Abstract
Sinuolinea species are myxozoans of the order Bivalvulida, suborder Variisporina, and family Sinuolineidae, which can be parasites for freshwater and marine fish. The aim of this study was to describe the occurrence of Sinuolinea niloticus n. sp. infecting Nile tilapia (Oreochromis niloticus) from aquaculture and from river sources with morphological and molecular analyses. Between March 2010 and November 2012, 116 Nile tilapia were randomly sampled from aquaculture net fishing (n = 56) in Mira Estrela, São Paulo, and from the Capivari River (n = 60) in Botucatu, São Paulo. The fishes that were sampled were examined by necropsy, microscopic observation and molecular techniques for detection and identification of the myxozoan causing disease in tilapia. All of the tissues that were sampled for analysis showed the presence of the parasite. It was observed by microscopy that the myxozoan belongs to the Sinuolinea genus. This identification was performed based on morphological characteristics and histopathology findings, such as structures consistent with myxozoan in the interstices in all analysed tissues, coagulative necrosis, haemorrhage, inflammatory processes, presence of melano-macrophages and eosinophils. The results of the molecular analyses revealed that the myxozoan detected and identified in this study is sister to a group of other Sinuolinea species. Because this is the first report of this parasite in Nile tilapia, the parasite was named S. niloticus n. sp. This is the first report of a Sinuolinea species in Brazil and in tilapia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first report of Sinuolinea spp. infecting fish was by Davis 1917. The author described that the parasite has roughly spherical spores with and without lateral processes, rounded capsules that are not convergent when viewed from above, capsular pores that are some distance apart and sometimes on nearly opposite sides of the spore, a sutural line forming a prominent ridge that takes a sinuous course around the spore and a sutural plane that is usually distinctly twisted on its axis.
Davis (1917) reported Sinuolinea arborescens as infecting the urinary bladder of Siphostoma floridae; Sinuolinea capsularis infecting the urinary bladder of Paralichthys albiguttus, Paralichthys dentatus and Spheroides maculates; and Sinuolinea dimorpha infecting the urinary bladder and ureters of Cynoscion regalis (Lom & Dyková, 2006). Since 1917, others species of Sinuolinea have been described: S. argyrosomi (Zhao & Song, 2003), S. arctica (Kodádková et al., 2014), S. cyclopterina (Basikalova, 1932), S. lesteri (Moser et al., 1989), S. mai (Zhao & Song, 2001), S. magna (Yoshino & Noble, 1973), S. murmanica (Basikalova, 1932), S. rebae (Tripathi, 1948), S. shandongensis (Zhao & Song, 2003), S. platycephali (Zhao & Song, 2003), S. phyllopteryxa (Garner et al., 2008), S. sinuosa (Shulman, 1953), S. triangulata (Shulman, 1966) and S. tetraodoni (El-Matbouli & Hoffmann, 1994).
The reports of Sinuolinea species cited above described only taxonomy and molecular characterisations, but Garner et al. (2008) described that the infection caused by a Sinuolinea species associated with renal tubular dilatation, hypertrophy of tubular epithelial cells, accumulation of cellular debris and some proteinaceous fluid in the tubules. This report describes the prevalence, morphological and molecular characterisations of Sinuolinea niloticus n. sp. infecting Nile tilapia (Oreochromis niloticus).
Materials and methods
Sampling
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Univ. Estadual Paulista (Permit Number: 161/2011-CEUA).
Between March 2010 and November 2012, every 2 months, 116 Nile tilapia (O. niloticus) were randomly sampled from aquaculture net fishing (n = 56) in Mira Estrela, São Paulo (19° 55′ 47.52″ S, 50° 08′ 36.56″ O) and from the Capivari River (n = 60) in Botucatu, São Paulo (22° 43′ 43″ S, 48° 22′ 29″ O; Table 1). At each sampling event (March, May, July, September, November), four fish were collected from both the aquaculture facility and from the river. The mortality in aquaculture during sampling was about 20–30 % in young and adult fish, which presented black skin and were located near from the edge of the tanks. The sampling of fish in aquaculture was random with net fishing, where one fish was taken in each of the four tanks. In the following months, the sampling was performed in the same way and tanks to avoid bias in the results. Then the fish were put in a cooler that contained 1 L of water for every 1 cm of fish for transportation. Oxygenation was performed using an aerator until arrival at the laboratory at the University Estadual Paulista (UNESP) of Botucatu, São Paulo, Brazil, for necropsy and parasite identification. The identification of Nile tilapia from aquaculture and river sources was according to FishBase data (Froese and Pauly 2015).
Samples and microscopy
Necropsy was performed according to Noga (2010). The organs sampled were the gut, heart, kidney, liver, muscle, spleen and stomach for light microscopy and histopathology.
For identification of the myxozoan structures, digestion and sedimentation of the tissues sampled were performed according to Grossel et al. (2003). These materials were analysed by microscopy, including Nomarski differential interference contrast (DIC) optics, as well as by molecular biology procedures. The measurements of spores were performed as described by Lom and Arthur (1989).
Histopathology
A 1-cm3 portion of each tissue was fixed in 10 % neutral buffered formalin followed by processing using standard histological techniques and embedding in paraffin (Bucke 1989). Haematoxylin and eosin were used for staining.
DNA extraction, PCR and sequencing
The samples destined for DNA extraction consisted of 20 mg of each organ collected (gut, heart, kidney, liver, muscle, spleen and stomach), as also 100 μL of a kidney primary cell culture from Nile tilapia infected by the myxozoan found in this report (not reported in this article), and 100 μL of material obtained for light microscopy identification. The samples were placed in sterile cryotubes containing 500 μL of buffer (50 mM EDTA, 50 mM Tris, and 150 mM NaCl, pH 8.0), 60 μL of 10 % SDS and 25 μL ribonuclease A (10 mg/mL). The samples were then subjected to three cycles of freezing and thawing (−80 °C for 4 h and 20 °C for 4 h), digested with 10 μL of proteinase K (10 mg/mL) at 60 °C for 1 h and washed in columns using the washing buffers of the Wizard® SV Genomic DNA Purification System (Promega®) kit according to the manufacturer’s instructions. The DNA was eluted in elution buffer (nuclease-free water) and kept at −20 °C until use.
The 18S small subunit ribosomal gene (18S SSU rDNA) was targeted with the primers ERIB1 and ERIB10 (Barta et al. 1997) for PCR reaction, ERIB1–Act1R (Hallet and Diamant, 2001) and MyxGen4F (Diamant et al., 2004)–ERIB10 for nested-PCR to obtain a complete SSU rDNA sequence from two overlapping fragments. Alternative nested PCR with MyxospecF–MyxospecR (Fiala, 2006) was performed to obtain a partial sequence in the case of negative results of the nested PCR. All reactions used 20 μL that was comprised of 3 μL of extracted genomic DNA, 10 μL of GoTaq Green Master Mix 2× (Promega®), 0.3 μM of each primer, 0.5 μL of DMSO and nuclease-free water (q.s.p.). The polymerase chain reaction (PCR) and nested PCR for all primers cited above were performed in a Veriti thermocycler (Life Technologies®) and consisted of an initial denaturation step of 95 °C for 3 min, followed by 40 cycles of 95 °C for 1 min, 48 °C for 1 min, and 72 °C for 1 min, finishing with terminal extension at 72 °C for 5 min and a hold at 22 °C. The PCR products were electrophoresed through a 1.5 % agarose gel stained with 1 % SYBRsafe (Invitrogen®), alongside of a HighRanger 1 kb DNA ladder (Norgen Biotek®). The amplicon of 1500 bp was excised from the gel and then purified with an Ilustra Microspin™ S-400 HR Columns kit (GE Healthcare®) according to manufacturer’s instructions.
The DNA amplified with universal primers ERIB1 and ERIB10 (Barta et al. 1997) of the kidney (target organ of S. niloticus n. sp.) of one fish from aquaculture and one from the river was submitted to the preparation of the amplicon for the deep sequencing system (Illumina MiSeq®) was performed according to Ullman et al. (2015). After sequencing, Sanger sequencing was performed with the same primers and same samples for confirmation of the first sequencing. For this, the purified amplicon was sequenced in both directions using ABI Big Dye Terminator Chemistry on an Applied Biosystems capillary 3500 Genetic Analyser. The quality of the electropherograms was assessed in Sequencing Analysis version 5.4 (Applied Biosystems). The first sequence was submitted to Denovo assembly using Geneious 7.1, followed by multiple alignments using the Denovo sequence and the one obtained by the Sanger method. After this step, a consensus sequence was made that was submitted to the Basic Local Alignment Search Tool (BLAST). To demonstrate the difference between the consensus sequence and other Sinuolinea species, MultAlin was used (Corpet 1988). After this analysis, it was performed PCR as describe above with all samples, and sequenced one sample consisted of a pool of all tissues collected by each sampling (one fish from aquaculture and one from the river by each month) by Sanger method for confirmation.
After analysis of the sequence, specific primers were designed (Sinuolineaf: 5′ TTC-AGC-CAC-ACG-AGA-TTG-AG 3′ and Sinuolinear: 5′ GGT-AGA-CACA-ACG-CTG-ATC-CA 3′) for real-time PCR (qPCR) using OligoPerfet Designer algorithm (https://tools.thermofisher.com/content.cfm?pageid=9716&icid=fr-oligo-6?CID=fl-oligoperfect). Quality of the primers was performed in the tool OligoAnalyzer 3.1 of Integrated DNA Technologies (https://www.idtdna.com/calc/analyzer). Specificity of the primers was determined using in silico analysis performed by primer OligoAnalzer 3.1 (https://www.idtdna.com/calc/analyzer), Auto Dimer Check software version 1.0, alignment using ClustalW in Molecular Evolutionary Genetics Analysis software version 6.0 and with a test of qPCR using two different myxozoans (Myxobolus spp. and Henneguya spp.). We observed that the primers designed only detected new species of Sinuolinea described in this paper. This was confirmed by analysis of sequencing (alignment), which presented 100 % of identity with the sequence obtained with Illumina® and Sanger method sequencing. The assay was conducted and analysed within the Applied Biosystems 7500 Fast Real-Time PCR Systems (Applied Biosystems). The 25 μL reaction mixture consisted of 12.5 μL of a GoTaq® qPCR Master Mix (Promega) 1×, 0.2 μM of each primer, 4 μL of DNA extracted and nuclease-free water (q.s.p.). All DNA extracted samples used in PCR and nested-PCR was performed by this reaction with these specific primers. The DNA sequenced was used as a positive control and nuclease-free water as a negative control. Cycling conditions were 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C, and 60 s at 60 °C. To confirm the specificity of the reaction, it sequenced by Sanger method 15 samples that were amplified (7 from O. niloticus infected by S. niloticus from aquaculture and 8 from the river) by qPCR.
For phylogenetic analysis, the following myxozoans were used to construct a phylogeny tree due to the genetic and morphological similarities among these species: Chloromyxum careni (accession number: HM641794), Cholomyxum leydig (accession number: DQ377710), Chloromyxum legeri (accession number: AY604197), Chlomoryxum riorajum (accession number: FJ1624481), Sinuolinea sp. (accession number: JX460906), S. dimorpha (accession number: JX460906), S. phyllopteryxa (accession number: DQ645952), S. niloticus n. sp.—our sequence (accession number: KR119066), Myxobolus arcticus (accession number: EU346378), Myxobolus curimatae (accession number: KP120979), Myxobolus honghuensis (accession number: JF340216), Myxobolus sandrae (accession number: EU346379), Myxospora sp. (accession number: JX460904), Myxidium sp. (accession number: GQ890672), Myxidium ceccarellii (accession number: KJ499821), Myxidium gadi (accession number: GQ890674 and GQ890675), Zschokkella balistoidi (accession number: KF179061), Sphaeromyxa kenti (accession number: JX443489), Sphaeromyxa longa (accession number: KM201344) and Sphaeromyxa sp. (accession number: KM201343). The sequence of C. careni (accession number: HM641795) was used as out-group. CLUSTAL_W (MEGA software version 5.1) was used for the initial sequence alignments.
For MEGA, the software recommended the general time reversible model, maximum likelihood, for our sequence as the most parameter-rich evolutionary model. Therefore, the settings used for analysis were nst = 6, with the gamma-distributed rate variation across sites, a proportion of invariable sites (rates – invgamma) and 1000 bootstraps.
Results and Discussion
Description
Species: Sinuolinea niloticus n. sp. (Fig. 1).
Host: Nile tilapia (Oreochromis niloticus).
Locality: Mira Estrela (19° 55′ 47.52″ S, 50° 08′ 36.56″ O) and Botucatu (22° 43′ 43″ S, 48° 22′ 29″ O), São Paulo, Brazil.
Sites of infection: Gut, heart, kidney, liver, muscle, spleen and stomach.
Spore characteristics: The spores were spheroids with a length range of 12.1 ± 1.6 μm (9.1–14.3). There were two spherical polar capsules on opposite sides in the middle of the spore close to the membrane, which had a diameter range of 5.9 ± 0.8 μm (4.1–8.1). The two valves were joined by the inner membrane of each polar capsule. The inner membrane followed the outer wall of the spore, and in the left polar capsule, the membrane passed through the middle (Fig. 2).
Illustration of Sinuolinea spp. A: S. niloticus n. sp. (present study). B: S. dimorpha, C: S. capsularis, D: S. capsularis, E: S. capsularis, F: S. arborescens, G: S. opacita, H: S. branchiophora (Davis, 1917), i: S. shandongensis, J: S. argyrosomi, K: S. platycephali (Zhao & Song, 2003), L: S. phyllopteryxa (Garner et al., 2008), M: S. arctica (Kodádková et al., 2014), N: S. tetraodoni (El-Matbouli & Hoffmann, 1994)
Deposited: Biotechnology Institute (IBTEC) with the registration numbers: IBTEC0001/2016 for DNA of S. niloticus n. sp. and IBTEC0002/2016 for tissues (gut, heart, kidney, liver, muscle, spleen and stomach) of Nile tilapia (O. niloticus) naturally infected by S. niloticus n. sp.
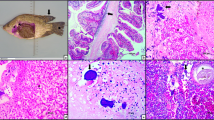
The measurements of the spores and polar capsules, hosts, sites of infection and prevalence of Sinuolinea spp. studied prior and during this study are listed in Table 2. These findings and morphological observations allowed confirmation that the myxozoan found in this study belonged to the Sinuolinea genus. Analysing the microscopic characteristics (Figs. 1 and 2), this parasite presented two valves on opposite sides in the middle of the spore close to the membrane, with the outer membrane following the outer wall of the spore and the membrane passing through the left polar capsule in the middle, which were structures that differed from other species described (Fig. 2), suggesting that the parasite found in this research was a new species of the Sinuolinea genus. Another interesting point is that the prevalence was very high, thus corroborating the occurrence reported by El-Matbouli and Hoffmann (1994). This value could be due to the contaminated habitat of the fish, environmental parameters, handling and density of the fish cultured.
Macroscopic lesions
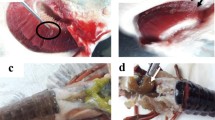
In this study, Nile tilapia sampled from aquaculture were observed to have congestion of the spleen; haemorrhage of the kidney; liquefaction of the kidney, muscle and the spleen; melanisation of the skin; renomegaly; scoliosis; and splenomegaly. However, fish caught from the river presented additional lesions, including atrophy of the spleen, congestion of the kidney, discolouration of the skin, haemorrhage of the spleen, hypertrophy of the kidney and spleen, ischaemia of the kidney and thinning of the kidney. However, only Garner et al. (2008) described lesions in Phyllopteryx taeniolatus infected with S. phyllopteryxa; in this animal, myxozoanosis caused a ventral abdominal purple discolouration and skin erosions, lesions that were observed only in Nile tilapia from aquaculture in the present study. The other lesions could be caused by parasitism, as the myxozoan was observed in all of the organs sampled. Although many lesions were observed, the most severe lesions were in the kidney (Tables 3, 4 and 5), suggesting that could be the target organ for S. niloticus n. sp.
Microscopic lesions
According to Noga (2010), most myxozoan infections of fish are relatively innocuous, eliciting only moderate host reactions. Heavy infections can be quite serious, resulting in mechanical damage from the pseudocysts or tissue necrosis and inflammation from the trophozoite feeding. Young fish are usually the most seriously affected by myxozoan infections. Histozoic forms usually cause more serious disease.
Garner et al. (2008) observed that the animals with myxozoans had biliary myxozoanosis, mineralised swim bladders, emaciation, renal tubular dilatation, hypertrophy of tubular epithelial cells, accumulation of cellular debris and some proteinaceous fluid in the tubules, in addition to a large number of developing and mature myxospores in the lumen of the renal tubules and collecting ducts. Comparing these findings with those of this present study, structures similar to myxozoans in the interstices were also observed in all tissues analysed, but the most affected organs were the kidney and the heart. Generally, coagulative necrosis, haemorrhage, inflammatory processes, the presence of melano-macrophages and eosinophils were observed in the gut, heart, kidney (Fig. 3), liver, muscle, spleen and stomach, as shown in Tables 4 and 5. These alterations could be caused by a myxozoan, as inflammatory cells, eosinophils and melano-macrophages are prevalent in this parasitism.
The prevalence of myxozoans by tissue analysed was higher in those tissues sampled from tilapia caught from the river (Fig. 4). Of all of the organs observed by microscopy, the heart, kidney, liver, muscle and stomach were the most parasitized. This could be due to the route of infection, justifying the presence of the parasite in the gut, stomach and liver, whereas other hypotheses include the contamination of the environment, low oxygen in the water or other pathogens that may stress the animals, which predispose them to this parasitism.
PCR, sequencing and phylogeny
According to Garner et al. (2008), the 18S SSU rRNA gene has proven useful in myxozoan identification from both freshwater and marine species (Fig. 5). The PCR amplified an approximately 1500 kb product of the 18S small subunit ribosomal gene (18S SSU rDNA) with the universal primers and a product of 150 pb with the specific primers designed in this study from the kidney, gall bladder, liver, spleen and stomach samples of all of the fish analysed in this study (100 % prevalence), similar to that amplified previously from a primary cell culture of Nile tilapia infected with Sinuolinea sp. (technique not presented in this paper), as observed by light microscopy, all of which were sequenced. Comparing the prevalence of positive samples by microscopy and PCR (Fig. 4) of the Nile tilapia sampled from aquaculture, it was observed that PCR detected more samples in the kidney, liver, spleen and stomach. However, in the tilapia sampled from the river, PCR and histology detected the same quantity of positive samples in the kidney and liver, but the molecular techniques were more sensitive in the spleen and stomach. These findings demonstrated the diagnostic importance of using histology and molecular techniques for the identification and characterisation of the pathogen causing myxozoanosis in fish.
The sequence obtained by the Illumina MiSeq® deep sequencing was confirmed by Sanger method. It was possible to obtain a longer sequence (1585 bp) using this technique and analysis with the Basic Local Alignment Search Tool showed a similarity of 89 % with Sinuolinea sp. KAB-2001 (Genbank: AF378346). However, Garner et al. (2008) noted that this sequence (Genbank: AF378346) contains a mixture of host and contaminant DNA segments. For this reason, multiple alignments with different sequences of Sinuolinea available in Genbank were made for similarity and phylogeny analysis. The morphology of our species is consistent with a Sinuolinea species, but does not match any nominal species. Furthermore, the DNA sequence analysis places this species as sister to known Sinuolinea species. As such, we propose a new species, designated as S. niloticus n. sp. due to its first description in the host Nile tilapia (O. niloticus).
This is the first report of Sinuolinea species infecting fish in Brazil, as there are only reports of this myxozoan in China (Zhao and Song 2003), Thailand (El-Matbouli and Hoffmann 1994), Svalbard (Kodádková et al. 2014) and the USA (Garner et al. 2008; Dyková et al. 2013). To date, there is still no report of this parasite in tilapia anywhere else in the world, with only Siphostoma floridae, Paralichthys albiguttus, P. dentatus, Spheroides maculates, Cynoscion regalis (Davis, 1917), Argyrosomus argentatus (Zhao & Song, 2003), Myoxocephalus scorpius (Kodádková et al., 2014), Cyclopterus lumpus (Basikalova, 1932), Hemiscyllium ocellatum Moser et al., 1989), Thamnacons septentrionalis (Zhao & Song, 2001), Coryphaenoides acrolepis, C. pectoralis (Yoshino & Noble, 1973), Ammodites tobianus (Basikalova, 1932), Solea solea, Coelorinchus coelorinchus (Tripathi, 1948), Nibea albiflora (Zhao & Song, 2003), Platycephalus indicus (Zhao & Song, 2003), P. taeniolatus (Garner et al., 2008), Boreogadus saida (Shulman, 1953), Sphaeroides vermicularis (Shulman, 1966) and Tetraodon palembangensis (El-Matbouli & Hoffmann, 1994) having been previously reported to infect tilapia, demonstrating the significance of this work.
This study highlights that those people involved with tilapia from aquaculture and river sources need to know about the presence of this parasite in this fish species, as this parasite can cause many alterations that can lead to loss in the production or even the mortality of the fish, depending on the on the quantity of the parasites and the immune response of the animals.
References
Barta JR, Martin DS, Liberator PA, Dashkevicz M, Anderson JW, Feighner SD, Elbrecht A, Perkins-Barrow A, Jenkins MC, Danforth D, Ruff MD, Profous-Juchelka H (1997) Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J Parasitol 83:262–271
Basikalova A (1932) Data on the parasitology on Murmansk fish. Sb. Nauchno-Promysl: Rabot na Murmane, Snabtekhizdat
Bucke D (1989) Histology. In: Austin DA, Austin B (eds) Methods for the microbiological examination of fish and shellfish. Ellis Horwood, Chichester, pp 69–97
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucl Acids Res 16(22):10881–10890
Davis HS (1917) Myxosporidia of the Beaufort region: a systematic and biological study. Fish Bull 35:201–243
Diamant A, Whipps CM, Kent ML (2004). A new species of Sphaeromyxa (Myxosporea:Sphaeromyxina: Sphaeromyxidae) in devil firefish, Pterois miles (Scorpaenidae), from the northern Red Sea: morphology, ultrastructure, and phylogeny. J Parasitol 90:1434–1442
Dyková I, Kodádková A, de Buron I, Fiala I, Roumillat WA (2013) Sinuolinea infections in the urinary system of Cynoscion species of Sinuolinea Davis, 1917 (Myxozoa: Myxosporea). Int J Parasitol Parasites Wildl 2:10–17
El-Matbouli M, Hoffmann RW (1994) Sinuolinea tetraodoni n. sp., a myxozosporean parasite of freshwater pufferfish Tetraodon palembangensis from Southeast Asia—light and electron microscope observations. Dis Aquat Org 19:47–54
Fiala I (2006). The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int J Parasitol 36:1521-1534
Froese R, Pauly D (2015) Fishbase. World wide web electronic publication., http://www.fishbase.org. Accessed 01 Aug 2015
Garner MM, Atkinson SD, Hallett SL, Bartholomew JL, Nordhausen RW, Reed H, Adams L, Whitaker B (2008) Renal myxozoanosis in weedy sea dragons, Phyllopteryx taeniolatus (Lacepède), caused by Sinuolinea phyllopteryxa n. sp. J Fish Dis 31:27–35
Grossel GW, Dykova I, Handlinger J, Munday BL (2003) Pentacapsula neurophila n. sp. (Multivalvulida) from the central nervous system of striped trumpeter, Latris lineate (Forster). J Fish Dis 26:315–320
Hallett SL, Diamant A (2001). Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis Aquat Org 46:197–212
Kodádková A, Dyková I, Tyml T, Ditrich O, Fiala I (2014) Myxozoa in high artic: survey on the central part of Svalbard archipelago. Int J Parasitol Parasites Wildl 3:41–56
Lom J, Arthur JR (1989) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12:151–156
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol 53:1–36
Moser M, Kent ML, Dennis D (1989) Gall bladder Myxosporea in coral reef fishes Heron Island, Australia. Aust J Zool 37:1–13
Noga EJ (2010) Fish disease: diagnosis and treatment. Blackwell Publishing, Iowa
Shulman SS (1953) Parasites of fish from the white sea. Izd Akad Nauk SSSR, Moskva, p 198
Shulman SS (1966) Myxosporidia of the USSR. Nauka Publishers, Moscow–Leningrad
Tripathi YR (1948) Some new Myxosporidia from Plymouth with a proposed new classification of the order. Parasitol 59:844–850
Ullman LS, Tozato CC, Malossi CD, da Cruz TF, Cavalcante RV, Kurissio JK, Cagnini DQ, Rodrigues MV, Biondo AW, Araújo Júnior JP (2015) Comparative clinical sample preparation of DNA and RNA viral nucleic acids for commercial deep sequencing system (Illumina MiSeq®). J Virol Methods 220:60–63
Yoshino TP, Noble RR (1973) Myxosporidia of macrourid fishes from southern California and Mexico. J Parasitol 59:844–850
Zhao Y, Song W (2001) Myxoproteus cheni sp. n. and Sinuolinea mai sp. n. (Myxosporea: Sinuolineidae) parasitic in the urinary bladder of marine fish (Thamnaconus septentrionalis) from the Yellow Sea, off the Qingdao coast of China. Acta Protozool 40:125–130
Zhao Y, Song W (2003) Studies on the morphology and taxonomy of three new myxosporeans of the genus Sinuolinea Davis, 1917 (Myxosporea: Sinuolineidae) infecting the urinary bladder of some marine fishes from the Shandong coast, China. Syst Parasitol 55(1):53–59
Acknowledgments
The authors would like to thank Prof. Dr. Reinaldo José da Silva from the Department of Parasitology, Biosciences Institute, Univ. Estadual Paulista (UNESP), for allowing the authors to use the Nomarski differential interference contrast (DIC) microscope of the laboratory of parasitology, which was crucial for identification of the myxozoan found in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Vaz Rodrigues, M., Francisco, C.J., Biondi, G.F. et al. Sinuolinea niloticus n. sp., a myxozoan parasite that causes disease in Nile tilapia (Oreochromis niloticus). Parasitol Res 115, 4307–4316 (2016). https://doi.org/10.1007/s00436-016-5214-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5214-z