Abstract
Sixty specimens of African sharptooth catfish Clarias gariepinus (Clariidae) were collected during the period of March 2014–February 2015 from boat landing sites and fishermen of the River Nile at Beni Suef Governorate, Egypt, and examined for the presence of digenean parasites. The morphology of these parasites was studied by using light microscopy to describe morphological and morphometrically measurements for different body parts. Three digenean species belonging to three different genera were collected from various organs within the examined fish species. A certain degree of site specificity was also observed, with Thaparotrema botswanensis being found only in the gall bladder of C. gariepinus, while both Pseudoholorchis clarii and Glossidium pedatum were found only in the intestine and thus seem to occupy a certain niche within their host. This study represent as the first record of T. botswanensis and G. pedatum from C. gariepinus, as well as the first report of the genera from the River Nile in Egypt. In addition, re-description of P. clarii clarifies measurements for some body parts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most important problems facing our world nowadays is food deficiency (Abdel-Ghaffar et al. 2015a). The protein deficiency is one of the major global challenges facing the third world today. In Egypt, the continuous increase in human population requires more food production to meet the consequent increasing demands (Abdel-Gaber et al. 2015a). With the increasing demands for animal protein, the poultry sources were expensive and insufficient. Fish were considered to compensate the continuous lack of such element due to its comparatively low price (Abdel-Ghaffar et al. 2015b; Abdelsalam et al. 2015; Abdel-Gaber et al. 2016). The catfishes are members of the order Siluriformes and comprise about 2800 species worldwide (Ayanda 2009; Eschmeyer 2014). There are three families in the African suborder Siluroidea that contain species suitable for aquaculture. These are the Claroteidae (formerly Bagridae), the Schilbeidae, and the Clariidae (Mwita 2014). The African sharptooth catfish, Clarias gariepinus (Burchell 1822), is a member of Clariidae family which is widely distributed in Africa and Middle East (Arafa and Reda 2002). It is one of the most important fish species for aquaculture and biological research (Yalcin et al. 2002; Chai 2007; Tepe et al. 2013; Abdel-Gaber et al. 2015b). However, parasitic infections are known to cause massive mortality in the fry and fingerling stages, especially in high-density aquaculture systems (Eissa et al. 2009; Jansen van Rensburg et al. 2013; Abdel-Gaber et al. 2015c). Fish helminthology in Africa is not as widely studied as other aspects of aquatic parasitology and fish biology (Imam et al. 1991; Galli et al. 1998; Abo Esa 2008). This is probably because helminth parasites mainly infect the internal organs, predominantly the gastrointestinal tract which, for humans, does not comprise the edible portion of the fish (Bhure and Nanware 2011; Omeji et al. 2013). Although fishermen and anglers regularly encounter encysted “grubs” (metacercariae) in the skin and muscles of fish, they regard them as just a nuisance, notwithstanding the biological and economic impact they may have on the fish species (Yooyen et al. 2006; Tasawar et al. 2007; Yilmaz et al. 2009).
Therefore, the objectives of the present study were to specifically identify and classify number of trematode parasites collected from C. gariepinus based on their morphological features and note their prevalence and mean intensity in the River Nile at Beni Suef Governorate, Egypt.
Materials and methods
Sample collection
A total of 60 fish specimens (C. gariepinus) were collected during the period of March 2014–February 2015 from boat landing sites and fishermen of the River Nile at Beni Suef Governorate, Egypt. All specimens ranged between 15 to 45 cm (total length) and 80 to 130 g (body weight). All samples were kept in full glass aquaria, supplied with chlorine free tap water with continuous aeration and filtration then transported immediately to Laboratory of Parasitology Research at Zoology Department in Faculty of Science, Beni Suef University, to follow the guidelines of identification according to Benech et al. (1923).
Parasitological examination
The collected fish samples were dissected, and the gastrointestinal tracts were examined carefully for parasitic infection under stereo- and light microscope. Relaxation for trematode parasites was carried out between two slides with drops of 70 % ethyl alcohol to the required point of relaxation observed under the binocular microscope. Care was usually taken to avoid the use of strong pressure, particularly on delicate parasites. All parasites were removed and fixed in 70 % ethanol. The collected parasites were washed in running water then soaked in semichon’s aceto-carmine for 3 h. After staining, worms were washed in distilled water and passed through ascending grades of ethyl alcohol 50, 70, 90, and 100 %, then transferred into xylol, clove oil and mounted in Canada balsam. Slides were then incubated at 60 °C for 24 h to driving the air bubbles according to Schmidt (1992). The identification of trematode parasite was established based on Sirikantayakul (1985). Photomicrographs were taken using Zeiss Axiovert 135 microscope supplied with a Canon Digital Camera. All drawings were made with the aid of camera Lucida (Weesner 1965).
Measurements
After the specimen was mounted on a slide, its outer borders and inner organs were measured. All measurements are in millimeter (mm) followed the guidelines of Bush et al. (1997) and were done after microscope calibration using a special slide with an additional ocular micrometer, minimum and maximum values given, followed by arithmetic mean (±SD) in parentheses.
Description
Family Opisthorchiidae Braun (1901)
Genus Thaparotrema Gupta (1955)
Thaparotrema botswanensis Jansen van Rensburg et al. (2013) (Figs. 1a–h, 4a; Table 1)
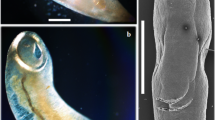
Photomicrographs of the adult digenea T. botswanensis. a The adult worm with oral sucker (OS) followed by the pharynx (PH), esophagus (E), ventral sucker (VS), genital pore (GP), intestinal caeca (IC), vitelline follicles (VF), seminal vesicle (SV), uterus (U), ovary (O), two testes (TE), and the body ended with excretory bladder (EB) opened outside with an excretory pore (EP). b–h High magnifications of different body parts showing: b, c Anterior end with oral sucker (OS) followed by the pharynx (PH) and esophagus (E). d Ventral sucker (VS) and genital pore (GP). e The Uterus (U) filled with eggs (EG). f Vitelline follicles (VF). g Eggs (EG). h Posterior part with the ovary (O), seminal receptacle (SR), two testes (TE), and body ended with excretory pore (EP)
Body was elongated, narrow with round extremities, maximum width at the level of the ventral sucker and measured 2.1–3.89 (3.11 ± 0.1) mm long and 0.32–0.54 (0.43 ± 0.1) wide. Oral sucker was terminal, rounded shape, and measured 0.19–0.39 (0.29 ± 0.01) mm long and 0.17–0.42 (0.31 ± 0.01) mm wide. Ventral sucker was slightly smaller than oral sucker, situated in the first quarter of the anterior part of the body and measured 0.14–0.33 (0.25 ± 0.01) mm long and 0.15–0.32 (0.20 ± 0.01) mm wide. Pre-pharynx was absent. Pharynx rounded and measured 0.04–0.92 (0.73 ± 0.01) mm long. Esophagus long, caecal bifurcation was pre-acetabular; caeca narrow, extend to almost end of the posterior extremity, and measured 0.03–0.2 (0.15 ± 0.01) mm long. Two testes, slightly oval, and tandem to diagonal within inter-caecal space near to the posterior extremity of the body. Posterior testis was slightly bigger than anterior one and measured 0.16–0.45 (0.28 ± 0.01) mm long and 0.14–0.34 (0.26 ± 0.01) mm wide. While the anterior testis measured 0.13–0.39 (0.22 ± 0.01)mm long and 0.15–0.37 (0.28 ± 0.01) mm wide. Cirrus sac was absent. Seminal vesicle was tubular commencing post-acetabular. Genital pore was median in position, just pre-acetabular. Ovary was smooth and inter-caecal. Mehlis’ gland was present and measured 0.09–0.28 (0.19 ± 0.01) mm long and 0.06–0.36 (0.19 ± 0.01)mm wide. Seminal receptacle situated obliquely anterior to the ovary. Laurer’s canal was not observed. Vitelline follicles were extending post-acetabular to the level of the ovary and slightly overlapping caeca. Uterus extensively coiled, inter-caecal, with ascending limb only, and sinistral to seminal vesicle. Eggs were operculated and measured 0.01–0.04 (0.03 ± 0.01) mm long and 0.002–0.01 (0.01 ± 0.001) mm wide. The excretory bladder was saccular and opened outside by sub-terminal excretory pore.
Taxonomic summary
Type host: African sharptooth catfish Clarias gariepinus (Burchell 1822) (F: Clariidae)
Site of infection: The gall bladder of infected fish
Host Locality: River Nile at Beni Suef Governorate, Egypt
Prevalence of infection: 11.66 % (7 out of 60 were infected)
Material deposition: Voucher specimens deposited in Zoology Department, Faculty of Science, Beni Suef University, Beni Suef, Egypt
Etymology: The specific name of the parasite (botswanensis) was derived from the Country name where the parasite was discovered and described for the first time
Remarks: The genus Thaparotrema Gupta 1955 was proposed to accommodate trematode parasites collected from the intestine and gall bladder of freshwater fish. This genus was erected for Thaparotrema vittalani Gupta 1955 that was described from the intestine of Bagrid catfish in India. Previously, this species was placed in the genus Opisthorchis later on Scholz (2008) transferred it to the genus Thaparotrema Gupta 1955 (Bray et al. 2008). In the present study, morphological and morphometric studies for this digenean parasite have close resemblance with T. botswanaensis described previously by Jansen van Rensburg et al. (2013) in having all characteristic features as elongated body, pre-pharynx absent, esophagus long, both testes oval and diagonal separated by excretory bladder with sigmoid stem between anterior and posterior testes and excretory pore is sub-terminal (Table 1). While it differs from the others as T. piscicola Bray et al. (2008) collected from gall bladder of Gymnarchus niloticus of Africa in having pharynx oval, ovary anterior to the seminal receptacle; excretory bladder with sigmoid stem and excretory pore is terminal; from T. pedicellatum Bray et al. (2008) collected from the intestine of Hemibagrus nemurus of Thailand in having oral sucker sub-terminal, pharynx oval, esophagus absent, testes separated by excretory bladder, ovary anterior to the seminal receptacle, uterine coils not much compacted, excretory bladder with sigmoid stem between anterior and posterior testes; from T. vitallani Gupta (1955) collected from intestine of Bagrid Catfish of India in having smaller body, testes diagonal near caecal end and separated by excretory bladder; anterior testis larger than the posterior one, uterine coils not compacted entirely, vitelline follicles not compacted highly, extending from post-bifucal level of intestine up to the level of the anterior testis, excretory bladder with sigmoid stem between anterior and posterior testes, and excretory pore is terminal.
Family Lepocreadiidae Odhner (1905)
Genus Pseudoholorchis Yamaguti (1958)
Pseudoholorchis clarii Bayoumy et al. (2013) (Fig. 2a-g, 4b; Table 2)
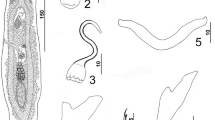
Photomicrographs of the adult digenea P. clarii. a The adult worm with oral sucker (OS) followed by the pharynx (PH), esophagus (E), ventral sucker (VS), cirrus pouch (CP), genital pore (GP), intestinal caeca (IC), vitelline follicles (VF), uterus (U), ovary (O), two testes (TE), and the body ended with excretory pore (EP). b–g High magnifications of different body parts showing: b, c Anterior end with oral sucker (OS) followed by the pharynx (PH) and esophagus (E). d, e Ventral sucker (VS) and cirrus pouch (CP). f Posterior part with excretory pore (EP). g Eggs (EG)
Body was elongated, dorsoventrally flattened with more or less a narrow anterior and broadly rounded posterior end. It measures 4.22–5.21 (4.92 ± 0.1) mm long and 1.1–1.98 (1.56 ± 0.1) mm wide. The oral sucker measured 0.29–0.41 (0.35 ± 0.01) mm long and 0.21–0.29 (0.25 ± 0.01) mm wide. The ventral sucker is spherical and larger than the oral one and measuring 0.42–0.51 (0.49 ± 0.01)mm long and 0.39–0.49 (0.46 ± 0.01)mm wide. The pharynx is distinct, spherical in shape measuring 0.062–0.078 (0.069 ± 0.001) mm long and 0.042–0.054 (0.049 ± 0.001) mm wide, while the esophagus is very short, looped and bifurcating into two long narrow intestinal caeca. The testes are two in number; anterior and posterior one. They are fusiform, tandem and lobed, separated from one another and are located in the middle third of the body. They are nearly equal in size and measuring 0.69–0.82 (0.76 ± 0.01) mm long and 0.19–0.29 (0.24 ± 0.01) mm wide. The cirrus pouch was filling the space between the ventral sucker and intestinal bifurcation containing tubular coiled seminal vesicle. Pars prostatica is distinct, thick-walled, and is surrounded by gland cells. The ejaculatory duct is short, narrow, and the genital atrium is indistinct. The genital pore is located anterior to the intestinal bifurcation just adjacent to the posterior margin of the oral sucker. The ovary measured 0.51–0.62 (0.59 ± 0.01) mm long and 0.13–0.19 (0.17 ± 0.01) mm wide is tri-lobed, entire and is found immediately in the pre-testicular region. Mehlis’ gland is found anterior to the ovary, and the uterine seminal receptacle occupies the posterior coils of the uterus. Laurer’s canal passes postero-ventrally and opens dorsally at the level of the anterior margin of the ovary. The uterus usually coils intercaecally between the anterior margin of the ovary and the posterior expanded portion of the seminal vesicle. It is filled with eggs and then it passes to the genital aperture with little or no coiling. Vitellaria extend from the level of the intestinal bifurcation to the posterior end of the body locating laterally and dorsoventrally to the caeca filling the post-testicular space. The excretory pore lies in the posterior extremity of the body.
Taxonomic summary
Type host: African sharptooth catfish Clarias gariepinus (Burchell 1822) (F: Clariidae)
Site of infection: The Intestine of infected fish
Host Locality: River Nile at Beni Suef Governorate, Egypt
Prevalence of infection: 20.0 % (12 out of 60 were infected)
Material deposition: Voucher specimens deposited in Zoology Department, Faculty of Science, Beni Suef University, Beni Suef, Egypt
Etymology: The specific name of the parasite (clarii) was derived from the host specific name where the parasite was discovered and described for the first time
Remarks: Previous studies recorded that few species of digenean parasites have been reported from family Lepocreadiidae (Bray and Cribb 1997). In the present study, one species of Lepocreadiidae was recovered from the intestine of the examined fish species. Comparing morphological and morphometric characteristics of the parasite under investigation, it is clear that there is a close similarity with previously species P. clarii described by Bayoumy et al. (2013) from C. gariepinus and P. pulcher previously described by Manter (1954) from Latridopsis ciliaris in having all the characteristic features of the genus Pseudoholorchis Yamaguti 1958 but with some measurement differences. The present work denoted that the present P. clarii parasite is easily distinguished from other species of the same genus by the following interspecific variations including the body shape which varied greatly from oval to sub-spherical; the oral sucker may be equal or larger than the acetabulum, the esophagus was short, and the intestinal furca have a T-shaped appearance, the ovary and testes were pre-acetabular in position, the ovary was lobed and was partly overlapping the right testis and vitellaria that extended halfway between the oral and ventral suckers.
Family Macroderoididae McMullen (1937)
Genus Glossidium Looss (1899)
Glossidium pedatum Looss (1899) (Fig. 3a-g, 4c; Table 3)
Photomicrographs of the adult digenea G. pedatum. a The adult worm with oral sucker (OS) followed by the pharynx (PH), esophagus (E), ventral sucker (VS), intestinal caeca (IC), vitelline follicles (VF), uterus (U), ovary (O), and the body ended with excretory pore (EP). b–g High magnifications of different body parts showing: b, c Anterior end with oral sucker (OS) followed by the pharynx (PH) and esophagus (E). d Ventral sucker (VS). e Eggs (EG). f Posterior part with excretory pore (EP). g Testes (TE)
Body was elongated with truncated posterior end and reaches the maximum width at the level of the ventral sucker. It measured 1.1–2.5 (1.9 ± 0.1) mm long and 0.32–0.45 (4.9 ± 0.01)mm wide. Cuticle covered with tiny spines which are dense in the anterior end of the body, and gradually diminishing posteriorly. Oral sucker was spherical in shape and measured 0.19–0.29 (0.22 ± 0.01) mm long and 0.18–0.28 (0.21 ± 0.01) mm wide. Ventral sucker was spherical in shape and measured 0.19–0.29 (0.22 ± 0.01)mm long and 0.18–0.28 (0.21 ± 0.01) mm wide. Both suckers are well-developed and equal in size. Pre-pharynx is short. Pharynx measured 0.08–0.12 (0.10 ± 0.01) mm long and it fairly large opens into the esophagus which may sometimes be indistinct. Intestinal caeca extend posteriorly, ending near posterior extremity of the body. Testes are tandem and oval in shape. Testes separated from each other by number of eggs. Anterior testis measured 0.15–0.29 (0.15 ± 0.01)mm long and 0.11–0.20 (0.17 ± 0.01) mm wide, while the posterior one measured 0.14–0.27 (0.17 ± 0.01)mm long and 0.13–0.24 (0.19 ± 0.01) mm wide. Cirrus sac was large, elongated, curves to right of the ventral sucker, contains bipartite seminal vesicle with posterior half being larger than the anterior part. Genital pore is median and immediately anterior to the ventral sucker. Ovary round to oval is situated posterior to the cirrus sac and measured 0.08–0.16 (0.12 ± 0.02) mm long and 0.13–0.23 (0.17 ± 0.01) mm wide. Uterus forming upward and downward loops reaching to the posterior end of the body, where it fills in almost whole post-testicular space. Numerous yellow to brown operculated eggs present which measured 0.03–0.06 (0.04 ± 0.001) mm long and 0.02–0.04 (0.03 ± 0.001) mm wide. Vitellaria irregularly shaped, extends laterally on either side of the body from ovarian level to the posterior margin of posterior testis. The excretory pore lies in the posterior extremity of the body.
Taxonomic summary
Type host: African sharptooth catfish Clarias gariepinus (Burchell 1822) (F: Clariidae)
Site of infection: The Intestine of infected fish
Host Locality: River Nile at Beni Suef Governorate, Egypt
Prevalence of infection: 16.66 % (10 out of 60 were infected)
Material deposition: Voucher specimens deposited in Zoology Department, Faculty of Science, Beni Suef University, Beni Suef, Egypt
Remarks: The genus Glossidium Looss 1899 comprises only two species: G. pedatum Looss 1899 and G. geminum (Mueller 1930). The latter was transferred by van Cleave and Mueller (1934) to the genus Alloglossidium Simer 1929 and was recently assigned by vande Vusse (1980) to the same genus. First described by Looss 1899 from the Nile Bagrid fish, Bagrus bayad (Forskål 1775) and Bagrus docmak (Forskål 1775) from the lower reaches of the Nile River; G. pedatum has also been reported from two other fish hosts: the catfish C. gariepinus in South Africa (Mashego 1977) and C. lazera in Egypt (Imam et al. 1991). In the present study, morphological and morphometric studies showed that the present digenean parasites recovered from the intestine has close resemblance with other G. pedatum described previously by Looss (1899), Fischthal (1973), Mashego (1977), Matla (2012), and Jansen van Rensburg et al. (2013) in having all characteristic features as it possesses a four-lobed pharynx, the ovary and testes are in the same position; the vitellaria extends from the ovarian level to the posterior margin of the posterior testis; the prepharynx has a sphincter-like structure situated just anterior to the pharynx (Table 3). On the basis of the abovementioned characteristics, specimens of the present material are thus assigned to G. pedatum.
Concluding remarks
Among the digenean parasites collected during the present investigation, only T. botswanensis, P. clarii, and G. pedatum were found infecting C. gariepinus. A certain degree of site specificity was also observed, with T. botswanensis being found only in the gall bladder of C. gariepinus, while both P. clarii and G. pedatum were found only in the intestine and thus seem to occupy a certain niche within their host. At any one time, a single C. gariepinus may host three species of digenean parasites, each occupying its own niche within the host. Not only these fish species infected with adult digenean parasites but they also host other ecto- and endoparasites, sometimes having a heavy parasite burden. C. gariepinus plays a notably important role in the life cycles of digenean parasites in the River Nile, as it acts as a definitive host for adults of the three recorded species. This study represents as the first record of T. botswanensis and G. pedatum from C. gariepinus, as well as the first report of the genera from the River Nile in Egypt. In addition, re-description of P. clarii with clarifying measurements for some body parts.
References
Abdel-Gaber R, Abdel-Ghaffar F, Morsy K, Bashtar A-R, Saleh R (2015a) New geographical record of Orientocreadium batrachoides (Digenea, Orientocreadiidae) of African sharptooth Catfish Clarias gariepinus in Egyptian water. Int J Anim Biol 1(6):286–291
Abdel-Gaber R, El Garhy M, Morsy K (2015b) Prevalence and intensity of helminth parasites of African catfish Clarias gariepinus in Lake Manzalah, Egypt. Adv Biosci Biotechnol 6:464–469
Abdel-Gaber R, Abdel-Ghaffar F, Bashtar A-R (2015c) Taxonomical and Phylogenetic characterizations of a new intestinal parasite Proenenterum myripristiae (Digenea, Lepocreadiidae) infecting pinecone soldier fish Myripristis murdjan (Beryciformes, Holocentridae) from red sea, Egypt. Int J Curr Res 7(12):23496–23503
Abdel-Gaber R, Abdel-Ghaffar F, Bashtar A-R, Morsy K, Saleh R (2016) Interaction between the intestinal parasite Polyonchobothrium clarias (Cestode: Ptychobothriidae) from the African sharptooth catfish Clarias gariepinus and heavy metal pollutants in an aquatic environment in Egypt. J Helminthol 8:1–11
Abdel-Ghaffar F, Abdel-Gaber R, Bashtar A-R, Morsy K, Mehlhorn H, Al Quraishy S, Saleh R (2015a) Hysterothylacium aduncum (Nematoda, Anisakidae) with a new host record from the common sole Solea solea (Soleidae) and its role as a biological indicator of pollution. Parasitol Res 114(2):513–22
Abdel-Ghaffar F, Abdel-Gaber R, Bashtar AR, Morsy K, Al Quraishy S, Saleh R, Mehlhorn H (2015b) Molecular characterization and new geographical record of Lecithochirium priacanthi (Digenea: Hemiuridae) infecting the moontail bullseye fish Priacanthus hamrur (Perciformes: Priacanthidae) from the Red Sea, Egypt. Parasitol Res 114:4471–4477
Abdelsalam M, Abdel-Gaber R, Mahmoud MA, Mahdy OA, Khafaga NIM, Warda M (2015) Morphological, molecular and pathological appraisal of Callitetrarhynchus gracilis plerocerci (Lacistorhynchidae) infecting Atlantic little tunny (Euthynnus alletteratus) in Southeastern Mediterranean. J Adv Res 7(2):317–326
Abo Esa JFK (2008) Study on some ectoparasitic diseases of catfish, Clarias gariepinus with their control by ginger Zingiber Officiale. Med Aqu J 1:1–9
Arafa SZ, Reda ES (2002) Cholinergic components of the nervous system of the digeneans Astiotrema reniferum, Orientocreadium batrachoides and Eumasenia aegypticus from the catfish Clarias gariepinus in Egypt. J Egypt Ger Soc Zool 38:75–91
Ayanda OI (2009) Comparison of parasitic helminthes infection between the sexes of Clarias gariepinus from Asa Dam Ilorin, North-Central Nigeria. Sci Res Essay 4:357–360
Bayoumy EM, Abd El-Monem S, Abd El-Atti MS (2013) New digenean parasite infecting the freshwater fish, Clarias gariepinus with special reference to its tegumental ultrastructure. World Appl Sci J 26(8):1046–1052
Benech V, Teugels GG, Gourene G (1923) Critere pratique pour distinguer deux poissons-chats africains, Clarias anguiIlans et C. gariepinus (Siluriformes; Clariidae). Cyb 17(1):83–5
Bhure DB, Nanware SS (2011) Studies on piscian trematode Orientocreadium batrachoides from Channa Gachua. R Res Sci Technol 3:13–4
Braun M (1901) Zur Revision der Trematoden der Voegel. Zentralblatt für Bakteriologie, 1(29):895–897.
Bray RA, Cribb TH (1997) The subfamily Aephnidiogeninae Yamaguti, 1934 (Digenea: Lepocreadiidae), its status and that of the genera Aephnidiogenes Nicoll, 1915, Holorchis Stossich, 1901, Austroholorchis n. g., Pseudaephnidiogenes Yamaguti, 1971, Pseudoholorchis Yamaguti, 1958 and Neolepocreadium Thomas, 1960. Sys Parasitol 36:47–68
Bray RA, Gibson D, Jones A. (2008) Keys to the trematoda. Vol. 3. CABI Publishing and the Natural History Museum, London, UK. ISBN 978-0-85199-588-5, XVI + 824 pp
Burchell WJ (1822) Travels in the interior of southern Africa: "Hints on emigration to the Cape of Good Hope, by the same author", v. 1., p. [1]-4. "The itinerary and register of the weather": p. [555]-573
Bush AO, Lafferty KD, Lotz J, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revised. J Parasitol 83(4):575–583
Chai JY (2007) Intestinal flukes. In: Murrell KD, Fried B (eds) Food-borne parasitic zoonoses: fish and plant-borne parasites, vol 11. Springer, New York, pp 53–115
Eissa AE, Moustafa M, El-Husseiny IN, Saeid S, Saleh O, Borhan T (2009) Identification of some skeletal deformities in some freshwater teleost raised Egyptian aquaculture. Chemosphere 77:419–425
Eschmeyer W (2014) Catalog of fishes. Electronic publication in “World Wide Web”. http://www.calacademy.org/research/ichthyology/catalog. Accessed 23 June 2014
Fischthal JH (1973) Three digenetic trematodes of Clarias mossambicus peters (Clariidae) from Ethiopia. Proc Helminthol Soc Wash 40(1):166–167
Forskål P (1775) Descriptiones animal iumavium, amphibiorum, piscium, insectorum, vermium; quæ in it inereorientali obser vavit Petrus Forskål. Post mortem auctorisedidit Carsten Niebuhr. Adjunct aestmateriamedica Kahirinaatque tabula marisrubri geographica. pp. 1-20, IXXXIV [=1-34], 1-164, 1 map. Hauniæ. (Möller).
Galli P, Crosa G, Ambrogi AO (1998) Heavy metals concentrations in acanthocephalans parasites compared to their fish host. Chemosphere 37:2983–2988
Gupta SP (1955) Trematode parasites of freshwater fishes. Indian J Helminthology 5:1–80
Imam EAE, El-Askalany MA, Rashad SM (1991) Studies on helminthes parasites of Synodentis schall and Bagrus bayad from beni-suef water resources. Assiut Vet Med J 24(48):137–152
Jansen van Rensburg C, van As JG, King PH (2013) New records of digenean parasites of Clarias gariepinus (Pisces: Clariidae) from the Okavango Delta, Botswana, with description of Thaparotrema botswanensis sp. n. (Platyhelminthes: Trematoda). Afr Invert 54(2):431–46
Looss A (1899) Weitere beiträge zur kenntnis der trematoden-fauna aegyptens, zugleich versuch einer natürlichen gliederung des genus Distomum retzius. Zool Jahrb: Abt für Systematik, Geographie und Biologie der Thiere 12:521–784
Manter HW (1954) Some digenetic trematodes from fishes of New Zealand. Tran Royal Soc New Zealand 82:475–568
Mashego SN (1977) A seasonal investigation of the ecto- and endoparasites of the Barbel, Clarias gariepinus (Burchell, 1822) in Lebowa, South Africa. M.Sc. thesis, University of the North.
Matla MM (2012) Helminth Ichthyo-parasitic fauna of a South African sub-tropical lake. PH.D. Thesis to Faculty of Science and Agriculture, University of Limpopo.
McMullen R (1937) Accessed through: The Interim Register of Marine and Nonmarine Genera at http://irmng.org/aphia.php?p=taxdetails&id=113256. Accessed 29 July 2016
Mueller JF (1930) The trematode genus Plagiorchis in fishes. Trans Am Microsc Soc 49:174–177
Mwita CJ (2014) The community of parasites infecting Clarias gariepinus in the Tanzanian waters: a case of lake Victoria. Open J Ecology 4:873–882
Odhner T (1905) Die Trematoden des arktischen Gebietes. Fauna Architects 4:289–372
Omeji S, Solomon SG, Uloko C (2013) Comparative study on the endo-parasitic infestation in clarias gariepinus collected from earthen and concrete ponds in Makurdi, Benue state, Nigeria. J Agriculture Vet Sci 2:45–49
Schmidt GD (1992) Essentials of parasitology. 5th ed. Brown, C. Publishers, USA.
Scholz T (2008) Family Opisthorchiidae Looss, 1899. In: Bray RA, Gibson DI, Jones A (eds) Keys to the Trematoda, vol 3. CABI Publishing and the Natural History Museum, Wallingford, Oxfordshire, pp 9–49
Simer PH (1929) Fish trematodes from the lower Tallahatchie river. Am Midl Nat 11:563–588
Sirikantayakul S (1985) Observations on the life cycle and egg shell of Orientocreadium batrachoides Tubangui, 1931(Trematoda: Allocreadiidae) in Clarias macrocephala Gunther 1864. Philipp J Sci 114:183–206
Tasawar Z, Umer K, Hayat CS (2007) Observations on lernaeid parasites of catlacatla from a fish hatchery in Muzaffargarh, Pakistan. The Pak Vet J 27:17–19
Tepe Y, Oğuz MC, Belk M, Őzgen R (2013) Orientocreadium batrachoides Tubangui, 1931 (Orientocreadiidae): The only trematode parasite of Clarias gariepinus Burchell, 1822 (Clariidae) from the Asi River (Southern Turkey). Tur Parazitol Derg 37:203–7
Van Cleave HJ, Mueller JF (1934) Parasites of oneida lake fishes. Part III. A biological and ecological survey of the worm parasites. Roosevelt Wildl Ann 3:161–334
vande Vusse FJ (1980) Revision of Alloglossidium Simer, 1929 (Trematoda: Macroderoididae) and description of A. microspinatum sp. n. from a leech. J Parasitol 66:667–670
Weesner FM (1965) General Zoological Techniques. The William and Wilkins Company. UnitedStates.
Yalcin Ş, Solak K, Akyurt I (2002) Growth of the catfish Clarias gariepinus (Clariidae) in the River Asi (Orontes) Turkey. Cyb 26:163–72
Yamaguti S (1958) Systema helminthum. Vol. I, The digenetic trematodes of vertebrates. Part 1. Intersci. Publ. Inc, New York, p 979
Yilmaz E, Cek Ş, Mazlum Y (2009) The effects of combined phytoestrogen administration on growth performance, sex differentiation and body composition of sharp tooth catfish Clarias gariepinus Burchell, 1822. Tur J Fish Aqua Sci 9:33–7
Yooyen T, Wongsawad C, Kumchoo K, Chaiyapo M (2006) A new record of Clinostomum philippinensis (Valasquez, 1959) in Trichogaster microlepis (Gunther, 1861) from Bung Borapet, Nakhon Sawan, Thailand. Southeast Asian J Trop Med Public Health 37:99–103
Acknowledgments
The authors extend their appreciations to members of Zoology Department, Faculty of Science, Cairo and Beni Suef Universities, Egypt for providing all facilities to complete this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals and have been approved and authorized by Institutional Animal Care and Use Committee (IACUC) at Zoology Department in Faculty of Science, Cairo University, Egypt.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abdel-Gaber, R., Sakarn, T., El-Shahawi, G. et al. Morphological re-description and new geographical records for three digenean parasites infecting African sharptooth catfish Clarias gariepinus (Pisces: Clariidae) in Egypt. Parasitol Res 115, 4251–4260 (2016). https://doi.org/10.1007/s00436-016-5203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5203-2