Abstract
Myxozoans can induce losses in production of marine and freshwater fish. Recently, a new parasitic myxozoan species, Sinuolinea niloticus, was identified and its importance highlighted. This study aimed to investigate Sinuolinea niloticus and its macroscopic and microscopic alterations in Nile tilapia (Oreochromis niloticus) specimens from fish farms in Botucatu, São Paulo, Brazil, as well as its molecular detection. Thirty tilapias from three different fish farms were collected and submitted to necropsy examination, where organs were sampled for histopathological and molecular analysis. The main necroscopic findings at all three fish farms were hemorrhaging, fin erosion, hepatomegaly, and splenomegaly. Microscopically, the main findings were degeneration of hepatocytes and hepatitis, epithelial and goblet cell hyperplasia in the gills, nephritis, gastritis, and presence of myxozoan-like structures in different organs. S. niloticus, detected by qPCR, showed a 90% prevalence among surveyed fish at farm 1 and 100% at farms 2 and 3. The high prevalence found in this study shows the importance of this myxozoan as a potential pathogenic agent of farmed O. niloticus that negatively affects fish health and, consequently, production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia represents one of the most important and widespread species in worldwide aquaculture. This scenario is due to its advantageous production characteristics such as rapid growth, adaptability to the environment, and resistance to stress and diseases (El-Sayed 2006).
In Brazil, the most cultivated species is the Nile tilapia (Oreochromis niloticus), which was introduced in 1971 and now it is widespread throughout the country (IBGE 2017). The last agricultural census showed that there are 110,072 fish farms in Brazil producing tilapias, of which 2929 of these farms are in São Paulo state and five in Botucatu city (IBGE 2017). According to the Dellova et al. (2019) Yearbook of Fish Farming, in 2018, tilapia production grew 11.9% over the previous year, reaching 400,280 t, which ranked Brazil fourth in world production, behind only China, Indonesia, and Egypt. Also, according to this publication, the state of São Paulo was the second producer in the country, with 69,500 t.
Conditions like poor water quality and stress predispose farmed fish to infections (Martins et al. 1999). As studied by Eiras (1994) and Eiras et al. (2010), some pathogens can cause high mortality rates in fish. Among these agents, myxozoans are important cosmopolitan parasites that can lead to serious damage in multiple organs, causing several lesions which may result in death and substantial economic losses to aquaculture companies and fisheries (Barassa et al. 2003; Sitjà-Bobadilla 2008; Noga 2010; Okamura et al. 2015).
Myxozoans are obligatorily histozoic (found in tissues) or coelozoic (found in the coelomic cavity) parasites, with aquatic annelids being the definitive hosts and fish the intermediate ones (Lom and Dykova 1992; Noga 2010). The organs affected described are diverse, sometimes with site tropism, and include fins, gills, gallbladder, urinary bladder, kidneys, and intestines (Fioravanti et al. 2004; Garner et al. 2008; Shin et al. 2014; Rodrigues et al. 2016).
Among myxozoans, the genus Sinuolinea, comprising approximately 20 species, can parasitize either marine or freshwater fish and cause injuries or even mortality, depending on the parasite loads and the immune status of the animals (Davis 1917; Garner et al. 2008; Rodrigues et al. 2016; Shin et al. 2019). Rodrigues et al. (2016) identified a new species of this genus (Sinuolinea niloticus) affecting O. niloticus that can lead to production losses, thus requiring its investigation in aquaculture.
This study aimed to investigate the presence and pathological lesions of S. niloticus parasites in tilapias from fish farms in Botucatu city, São Paulo state, Brazil.
Material and methods
Sampling
Before the execution of this study, the project was submitted to the Committee for Ethics in Animal Use of FMVZ – UNESP Botucatu and approved under the number 0096/2019.
For this project, a total of 30 fish (O. niloticus) were caught at three fish farms in Botucatu city, São Paulo state, Brazil, in April of 2019 (10 animals from each farm). The capture of fish was performed until 10 fish were obtained, regardless of their appearance (healthy or unhealthy), using natural fishing with telescopic rods, hook number 4 without a gaff, and monofilament line number 0.28, with commercial feed P28. After this procedure, O. niloticus specimens were transported to the laboratory in a styrofoam box containing recyclable ice in equal proportions (1 kg of ice to 1 kg of fish) for necropsy and collection of organs for histopathological and molecular analysis.
The longest time of transportation to the laboratory was 23 min. Fish were alive when they reached the laboratory. Then, they were euthanized according to the guidelines provided by the Committee for Ethics in Animal Use of FMVZ – UNESP Botucatu.
Necropsy and histopathology
Necropsy was performed according to Noga (2010). The organs sampled were the eyes, gill, gonads, gut, heart, kidneys, liver, muscle, spleen, and stomach, for histopathology and molecular analysis. As described by Bancroft and Gamble (2008), a 1-cm3 fragment of each tissue was fixed in 10% neutral buffered formalin, followed by processing using standard histological techniques and stained with hematoxylin and eosin.
Molecular techniques
The organ tissues collected from each fish were pooled, and 20 mg of each sample was used for molecular analysis. The DNA was extracted using the kit Wizard® SV Genomic DNA Purification System (Promega Corporation®) according to the manufacturer’s instructions. The DNA was eluted in elution buffer (nuclease-free water) and kept at − 20 °C until use. The purity and quantification of extracted DNA were measured using a 260/280 absorbance rate in a Nanodrop 2000c (Thermo Fisher Scientific®). Only DNA with a ratio > 1.7 (260/280 rate) was used in this study. The O. niloticus samples used as the positive control had been naturally infected by the myxozoan S. niloticus previously identified by PCR and submitted to GenBank under the number KR119066 as described by Rodrigues et al. (2016) after sequencing and phylogeny analysis.
Lesions and structures compatible with myxozoan were observed in the histopathology, and therefore, qPCR (real-time polymerase chain reaction) was performed. For this, the myxozoan reaction mixture consisted of 10 μL Gotaq qPCR Master Mix 1X (Promega), 300 nM of each primer (Sinuolinea F and Sinuolinea R), 3 μL de DNA and nuclease-free water to adjust the final volume to 20 μL. Cycling conditions were performed as described by Rodrigues et al. (2016), i.e., 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 60 s at 60 °C. The positive result was confirmed by melting curve analysis compared with the positive control.
Statistical analysis
At each fish farm sampled, the prevalences of macroscopic and microscopic lesions, and S. niloticus were calculated. The statistical difference of myxozoan positivity was evaluated by molecular techniques between fish farms using the chi-square test with a 95% confidence interval. Statistical difference was adopted as p < 0.05. All statistical analyses were performed in Statistica v. 10 (Stat Soft 2011) and visualized in GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, USA, https://www.graphpad.com.
Results and discussion
Pathology
Oreochromis niloticus sampled from fish farm 1 presented primary lesions in the skin, spleen, and liver, while all of them presented hemorrhaging and 80% showed skin erosions of the fins. Other relevant lesions were hepatomegaly (100%), steatosis (80%), splenomegaly (60%), congestion and mucus production in the gills (40%), and lamellar fusion (30%).
Tilapias from fish farm 2 presented hemorrhaging in fins (100%), erosion of the fins (90%), hepatomegaly (80%), splenomegaly (60%), liver pallor (30%), hemorrhaging in the gills (30%), and lamellar fusion (30%). Specimens at fish farm 3 showed hepatomegaly (100%) and liver pallor (90%), hemorrhaging and erosion in fins (80% and 90%), splenomegaly (80%), petechiae on the skin (30%), and whitish areas in the gills (30%) (Table 1 and Fig. 1).
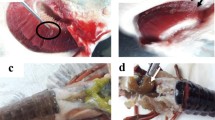
a In situ analysis shows congestion and hemorrhaging of gills (asterisk), hepatomegaly (arrowhead), whitish areas in the dorsal fin (arrow), and erosion of caudal fin (circle). b Intestine with inflammatory infiltrate (arrow). c Kidney infected by myxozoan cysts (arrow) and tubular degeneration (asterisk). d Liver infected by myxozoan cysts (arrowhead), hepatocyte degeneration (asterisk), and inflammatory infiltrate (arrow). e Stomach with myxozoan cysts (arrow). f Spleen with myxozoan cysts (arrow) and granuloma (asterisk)
However, Rodrigues et al. (2016) sampled O. niloticus from one fish farm and one river, and observed lesions dissimilar to our study, except for splenomegaly. The tilapias from fish farm presented congestion of the spleen, hemorrhaging of the kidney, liquefaction of the kidney, muscle and the spleen, melanization of the skin, renal hypertrophy, scoliosis, and splenomegaly. The fish caught from the river presented additional lesions: splenic atrophy, kidney congestion, skin discoloration, splenic hemorrhaging, hypertrophy of the kidney and spleen, kidney ischemia, and kidney thinning.
In our study, macroscopic lesions were undetectable in the kidney, probably because of the short fish length and therefore the size of this organ. Moreover, the steatosis found at fish farms 1 and 3 may be attributable to the diet offered by the farmers.
Furthermore, Garner et al. (2008) described a ventral abdominal purple discoloration and skin erosions in Phyllopteryx taeniolatus infected with S. phyllopteryx, lesions that were not observed in our study, and found only in O. niloticus from aquaculture by Rodrigues et al. (2016). Despite the disparity, the lesions described herein might have been caused by parasitism, especially in the heart, kidney, liver, spleen, stomach, and gut, organs where myxozoans were observed.
Microscopically, Oreochromis niloticus sampled from fish farm 1 displayed hepatocyte degeneration (100%), inflammation and hyperplasia in the gills (90% and 60%), inflammatory process and hemorrhaging in the heart (80% and 70%), congestion, melanomacrophages and inflammatory process in the liver (50%), myxozoan-like structures placed in the liver (40%), gills and heart (30%). The lamellae were hemorrhagic and fused (40%).
Tilapias from fish farm 2 presented hyperplasia and inflammatory process in the gills (90%), heart inflammation, hemorrhage, edema and degeneration (80%, 40%, 30%, and 30%), hepatocyte degeneration, congestion, inflammation, melanomacrophages, and necrosis (90%, 50%, 30%, 30%, and 50%).
In tilapias from fish farm 3, epithelial and goblet cell hyperplasia were found in the gills (80%), some of which showed associated inflammatory process (40%). The liver presented degeneration (100%), hemosiderin (60%), myxozoan-like structures (50%), inflammatory process (40%), and congestion (30%). Lastly, there was heart degeneration (60%), inflammatory process, and edema (50% each) (Fig. 1 and Table 2).
Histologically, there were myxozoan cysts causing inflammation and tissue injury in the liver, kidneys, spleen, stomach, gut, and heart, similar to those described by Rodrigues et al. (2016), that were subsequently all positive in our molecular results, confirming the etiologic agent. The lesions found may lead to a decreased surface area for oxygen absorption and organ malfunction, which results in a deficit in productivity, facilitates secondary infections, and may cause death. Consequently, economic losses are substantially increased.
Severe infections may also result in mechanical damage from the pseudocysts or tissue necrosis and inflammation, which can lead to production loss or even fish mortality, depending on the immune response and parasite load (Luque 2004; Rodrigues et al. 2016).
Furthermore, although myxozoan is not considered zoonotic, the injuries caused to the fish should predispose them to secondary diseases that may be zoonotic, for example, at fish farm 3, where 10% of the animals tested positive for Aeromonas sp. and Mycobacterium sp., results not presented in this manuscript.
Molecular results
The samples analyzed showed a high prevalence of S. niloticus in tissues of O. niloticus. Fish sampled from fish farm 1 presented 90% of this parasite versus 100% at farms 2 and 3. Thus, a product of 150 bp was amplified as expected by primers used for S. niloticus while the amplification curve and melting analysis compared to positive control demonstrated the positivity of the samples collected in this study. All positive tests had 100% homology with Sinuolinea niloticus.
The high prevalence of this myxozoan species in this study (90 to 100%) is similar to the findings of Rodrigues et al. (2016) in O. niloticus infected with S. noliticus, and similar to those of El-Matbouli and Hoffmann (1994) studying S. tetraodoni in the pufferfish (Tetraodon palembangensis) which both showed 100% prevalence.
Therefore, it might be interesting to investigate the predisposing factors for this pathogen in order to adopt prophylactic measures.
In addition, we investigated known bacteria and microsporidia infecting tilapia because histopathology showed bacterial colonies. We tested using PCR and qPCR for Aeromonas sp., Edwardsiella sp., Francisella sp., Mycobacterium sp., Nucleospora braziliensis, and Streptococcus sp. Only fish farm 3 tested positive for two of these agents, with 10% of the animals being positive for Aeromonas sp. and Mycobacterium sp., results not presented in this manuscript.
Finally, we did not test for viral pathogens because we could not afford the extra costs.
Conclusion
The high prevalence of S. niloticus at the fish farms investigated in this study and the lesions found corroborate the importance of S. niloticus as a pathogenic agent of Nile tilapia (O. niloticus) that causes injuries and can negatively affect fish health and, consequently, production.
References
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier, London
Barassa B, Adriano EA, Arana S, Cordeiro NS (2003) Henneguya curvata n. sp. (Myxosporea: Myxobolidae) parasitizing the gills of Serrasalmus spilopleura (Characidae: Serrasalmidae), a south American freshwater fish. Folia Parasitol 50:151–153. https://doi.org/10.14411/fp.28003.026
Davis HS (1917) Myxosporidia of the Beaufort region: a systematic and biological study. Washington Bull U S Bureau Fish 35:201–243
Dellova D, França D, Donati G, Cezário G, Marini H, Villa Real J, Lino J, Priebsch K, Albuquerque L, Oliveira M, Vieira R (2019). Associação Brasileira da Piscicultura - Peixe Br. Anuário Peixe BR da Piscicultura. https://www.peixebr.com.br/Anuario2019/AnuarioPeixeBR2019.pdf. Accessed 17 November 2019
Eiras JC (1994) Elementos de ictioparasitologia. Fundação Eng, António de Almeida, Portugal, 339p
Eiras JC, Monteiro CM, Brasil-Sato MC (2010) Myxobolus franciscoi sp. nov. (Myxozoa: Myxozporea: Myxobolidae) a parasite of Prochilodus argenteus (Actinopterygii: Prochilodontidae) from the Upper São Francisco River, Brazil, with a revision of Myxobolus spp. from South America. Zoologia 27:131–137
El-Matbouli M, Hoffmann RW (1994) Sinuolinea tetraodoni n. sp., a myxosporean parasite of freshwater pufferfish Tetraodon palembangensis from Southeast Asia - light and electron microscope observations. Dis Aquat Org 19:47–54. https://doi.org/10.3354/dao019047
El-Sayed AFM (2006) Tilapia culture. Elsevier, London
Fioravanti ML, Caffara M, Florio D, Gustinelli A, Marcer F (2004) Sphaerospora dicentrarchi and S. testicularis (Myxozoa: Sphaerosporidae) in farmed European seabass (Dicentrarchus labrax) from Italy. Folia Parasitol 51:208–210
Garner MM, Atkinson SD, Hallett SL, Bartholomew JL, Nordhausen RW, Reed H, Adams L, Whitaker B (2008) Renal myxozoanosis in weedy sea dragons, Phyllopteryx taeniolatus (Lacepède), caused by Sinuolinea phyllopteryx n. sp. J Fish Dis 31:27–35
Instituto Brasileiro de Geografia e Estatística - IBGE (2017). Censo Agropecuário 2017. https://sidra.ibge.gov.br/tabela/6661#resultado. Accessed 18 October 2019
Lom J, Dykova I (1992) Protozoan parasites of fishes. In: Fast AW, Lester LJ (eds) Developments in aquaculture and fisheries science. Elsevier, Amsterdam, pp 26–315
Luque JL (2004) Biologia, epidemiologia e controle de parasitas de peixes. In: Colégio Brasileiro de Parasitologia Vaterinária (ed) Anais 13° Congresso Brasileiro de Parasitologia Veterinária & I Simpósio Latino-Americano de Ricketisioses. Ouro Preto, MG, Brazil, pp 161–164
Martins ML, de Souza VN, de Moraes JRE, de Moraes FR (1999) Gill infection of Leporinus macrocephalus Garavello & Britski, 1988 (Osteichthyes: Anostomidae) by Henneguya leporinicola n. sp. (Myxozoa: Myxobolidae) Description, histopathology and treatment. Rev Bras Biol 59(3):527–534
Noga EJ (2010) Fish disease: diagnosis and treatment. Blackwell Publishing, Iowa
Okamura B, Gruhl A, Bartholomew JL (2015) An introduction to Myxozoan evolution, ecology and development. In: Okamura B, Gruhl A, Bartholomew JL (ed) Myxozoan evolution, ecology and development. Springer, Budapest, pp 1–20
Rodrigues MV, Francisco CJ, Biondi GF, Araújo Júnior JP (2016) Sinuolinea niloticus n. sp., a myxozoan parasite that causes disease in Nile tilapia (Oreochromis niloticus). Parasitol Res 115(11):4307–4316
Shin SP, Nguyen VG, Jeong JM, Jun JW, Kim JH, Han JE, Baeck GW, Park SC (2014) The phylogenetic study on Thelohanellus species (Myxosporea) in relation to host specificity and infection site tropism. Mol Phylogenet Evol 72:31–34
Shin SP, Jin CN, Sohn HC, Lee J (2019) Sinuolinea capsularis (Myxosporea: Sinuolineidae) isolated from urinary bladder of cultured olive flounder Paralichthys olivaceus. Korean J Parasitol 57(2):127–134. https://doi.org/10.3347/kjp.2019572127
Sitjà-Bobadilla A (2008) Fish immune response to Myxozoan parasites. http://www.Parasite-Journal.org. Parasite 15:420–425. https://doi.org/10.1051/parasite/2008153420
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All applicable guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Battazza, A., da Silva Brasileiro, F.C., Machado, E.F. et al. Identification and characterization of Sinuolinea niloticus from Nile tilapia (Oreochromis niloticus) farmed in Botucatu, Brazil. Aquacult Int 28, 1899–1906 (2020). https://doi.org/10.1007/s10499-020-00565-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00565-6