Abstract
Hysterothylacium aduncum (Nematoda, Anisakidae) was isolated from the intestine of the common sole Solea solea (Family, Soleidae) collected from coasts along Alexandria City at the Mediterranean Sea in Egypt, during the period from May to September 2013. Light and scanning electron microscopy revealed that this nematode parasite belongs to the family Anisakidae in the genus Hysterothylacium. The type species is named H. aduncum, based on the presence of three interlocked lips with the interlabium in between, the presence of cephalic papillae, and large numbers of caudal papillae in males. Body measurements showed that the male worms were smaller than females measuring 13.9–18 mm (16.2 ± 0.2) in length and 0.26–0.34 mm (0.30 ± 0.01) in width. Females measured 20.5–24.5 mm (22.7 ± 0.2) in length and 0.41–0.52 mm (0.45 ± 0.01) in width. The morphological characteristics of this species was confirmed by molecular analysis of 18S rDNA for these parasites followed by comparison between sequence data for them with those obtained from the Genbank showing that H. aduncum is deeply embedded in the genus Hysterothylacium with a sequence similarity between 95.5–94.3 % with close relationships to other H. aduncum specimens and Hysterothylacium sp.. Furthermore, it was shown that this parasitic nematode is able to accumulate larger concentrations of heavy metals such as Fe, Cu, Cd, and Ni within its tissues than of its host fish and thus it can be used as a useful bio-indicator of water pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among many parasites found in fish, nematodes are those of the highest importance (Dural et al. 2011; Abdel-Ghaffar et al. 2013). Ascaridoid nematodes (family Anisakidae) have been recorded worldwide naturally parasitizing approximately 200 fish species (Køie et al. 1995) and 25 cephalopod species (Hochberg 1990). Marine mammals as well as humans can also become accidental hosts by ingesting fish infected with third-stage larvae (Szostakowska et al. 2002). Anisakid nematodes which are common in bony fish are represented by the following genera: Anisakis Dujardin, 1845; Contracaecum Railliet and Henry, 1912; Hysterothylacium Ward and Magath, 1917; Paranisakiopsis Yamaguti, 1941; and Pseudoterranova Mozgovoy, 1951. The genus Hysterothylacium comprises more than 59 species and is considered as one of the most ubiquitous parasitic nematode species in fishes of the North Atlantic (Navone et al. 1998; Balbuena et al. 2000; Klimpel et al. 2006). It can be assumed that life cycles within the genus Hysterothylacium are principally similar in all species. Sexually mature adults and the fourth larval stages (L4) are mainly found in the lumen of the stomach and intestine of the fishes, which act as definitive hosts, while the third larval stages (L3) are found in the fish mesenteries. More than 100 different benthic and planktonic invertebrate species act as intermediate and/or paratenic hosts (Navone et al. 1998). In the North Atlantic, the North Sea, the Baltic Sea, the Mediterranean Sea and in adjacent temperate and cold waters, the species Hysterothylacium aduncum Rudolphi, 1802, is a very common fish parasite, but its taxonomic status is highly ambiguous (Margolis and Arthur 1979; Palm et al. 1999; Klimpel et al. 2006). Several authors assumed that H. aduncum is a single species parasitizing opportunistically in various marine fish species (Szostakowska et al. 2002; Morsy et al. 2013). In contrast, Hartwich (1975) recognized three distinctive species: H. aduncum mainly found in clupeid hosts like sprat Sprattus sprattus and herring Clupea harengus, Hysterothylacium gadi from gadoid fish, and Hysterothylacium auctum, which was frequently identified in the eelpout Zoarces viviparous. Petter and Cabaret (1995) reported a great variability between parasitic specimens collected from different fish species in North Atlantic waters, but they proposed only two subspecies on the basis of biometrical data. Thus, it would not be surprising if future molecular studies revealed that H. aduncum represents a complex of an unknown number of sibling species (Balbuena et al. 2000). Recently, various studies have demonstrated that the internal transcribed spacers (ITS) of nuclear ribosomal DNA (rDNA) and polymerase chain reaction-based results provide genetic markers of the appropriate DNA target sequence for the exact identification and delineation of parasitic nematodes of the family Anisakidae and Ascarididae (Mašová et al. 2010; Testini et al. 2011).
A variety of organisms have been investigated to evaluate their potential as biological indicators for different types of pollution in the aquatic environment. The relationship between pollution and parasitism in aquatic organisms and the potential role of parasites as water quality indicators have received increasing attention during the past two decades (Dural et al. 2011). However, until now, little is known on the accumulation of toxins within parasites. A variety of wild freshwater and marine fish are subjected to infection by different species of parasites. Particularly, intestinal helminths can accumulate heavy metals at concentrations that are magnitudes higher than those in the host tissues or the environment (Schludermann et al. 2003; Morsy et al. 2012). This aspect suggests that parasites may serve as useful indicators for biologically available metals (Galli et al. 2001).
In the present study, morphological and molecular analyses of H. aduncum parasitizing the common sole S. solea captured at the coasts of Alexandria City in the Mediterranean Sea, Egypt were investigated. Also, the bioaccumulation effect of these parasites for some heavy metals was studied to determine whether this anisakid species is a useful bio-indicator species for water pollution or not.
Materials and methods
In the period between May to September 2013, anisakid worms of the genus Hysterothylacium were collected from 80 specimens of the common sole Solea solea (Soleidae) captured at the coasts of Alexandria City along the Mediterranean Sea, Egypt. The fishes were transported alive with good aeration and cooling to the Parasitology Laboratory of the Zoology Department, Faculty of Science, Cairo University. The fishes were identified according to Desoutter (1992) and examined externally for external lesions or parasitic cysts. They were dissected carefully and examined thoroughly for endoparasitic infections. The contents of the digestive tract were poured into physiological saline solution and examined by dissecting microscopy. The nematode larvae were collected, fixed, and stored in 70 % ethanol. For light microscopy, worms were cleared in lactophenol and photographed by help of a Zeiss microscope supplied by a Canon digital camera. The taxonomic identification followed the guidelines of Petter and Maillard (1988), Incorvaia and Díaz de Astarloa (1998), Timi et al. (2001), Bicudo et al. (2005), and Felizardo et al. (2009). For scanning electron microscopy, samples were fixed in 2.5 % glutaraldehyde solution buffered in 0.1 M sodium cacodylate. Samples were dehydrated in an ethanol series, CO2 critical-point dried, coated by gold, and finally examined and photographed using an Etec Autoscan Jeol SEM under an accelerating voltage of 20 Kv. Measurement ranges were taken in millimeters—mean values were given in parentheses unless otherwise indicated. For molecular analysis, worms were washed with phosphate buffer saline and homogenized in liquid nitrogen. DNA was extracted using a QIAamp® DNA Mini Kit (Quiagen, GmbH, Germany) following the manufacturer’s protocol. Polymerase chain reaction (PCR) was carried out to amplify the target 18S rDNA using the primers NC5 (forward: 5’-TAGGTGAACCTGCG GAAGGATCATT-3’) and NC2 (reverse: 5’-TTAGTTTCTTTTCCTCC GCT-3’). The PCR product were directly sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) with 310 Automated DNA Sequencer (Applied Biosystems, USA) using the same primers for annealing. The net sequence data was aligned using CLUSTAL-X multiple sequence alignment (Thompson et al. 1997) and compared with some of the previously recorded sequence data for species of the same genus obtained from the Genebank to analyze intra-specific differences. The dendrogram was built up using the multiple-alignment algorithm in Megalign (DNASTAR, Window version 3.12e). Phylogenetic relationships were inferred using maximum-likelihood (ML) (Felsenstein 1981). The obtained sequences have been deposited in the Genebank under accession number KC004227. Heavy metal analyses in different organs (liver, gills, kidney, and muscles) of infected and non-infected fish and also in nematode tissues were carried out according to the procedure described by UNEP/FAO/IOC/IAEA (1984). Tissues were digested with specific volumes of concentrated nitric acid and per-chloric acid (2:1 v/v) at 60 °C for 3 days. All samples were diluted with bi-distilled water and assayed using inductively coupled plasma-atomic emission spectrometry (Varian model-Liberty Series II). The metals concentrations of Pb, Zn, Fe, Cd, Cu, and Ni were recorded as microgram metal per gram wet weight and carried out at band width of 0.2 nm and wavelength range of 190–900 nm with 250 nm Elbert mount diffraction grating monochromator.
Results
Thirty five out of eighteen specimens of the examined fish were found to be naturally infected by nematode worms with a percentage of 43.75 %. The infection was recorded in the intestine of infected fish and increased during winter to be 47.5 % (19 out of 40) and fall to 40.0 % (16 out of 40) during summer.
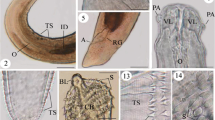
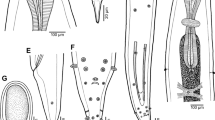
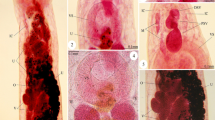
Morphological examination of the recovered worms revealed that the adult worms are characterized by an elongated cylindrical body with its anterior end characterized by the presence of a mouth surrounded by three lips, one dorsal and two sub-ventral ones, which can be interlocked leaving interlabia in between (Figs. 1, 2, 3, 9, 10, 11). Lips (approximately equal in size) were provided with papillae-like structures (Figs. 1, 2, 3, 10). The worm possess a long anterior muscular esophagus, which measured 1.5–3.1 mm (2.5 ± 0.02) in length, was longer in females than in males, and was followed by the intestinal caecum. An excretory pore occurred slightly posterior to the level of the nerve ring. The worm body was covered by a cuticle which was transversely striated (Fig. 4). The tail of the worms was tipped by a single minute thorn called mucron. The body of the male worm measured 13.9–18 mm (16.2 ± 0.2) in length and 0.26–0.34 mm (0.30 ± 0.01) in width at the level of the esophagus. The posterior end was provided with two unequal spicules (Figs. 5, 12, 13). The left one measured 0.69–0.85 mm (0.76 ± 0.2) in length while the right one reached 0.73–0.88 mm (0.81 ± 0.2) in length and possessed caudal papillae (Figs. 6, 12, 14). The body of the female worm was 20.5–24.5 mm (22.7 ± 0.2) long and 0.41–0.52 mm (0.45 ± 0.01) wide and terminated in a conical tail bearing the anus and a short mucron (Figs. 7, 15). The larvae ended in a long cactus-like tail (Fig. 8).
Photomicrographs of Hysterothylacium aduncum showing high magnifications of 1–3 the anterior end being equipped by three interlocked lips (L) with interlabium (IL) in between and provided with papillae (P). Note the presence of a surrounding cuticle (C), esophagus (OE). 4 Mid-part of the body showing transverse striations (TS) of cuticle. 5–8 Posterior parts of 5 male with two spicules (SP), short mucron (M), and caudal papillae (P). 6 Caudal papillae (P). 7 Female terminated with short mucron (M) with rectum (R) and anal region (A). 8 Larva with a cactus tail or mucron (M)
Scanning electron micrographs of H. aduncum at high magnifications. 9–11 The anterior end with the three interlocked lips (L), interlabium (IL), and papillae (P). 10–15 Posterior parts of 12–14 male with long spicules (SP), caudal papillae (P), and mucron (M). 15 Female with short mucron (M) and anus (A) region
Taxonomic summary
H. aduncum Rudolphi, 1802.
Family: Anisakidae Railliet and Henry, 1912.
Host: Common sole S. solea fish Linnaeus, 1758 (Family: Soleidae).
Locality: Coasts of Alexandria City, Mediterranean Sea, Egypt.
Site of infection: The adult worms as well as their larvae were found in the intestine of the infected fish.
Prevalence rate of infection: 35 (47.5 %) out of 80 specimens of the examined fish were infected.
Molecular analyses of the recovered worms demonstrated that the 18S rDNA sequences (771–789 bp) showed 95.5 % similarity to HQ270430, JX413597, and HM437225 for different deposited sequences of previously described H. aduncum, 95.0 % similarity to HQ270433 of Hysterothylacium sp., 94.3 % similarity to AF115571 of H. auctum, and 92.3 % similarity to AB277824 of Pseudoterranova decipiens. These nematodes revealed low genetic divergent values. The highest BLAST scores were aligned and compared with our present sample shown in Fig. 16. Sequence alignment resulted in the fact that the major clade of the constructed dendrogram clustered all Anisakidae species with sequence similarities between 95.5–94.3 %. This showed that H. aduncum is deeply embedded in the genus Hysterothylacium with close relationships to other H. aduncum worms and to Hysterothylacium sp. Thus, they are sister taxons with high bootstrap values (Fig. 17). The minor clade contains 18S rDNA sequences of Porrocaecum depressum and Ascaris suum as outgroups and in addition other taxons with high divergence values.
Heavy metal analyses of the infected fish organs (liver, gills, kidney, and muscles) showed that toxic metals like Pb, Zn, Fe, Cd, Cu, and Ni were detected in all of the tested samples. The concentration rates (mg/g) of these metals in the organs of the examined fish tissues and its parasitizing nematodes were given in Table 1. It was shown that trace metal accumulations in fish tissues occurred in the following descending order: Fe > Cu > Cd > Zn > Ni > Pb. The concentrations of Zn, Fe, Cd, and Cu in fish tissues were found in the following grading order: liver > gills > muscle > kidney. Gills can accumulate large amounts of both Pb and Ni when compared to other fish tissue. The concentrations of Fe, Cu, Cd, and Ni were higher in the nematode parasites than in the tissues of infected fish and were found to be exceeding the US Environmental Protective Agency (0.1 μg−1 (epa)).
Discussion
The common sole S. solea Linnaeus (1758) is a representative of flatfishes which are normally distributed in the continental shelf seas of the Eastern Atlantic Ocean (Froese and Pauly 2007). In the present study, it was found that S. solea, which inhabits also the coasts of the Mediterranean Sea, was infected by H. aduncum which is a common nematode parasite that is not very host-specific neither as adult nor as larval stages (Klimpel et al. 2006). The fish host can heavily accumulate different stages of H. aduncum by ingesting crustaceans, chaetognaths, and small fish that are infested and serve as carriers (Rossin et al. 2011). The percentage of infection with this anisakid parasite was found here to reach 47.5 % being similar to the results of Genc et al. (2005), who stated that Hysterothylacium sp. infected different species of sparid and elasmobranch fishes with percentage of infection ranging between 7.69–78.57 %. Vidal-Martínez et al. (1994) reported that morphological differentiation of nematode parasites belonging to the genus Hysterothylacium is extremely difficult, since all species show the same characteristics. The present parasite is similar to H. aduncum Rudolphi, 1802; Hysterothylacium seriolae Yamaguti, 1941; Hysterothylacium fortalezae Klein, 1973; Hysterothylacium arnoglossi Petter and Maillard, 1987; Hysterothylacium scomberoidei Bruce and Cannon, 1989; Hysterothylacium physiculi Moravec and Nagasawa, 2000; and Hysterothylacium winteri Torres and Soto, 2004, since it shows also three cephalic lips as long as wide, an identical position and anatomy of the foregut, an excretory pore just behind the nerve ring, a very short intestinal caecum (slightly longer than the ventriculus), a very long ventricular appendix, distinct lateral alae, relatively short spicules (not over 1.0 mm), and tail tips of both sexes being covered with numbers of nodular protuberances. Furthermore, this worm is morphometrically very similar to other comparable species of H. aduncum reported by Rudolphi (1802) and Morsy et al. (2012) and is located in the same range of measurements for different body parts.
Molecular approaches such as PCR and direct sequencing of ITS for rDNA have proven to be particularly useful for the accurate and effective identification of all life stages of ascaridoid nematodes (Mašová et al. 2010; Testini et al. 2011; Knoff et al. 2012). In the present study, the lengths of the PCR products of H. aduncum were found to be 771–789 bp being identical to that reported previously in other studies by Martin-Sánchez et al. (2005) and Setyobudi et al. (2011). Valentini et al. (2006) showed that nucleotide differences in the ITS sequences among anisakid species were significantly greater than that within a species. Interspecific differences in 5.8S rDNA sequence were considerably lower than in the ITS, which is not unexpected because the rDNA gene is relatively conserved in nematodes. Similarly, the present study demonstrated that nucleotide differences were also highly conserved. Interspecific differences were 1.4–14.9 % with minor differences between H. aduncum and other Hysterothylacium species. The phylogenetic tree topologies observed within two clades of Hysterothylacium species grouped specimens of H. aduncum, H. auctum, Pseudoterranova decipiens, and Raphidascaris trichiuri in the same group. This is similar to the 18S rDNA MP tree topology obtained by Smythe et al. (2006). This demonstrates that H. aduncum represents a taxonomic unit genetically distant from other anisakids, even from other Hysterothylacium species. In agreement with the observation of Nadler and Hudspeth (2000), Hysterothylacium does not represent a monophyletic group, since the presence of P. decipiens and R. trichiuri suggest the existence of a polyphyletic group for H. aduncum on the 18S rDNA tree.
Some organisms are able to provide information on the chemical state of their environment through their presence or absence (Florence et al. 1992; Muir et al. 1992). Recent studies have been focused on the use of parasites as biological indicators for heavy metal accumulation (Trucekova et al. 2002). In the present study, the heavy metal analysis demonstrated that distribution of Zn, Fe, Cd, and Cu in different organs of infected fishes followed the order: liver > gills > muscles>. These results are in agreement with those obtained by Taweel et al. (2011) and Morsy et al. (2012) who studied the accumulation of different heavy metal in Tilapia and European seabass fish. The investigation shows that the liver and gills are the most important organs for assessing metal accumulation, since the levels of heavy metals in the gills reflect the concentrations of metals in the surrounding water, where the fish live, while the concentrations of metals in the liver represent the storage of metals in the fish body. These obtained results are consistent with those reported by other authors (Florence et al. 1992; Muir et al. 1992; Roméo et al. 1999; Yilmaz et al. 2007). In the present study, comparisons were done between the metal concentrations in the recovered nematode parasites and those in the different organs of the infected fish. These results showed that these nematodes accumulate more Fe, Cu, Ni, and Cd than the tissues of the infected fish. These results agreed with Azmat et al. (2008), who reported that the high level of toxic metals in Echinocephalus sp. and Ascaris sp. within its host suggests that these nematode parasites may be used as sensitive indicators for heavy metals in the aquatic ecosystem.
Recent field study have demonstrated that the 18S rDNA gene of H. aduncum yielded a unique sequence that confirms the taxonomic position in Raphidascarididae with new host and locality record in Egypt. In addition, particular fish nematode parasites can accumulate toxic metals from the aquatic environment. Thus, the application of certain parasites as sentinel organisms could provide a promising new domain for future research in environmental research.
References
Abdel-Ghaffar F, Bashtar AR, Mehlhorn H, Abdel-Gaber R, Al Quraishy S, Saleh R (2013) Morphological and phylogenetic analysis of Echinocephalus carpiae n. sp. (Nematoda: Gnathostomatidae) infecting the common carp Cyprinus carpio inhabiting Burullus Lake as a new host record in Egypt. Parasitol Res 112:4021–4028
Azmat R, Fayyaz S, Kazi N, Mahmood SJ, Uddin F (2008) Natural bio-remediation of heavy metals through nematode parasite of fish. Biochemistry 7:139–143
Balbuena JA, Karlsbakk E, Kvenseth AM, Saksvik M, Nylund A (2000) Growth and migration of third-stage larvae of Hysterothylacium aduncum (Nematoda: Anisakidae) in larval herring Clupea harengus. J Parasitol 86:1271–1275
Bicudo AJA, Tavares LER, Luque JL (2005) Metazoários parasitos da cabrinha Prionotus punctatus (Bloch 1793) (Osteichthyes: Triglidae) do litoral do Estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 14:27–33
Bruce NL, Cannon LRG (1989) Hysterothylacium, Iheringascaris and Maricostula new genus, nematodes (Ascaridoidea) from Australian pelagic marine fish. J Nat Hist 23:1397–1441
Desoutter M (1992) Soleidae. p. 860-865. In: Levêque C, Paugy D, Teugels GG (eds) Faune des poissons d’eaux douces et saumâtres d’Afrique de l’Ouest Tome 2. Faune Tropicale n° 28. Musée Royalle de l’Afrique Centrale, Tervuren, Belgique and O.R.S.T.O.M, Paris, p 902
Dujardin F (1845) Histoire naturelle des helminthes ou vers intestinaux. Libre Encycopedidique de Roret (Suites a Buffon), Paris, p 652
Dural M, Genc E, Sangun MK, Güner Ö (2011) Accumulation of some heavy metals in Hysterothylacium aduncum (Nematoda) and its host sea bream, Sparus aurata (Sparidae) from North-Eastern Mediterranean Sea (Iskenderun Bay). Environ Monit Assess 174:147–155
Felizardo NN, Knoff M, Pinto RM, Gomes DC (2009) Larval anisakid nematodes of the flounder, Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) from Brazil. Neotropical Helminthol 3:57–64
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Florence T, Morrison GM, Stauber JL (1992) Determination of trace element speciation and the role of speciation in aquatic toxicity. Sci Total Environ 125:1–13
Froese R, Pauly D (2007) Fishbase, 2007/10. World Wide Web electronic publication, www.fishbase.org, version (10/2007)
Galli P, Crosa G, Mariniello L, Ortis M, D’Amelio S (2001) Water quality as a determinant of the composition of fish parasite communities. Hydrobiology 452:173–179
Genc ER, Genc AM, Genc EV, Cengizler I, Can FM (2005) Seasonal variation and pathology associated with helminthes infecting two serranids (Teleostei) of Iskenderun Bay (Northeast Mediterranean Sea), Turkey. Turk J Fish Aquat Sci 5:29–33
Hartwich G (1975) Schlauchwürmer, Nemathelminthes, Rund oder Fadenwürmer, Nematoda parasitische Rundwürmer vonWirbeltieren. I. Rhabditida und Ascaridida. Die Tierwelt Deutschlands, Fischer, Jena
Hochberg FG (1990) Diseases of Mollusca: Cephalopoda. In: Kinne O (ed) Diseases of marine animals, Vol. III: introduction, Cephalopoda, Annelida, Crustacea, Chaetognatha, Echinodermata and Urochordata. Biologische Anstalt Helgoland, Hamburg, pp 47–227
Incorvaia IS, Díaz de Astarloa JM (1998) Estudio preliminar de las larvas (Nematoda: Ascaridida) parásito de Paralichthys orbignyanus (Valenciennes, 1839) y Paralichthys patagonicus (Pisces: Pleuronectiformes). Bol Chil Parasitol 53:38–42
Klein VLM (1973) Helmintos parasitos das espécies Scomberomorus cavalla Cuvier) Scomberomorus maculatus (Mitchill) do litoral cearense, Contracaecum fortalezae sp. n. (Nematoda, Ascaridoidea). Mem Inst Oswaldo Cruz 71(1/2):199–202
Klimpel S, Palm HW, Busch MW, Kellermanns E, Ruckert S (2006) Fish parasites in the Arctic deep-sea: poor diversity in pelagial fish species vs. heavy parasite load in a demersal fish. Deep Sea Res I 53:1167–1181
Knoff M, Felizardo NN, Iniguez AM, Maldonado A, Torres EJL, Pinto RM, Gomes DC, Pinto RM (2012) Genetic and morphological characterisation of a new species of the genus Hysterothylacium (Nematoda) from Paralichthys isosceles Jordan, 1890 (Pisces: Teleostei) of the Neotropical region, state of Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 107:186–193
Køie M, Berland B, Burt MDB (1995) Development to third stage larvae occurs in the eggs of Anisakis simplex and Pseudoterranova decipiens (Nematoda, Ascaridoidea, Anisakidae). Can J Fish Aquat Sci 52(Suppl 1):134–139
Linnaeus C (1758) Caroli Linnaei “Systema Naturae”. Ed. Decima, Reformata Holmiae 1758, pp 244–245
Margolis L, Arthur JR (1979) Synopsis of the parasites of fish of Canada. Bull Fish Res Board Can 199:1–269
Martin-Sánchez J, Artacho-Reinoso ME, Díaz-Gavilán M, Valero-López A (2005) Structure of Anisakis simplex s.l. population in a region sympatric for A. pegreffii and A. simplex s.s. Absence of reproductive isolation between both species. Mol Biochem Parasitol 141:155–162
Mašová Š, Moravec F, Baruš V, Seifertová M (2010) Redescription, systematic status and molecular characterization of Multicaecum heterotis Petter, Vassiliadès et Marchand, 1979 (Nematoda: Heterocheilidae), an intestinal parasite of Heterotis niloticus (Osteichthyes: Arapaimidae) in Africa. Folia Parasitol 57:280–288
Moravec F, Nagasawa K (2000) Two remarkable nematodes from sharks in Japan. J Hist 34:1–13
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mehlhorn H, Al Quraishy S, El-Mahdi M, Al-Ghamdi A, Mostafa N (2012) First record of anisakid juveniles (Nematoda) in the European seabass Dicentrarchus labrax (family: Moronidae), and their role as bio-indicators of heavy metal pollution. Parasitol Res 110:1131–1138
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mostafa N (2013) New host and locality records of two nematode parasites Dujardinnascaris mujibii (Heterocheilidae) and Hysterothylacium aduncum (Anisakidae) from the common seabream Pagrus pagrus: a light and scanning electron microscopic study. Parasitol Res 112: 807–815
Mozgovoy AA (1951) Ascaridata of animals and man, and the diseases caused by them. Osnovy nematodologii. II. Izdatielstwo AN SSSR, Moskva, p 616
Muir DCG, Wagemann R, Hargrave BT, Thomas DJ, Peakall DB, Norstrom RJ (1992) Arctic marine ecosystem contamination. Sci Total Environ 122:75–134
Nadler SA, Hudspeth DSS (2000) Phylogeny of the Ascaridoidea (Nematoda: Ascaridida) based on three genes and morphology: hypotheses of structural and sequence evolution. J Parasitol 86:380–393
Navone GT, Sardella NH, Timi JT (1998) Larvae and adults of H. aduncum (Rudolphi 1802) (Nematoda: Anisakidae) in fish and crustaceans in the South West Atlantic. Parasite 5:127–136
Palm HW, Klimpel S, Bucher C (1999) Checklist of metazoan fish parasites of German coastal waters. Ber Inst Meereskunde Christ-Albrechts-Univ Kiel 307:1–148
Petter AJ, Cabaret J (1995) Ascaridoid nematodes of teleostean fish from the eastern North Atlantic and seas of the North of Europe. Parasite 2:217–230
Petter AJ, Maillard C (1987) Larves d’ascarides parasites des poisons en Méditerranée occidentale. Bull Mus Natl Hist Nat Paris Ser 10A:347–369
Petter AJ, Maillard C (1988) Larves d’ascarides parasites de poisons en Méditerranée occidentale. Bull Mus Hist Nat 10:347–369
Railliet A, Henry A (1912) Parasitic nematodes du genera Camallanus. Buffalo Soc Pathol 8:270
Roméo M, Siau Y, Sidoumou Z, Gnassia-Barelli M (1999) Heavy metal distribution in different fish species from the Mauritania Coast. Sci Total Environ 232(3):169–175
Rossin MA, Datri LL, Incorvaia IS, Timi JT (2011) A new species of Hysterothylacium (Ascaridoidea, Anisakidae) parasitic in Zenopsis conchifer (Zeiformes, Zeidae) from Argentinean waters. Acta Parasitol 56:310–314
Rudolphi CA (1802) Fortsetzung der Beobachtungen ueber die Eingeweidewuermer. Arch Zool U Zool 2:65
Schludermann C, Koneony R, Laimgruber S, Auteurs JW (2003) Fish macroscopic parasites as indicator of heavy metal pollution in river sites in Austria. Parasitology 30:201–238
Setyobudi E, Jeon CH, Lee CH, Seong KB, Kim JH (2011) Occurrence and identification of Anisakis spp. (Nematoda: Anisakidae) isolated from chum salmon (Oncorhynchus keta) in Korea. Parasitol Res 108:585–592
Smythe AB, Sanderson MJ, Nadler SA (2006) Nematode small subunit phylogeny correlates with alignment parameters. Syst Biol 55(6):972–992
Szostakowska B, Myjak P, Kur J (2002) Identification of anisakid nematodes from the Southern Baltic Sea using PCR-based methods. Mol Cell Probes 16:111–118
Taweel AA, Shuhaimi-Othman M, Ahmad AM (2011) Heavy metals concentration in different organs of Tilapia fish (Oreochromis niloticus) from selected areas of Bangi, Selangor, Malaysia. Afr J Biotechnol 10(55):11562–11566
Testini G, Papini R, Lia RP, Parisi A, Dantas-Torres F, Traversa D, Otranto D (2011) New insights into the morphology, molecular characterization and identification of Baylisascaris transfuga (Ascaridida, Ascarididae). Vet Parasitol 175:97–102
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Timi JT, Sardella NH, Navone GT (2001) Parasitic nematodes of Engraulis anchoita Hubbs et Marini, 1935 (Pisces, Engraulidae) of the Argentine and Uruguayan coasts, South West Atlantic. Acta Parasitol 46:186–193
Torres P, Soto MS (2004) Hysterothylacium winteri sp. n. (Nematoda: Anisakidae), a parasite of Chilean rock cod, Eleginops maclovinus (Perciformes: Eleginopidae), from South Chile. Folia Parasitol 51:55–60
Trucekova L, Hanzelova M, Spakulova M (2002) Concentration of heavy metals and its endoparasites in the polluted water reservoir in eastern Slovakia. Helminthology 39:1–23
UNEP/FAO/IAEA/IOC (1984) Sampling of selected marine organisms and sample preparation for trace metal analysis: Reference methods for marine pollution studies. Rev. 2:19
Valentini A, Mattiucci S, Bondanelli P, Webb SC, Mignucci-Giannone AA, Colom-Llavina MM, Nascetti G (2006) Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences and comparison with allozyme data. J Parasitol 92:156–166
Vidal-Martínez VM, Osorio-Sarabia D, Overstreet RM (1994) Experimental infection of C. multipapillatum (Nematoda: Anisakinae) from Mexico in the domestic cat. J Parasitol 80:576–579
Ward HB, Magath TB (1917) Notes on some nematodes from fresh water fish. J Parasitol 3:57–65
Yamaguti S (1941) Studies on the helminth fauna of Japan. Part II. Nematodes of fish. Jpn J Zool 9:343–396
Yilmaz F, Ozodemir N, Demirak A, Tuna AL (2007) Heavy metal level in two fish species Leuscius cephalus and Lepomis gibbosus. Food Chem 100:830–835
Acknowledgments
This work was supported by the Faculty of Science, Cairo University, Cairo, Egypt. Also, the authors extend appreciations to the Deanship of Scientific Research at King Saud University for helping in the work through the international research group project IRG14-23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Ghaffar, F., Abdel-Gaber, R., Bashtar, AR. et al. Hysterothylacium aduncum (Nematoda, Anisakidae) with a new host record from the common sole Solea solea (Soleidae) and its role as a biological indicator of pollution. Parasitol Res 114, 513–522 (2015). https://doi.org/10.1007/s00436-014-4213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4213-1