shing H, Flink K (1997) A comparison of the sensitivity of the InPouch TV, Diamond’s and Trichosel media for detection of Trichomonas vaginalis. Genitourin Med 73:297–298
Bradic M, Warring SD, Tooley GE, Scheid P, Secor WE, Land KM, Huang PJ, Chen TW, Lee CC, Tang P, Sullivan SA, Carlton JM (2017) Genetic indicators of drug resistance in the highly repetitive genome of Trichomonas vaginalis. Genome Biol Evol 9:1658–1672
Byun JM, Jeong DH, Kim YN, Lee KB, Sung MS, Kim KT (2015) Experience of successful treatment of patients with metronidazole-resistant Trichomonas vaginalis with zinc sulfate: a case series. Taiwan J Obstet Gynecol 54:617–620
Cherpes TL, Wiesenfeld HC, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL (2006) The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis 33:747–752
Conrad MD, Gorman AW, Schillinger JA, Fiori PL, Arroyo R, Malla N, Dubey ML, Gonzalez J, Blank S, Secor WE, Carlton JM (2012) Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis 6:e1573
Fouts AC, Kraus SJ (1980) Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 141:137–143
Gandhi NR, Brust JC, Moodley P, Weissman D, Heo M, Ning Y, Moll AP, Friedland GH, Sturm AW, Shah NS (2014) Minimal diversity of drug-resistant Mycobacterium tuberculosis strains, South Africa. Emerg Infect Dis 20:426–433
Grama DF, Casarotti LS, Silva LS, Mendon DF, Viana C, Cury MC (2013) Prevalence of Trichomonas vaginalis and risk factors in women treated at public health units in Brazil: a transversal study. Trans R Soc Trop Med Hyg 107:584–591
Hegazy MM, El-Tantawy NL, Soliman MM, El-Sadeek ES, El-Nagar HS (2012) Performance of rapid immunochromatographic assay in the diagnosis of Trichomoniasis vaginalis. Diagn Microbiol Infect Dis 74:49–53
Hussein AH, Saleh MH, Nagaty IM, Ghieth KA, El-Azab NA (2015) Prevalence, clinical criteria and sociodemographic predictors of Trichomonas vaginalis infection in suspected Egyptian women, using direct diagnostic techniques. Iran J Parasitol 10:432–440
Hussien EM, El-Sayed HZ, El-Moamly AA, Helmy MM, Shaban MM (2005) Molecular characterization of Egyptian Trichomonas vaginalis clinical isolates by HSP70 restriction fragment length polymorphism. J Egypt Soc Parasitol 35:699–710
Kalra B, Kalra S (2017) Vulvovaginitis and diabetes. J Pak Med Assoc 67:143–145
Kazemi F, Hooshyar H, Zareikar B, Bandehpour M, Arbabi M, Talari S, Alizadeh R, Kazemi B (2010) Study on ITS1 gene of Iranian Trichomonas vaginalis by molecular methods. Iran J Parasitol 5:9–14
Kissinger P, Secor WE, Leichliter JS, Clark RA, Schmidt N, Curtin E, Martin DH (2008) Early repeated infections with Trichomonas vaginalis among HIV-positive and HIV-negative women. Clin Infect Dis 46:994–999
Kulda J, Vojtĕchovská M, Tachezy J, Demes P, Kunzová E (1982) Metronidazole resistance of Trichomonas vaginalis as a cause of treatment failure in trichomoniasis—a case report. Br J Vener Dis 58:394–399
Meri T, Jokiranta TS, Suhonen L, Meri S (2000) Resistance of Trichomonas vaginalis to metronidazole: report of the first three cases from Finland and optimization of in vitro susceptibility testing under various oxygen concentrations. J Clin Microbiol 38:763–767
Narcisi EM, Secor WE (1996) In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother 40:1121–1125
Negm AY, El-Haleem DA (2004) Detection of trichomoniasis in vaginal specimens by both conventional and modern molecular tools. J Egypt Soc Parasitol 34:589–600
O’Rourke M, Ison CA, Renton AM, Spratt BG (1995) Opa-typing: a high resolution tool for studying the epidemiology of gonorrhoea. Mol Microbiol 17:865–875
Paulish-Miller TE, Augostini P, Schuyler JA, Smith WL, Mordechai E, Adelson ME, Gygax SE, Secor WE, Hilbert DW (2014) Trichomonas vaginalis metronidazole resistance is associated with single nucleotide polymorphisms in the nitroreductase genes ntr4Tv and ntr6Tv. Antimicrob Agents Chemother 58:2938–2943
Poole DN, McClelland RS (2013) Global epidemiology of Trichomonas vaginalis. Sex Transm Infect 89:418–422
Rabiee S, Bazmani A, Matini M, Fallah M (2012) Comparison of resistant and susceptible strains of Trichomonas vaginalis to metronidazole using PCR method. Iran J Parasitol 7:24–30
Radonjic IV, Dzamic AM, Mitrovic SM, Arsic Arsenijevic VS, Popadic DM, Kranjcic Zec IF (2006) Diagnosis of Trichomonas vaginalis infection: the sensitivities and specificities of microscopy, culture and PCR assay. Eur J Obstet Gynecol Reprod Biol 126:116–120
Schwebke JR, Barrientes FJ (2006) Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob Agents Chemother 50:4209–4210
Stark JR, Judson G, Alderete JF, Mundodi V, Kucknoor AS, Giovannucci EL, Platz EA, Sutcliffe S, Fall K, Kurth T, Ma J, Stampfer MJ, Mucci LA (2009) Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: Physicians’ Health Study. J Natl Cancer Inst 101:1406–1411
Snipes LJ, Gamard PM, Narcisi EM, Beard C, Lehmann T, Secor WE (2000) Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol 38:3004–3009
Yarlett N, Hof H, Yarlett NC (1987) Activities of metronidazole and niridazole against Trichomonas vaginalis clinical isolates. J Antimicrob Chemother 19:767–770
Younis EZ, Elamami AH (2016) Trichomonas vaginalis infection in women with Type 2 diabetes mellitus and vaginal discharge in Benghazi, Libya. Ibnosina J Med Biomed Sci 8:109–113
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Institutional Review Board of the Faculty of Medicine, Mansoura University, Egypt (IRB reference no: 16.06.83).
Statement of informed consent
Prior to enrollment, informed consent was gained from each participant. Women with confirmed trichomoniasis were treated and followed up.
Rights and permissions
About this article
Cite this article
Abdel-Magied, A.A., El-Kholya, ES.I., Abou El-Khair, S.M. et al. The genetic diversity of metronidazole susceptibility in Trichomonas vaginalis clinical isolates in an Egyptian population. Parasitol Res 116, 3125–3130 (2017). https://doi.org/10.1007/s00436-017-5627-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5627-3