Abstract
The present study attempts to establish the sperm ultrastructure baseline for Taenia hydatigena, which is essential for the future research on the location of specific proteins involved in spermatogenesis in this species. Thus, the ultrastructural organisation of the mature spermatozoon is described by means of transmission electron microscopy. Live tapeworms were obtained from an experimentally infected dog in the Department of Pathology and Public Health of the Agronomic and Veterinary Institute Hassan II of Rabat (Morocco). The spermatozoon of T. hydatigena is a filiform cell, which is tapered at both extremities and lacks mitochondria. It exhibits all the characteristics of type VII spermatozoon of tapeworms, namely a single axoneme, a crested body, spiralled cortical microtubules and nucleus, a periaxonemal sheath and intracytoplasmic walls. Other interesting characteristics are the presence of a 2000 nm long apical cone in its anterior extremity and only the axoneme in its posterior extremity. The ultrastructural characters of the spermatozoon of T. hydatigena are compared with those of other cestodes studied to date, with particular emphasis on representatives of the genus Taenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among cestodes, the genus Taenia includes numerous species with a great medical or veterinary importance. Taenia hydatigena is a cosmopolitan tapeworm that presents an indirect life cycle including a definitive host, which is parasitized by the adult worm and an intermediate host that is infected by the larval stage of the cysticercus type. Thus, the adult stage of T. hydatigena mainly infects the intestine of dogs and other canids such as wolves or foxes, but it has also been reported in felids and the brown bear. Its larval stage, namely Cysticercus tenuicollis, is common and widespread in domesticated ruminants and causes cysticercosis of the liver and serous membranes in cattle and swines (Verster 1969; Urquhart et al. 1987; Loos-Frank 2000).
The usefulness of sperm ultrastructure as an important source of characters for phylogenetic inference has been clearly demonstrated within Platyhelminthes in general and but particularly in cestodes (Justine 1991a, b, 1998, 2001; Bâ and Marchand 1995; Miquel et al. 1999, 2007; Levron et al. 2010). Presently, it is generally accepted that the integration of data obtained in both morphology and molecular biology studies is essential to provide a valid basis for a better knowledge of systematics and evolutionary relationships of Platyhelminthes (Hoberg et al. 1997; Littlewood et al. 1998; Olson et al. 2001; Waeschenbach et al. 2007, 2012). Concerning tapeworms, most works refer to the representatives of the order Cyclophyllidea with more than 50 studied species (for a review, see Marigo 2011). Concerning the family Taeniidae, there are ultrastructural and spermatological studies for two species of the genus Echinococcus (Morseth 1969; Barrett and Smyth 1983; Shi et al. 1994) and eight of the genus Taenia, namely T. crassiceps (Willms et al. 2004; Willms and Robert 2007), T. hydatigena (Featherston 1971), T. mustelae (Miquel et al. 2000), T. parva (Ndiaye et al. 2003), T. pisiformis (Tian et al. 1998a, b), T. saginata (Tian et al. 1998a, b; Bâ et al. 2011), T. solium (Tian et al. 1998a, b; Willms et al. 2003) and T. taeniaeformis (Miquel et al. 2009a, b). However, for some of these taeniids, the available studies are briefly illustrated with TEM micrographs and the authors do not explain the complete ultrastructural organisation of their sperm cells. This is the case of T. crassiceps, T. hydatigena, T. pisiformis and T. solium and also the species of the genus Echinococcus. In this sense, a comprehensive analysis of such ultrastructural data is essential for a comparison of different species and to extract useful conclusions.
The present study, in the framework of the PARAVAC project, constitutes a preliminary approach for a future immunohistochemical analysis for the location of some antigenic proteins during sperm development. In this sense, the major objective of the present work concerns the study of the ultrastructure of the mature spermatozoon of T. hydatigena in order to draw a complete description of the male gamete for comparison with other cyclophyllideans, in particular with taeniids. Moreover, establishing the baseline of T. hydatigena sperm ultrastructure is essential for the posterior investigation of the expression sites of these proteins during spermatogenesis.
Materials and methods
Live specimens of T. hydatigena were isolated from the intestine of an experimentally infected dog in the Department of Pathology and Public Health of the Agronomic and Veterinary Institute Hassan II of Rabat (Morocco) after oral administration of arecoline bromhydrate.
Adult tapeworms recovered from faeces were immediately rinsed with a 0.9 % NaCl solution. Later, they were fixed in cold (4 °C) 2.5 % glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.4 for a minimum of 2 h, rinsed in 0.1 M sodium cacodylate buffer at pH 7.4, post-fixed in cold (4 °C) 1 % osmium tetroxide with 0.9 % potassium ferricyanide [K3Fe (CN) 6] in the same buffer for 1 h, rinsed in milliQ water, dehydrated in an ethanol series and propylene oxide, embedded in Spurr’s resin and polymerised at 60 °C for 72 h.
Ultrathin sections (60–90 nm thick) of mature segments at the level of the vas efferens were obtained in a Reichert-Jung Ultracut E ultramicrotome. Sections were placed on 200-μm mesh copper grids and double-stained with uranyl acetate and lead citrate according to the Reynolds (1963) methodology.
The grids were examined in a JEOL 1010 transmission electron microscope operated at 80 kV, in the “Centres Científics i Tecnològics” of the University of Barcelona (CCiTUB).
Results
The observation of numerous ultrathin sections from areas of mature proglottids containing seminal ducts by means of TEM has enabled us to establish the main ultrastructural characteristics and the pattern of ultrastructural organisation of the mature spermatozoon of T. hydatigena (Figs. 1, 2 and 3). It is a filiform cell, tapered at both ends and lacking mitochondria. Four consecutive regions (I–IV) with differential ultrastructural features can be distinguished.
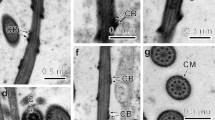
Anterior regions (I and II) of the spermatozoon of Taenia hydatigena. a Longitudinal section of region I. b Complete longitudinal section of the apical cone showing the anterior spermatozoon extremity. c, d Cross-sections of the apical cone at two different levels. e Cross-section at the level of the centriole. f Cross-section of region I showing the axoneme. g Transitional cross-section between regions I and II. Arrowhead indicates the extreme reduction of the crested body. h Cross-section at the level of the anterior area of region II. Note the appearance of the periaxonemal sheath. i, j Longitudinal sections of anterior and posterior areas of region II. k Cross-section at the level of the posterior area of region II. AC apical cone, ASE anterior spermatozoon extremity, Ax axoneme, C centriole, CB crested body, CM cortical microtubules, IW intracytoplasmic walls, PS periaxonemal sheath. Bars (a, i, j) 500 nm and (b–h, k) 300 nm
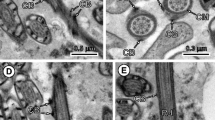
Posterior regions (III and IV) of the spermatozoon of Taenia hydatigena. a Longitudinal section showing the transition between regions II and III. b Detail of this transitional area showing the appearance of the nucleus between the periaxonemal sheath and the axoneme (arrowheads). c Longitudinal section showing the transition between regions III and IV. d–f Cross-sections at the level of anterior, middle and posterior areas of region III. g Cross-section of region IV showing the axoneme surrounded by the plasma membrane. h Cross-section at the level of the disorganisation of the axoneme. i Detail of the posterior spermatozoon extremity. Ax axoneme, CC central core, CM cortical microtubules, D doublets, DM electron-dense material, IW intracytoplasmic walls, N nucleus, PS periaxonemal sheath, PSE posterior spermatozoon extremity. Bars (a, c) 500 nm, (d–i) 300 nm and (b) 200 nm
Schematic reconstruction of the spermatozoon of Taenia hydatigena. AC apical cone, ASE anterior spermatozoon extremity, Ax axoneme, C centriole, CB crested body, CM cortical microtubules, IW intracytoplasmic walls, N nucleus, PM plasma membrane, PS periaxonemal sheath, PSE posterior spermatozoon extremity
Region I (Figs. 1a–g and 3(I)) constitutes the anterior extremity of the mature spermatozoon. Its maximal width is about 310 nm at the level of the anterior portion of this region where the axoneme begins. The two main characteristics of region I are the apical cone and the crested body. From the anterior tip of the spermatozoon to the centriole, there is an apical cone (Fig. 1a–c) over 2000 nm long and 225 nm wide, constituted by electron-dense material. The crested body is a single helical cord that externally surrounds the male gamete throughout region I. The crested body has a maximum thickness of about 80 nm at the level of the apical cone and the centriole (Fig. 1c–e). Later, when the axoneme is already formed, the thickness of the crested body decreases progressively toward the end of region I (Fig. 1f, g). The axoneme, centrally located, is characterised by its 9 + ‘1’ Ehlers’ trepaxonematan pattern. Thus, it presents nine peripheral doublets with their arms and a central element. This central structure is not a microtubule and constitutes the principal characteristic of the trepaxonematan pattern axonemes. The axoneme is surrounded by a thin layer of electron lucent cytoplasm and by an electron-dense submembranous layer of cortical microtubules spirally arranged at an angle of about 50° in relation to the hypothetical longitudinal axis of the sperm cell.
Region II (Figs. 1h–k, 2a and 3 (II)) is devoid of crested body being characterised by the appearance of both periaxonemal sheath (Figs. 1h–k, 2a) and intracytoplasmic walls (Figs. 1j, 2a). The periaxonemal sheath is a striated layer that surrounds the axoneme and the intracytoplasmic walls consist in transverse structures that connect the periaxonemal sheath with the peripheral layer of spiralled cortical microtubules (Figs. 1j, 2a). Another interesting aspect concerning region II is the progressive increase of the width of the sperm cell, until a maximal width of about 415 nm in posterior areas of this region, where intracytoplasmic walls are well developed.
Region III (Figs. 2a–f and 3 (III)) corresponds to the nuclear area of the mature spermatozoon. Its maximal width is about 475 nm, at the level of anterior portions of this region. The nucleus forms a loose spiral around the axoneme, presenting a horseshoe shape (Fig. 2d) or sometimes an annular shape (Fig. 2e, f) in cross-sections. The nucleus is localised between the rods of the periaxonemal sheath and the axoneme (Fig. 2a, b). Along this region, there is a progressive reduction and disappearance of the periaxonemal sheath, intracytoplasmic walls and cortical microtubules (Fig. 2d–f).
Region IV (Figs. 2c, g–i and 3 (IV)) constitutes the posterior spermatozoon extremity. It is a short region about 2500 nm long and characterised by the sole presence of the axoneme surrounded by the plasma membrane (Fig. 2c, g). Its maximal width is around 200 nm. Near the posterior spermatozoon extremity, the axoneme becomes disorganised (Fig. 2h) and the posterior tip of the male gamete consists of electron-dense material surrounded by the plasma membrane (Fig. 2c, i).
Discussion
The mature spermatozoon of T. hydatigena exhibits the general pattern of type VII spermatozoon of Levron et al. (2010). According to these authors, the sperm cells of cestodes are classified in seven types taking into account several characteristics such as the number of axonemes, the spiralled or parallel arrangement of the nucleus and cortical microtubules and the presence or absence of crested bodies, periaxonemal sheath and intracytoplasmic walls.
The spermatozoon of Taenia species shows a single axoneme, a crested body, spiralled cortical microtubules and nucleus, a periaxonemal sheath and transverse intracytoplasmic walls. These and other additional structures are analysed in the present study.
Anterior spermatozoon extremity and related structures: apical cone and crested body
As occurs in all Taenia species, the mature spermatozoon of T. hydatigena is capped in its anterior extremity by an electron-dense structure that constitutes the so-called apical cone. The length of the apical cone has been evaluated for only three of the studied Taenia species, and it is around 2000 nm in T. hydatigena and T. mustelae (Miquel et al. 2000; present study) and around 1200 nm in T. saginata (Bâ et al. 2011). In T. parva and T. taeniaeformis, the length of the apical cone was estimated to be longer than 1900 and 1350 nm, respectively (Ndiaye et al. 2003; Miquel et al. 2009a).
At the level of the apical cone, an electron-dense crested body initiates its helical course around the spermatozoon, as described in other Taenia species. In fact, the presence of a crested body (or bodies) always characterises the anterior extremity of the spermatozoon (Bâ and Marchand 1995), and it constitutes a synapomorphy for the Eucestoda or for a part of them (see Justine 1998, 2001). Among cyclophyllideans, the number of crested bodies varies from 1 to 12 (see Marigo 2011). However, the presence of one or two crested bodies is the most frequent situation among cestodes. Only hymenolepidids and certain anoplocephalids exhibit more than two crested bodies in their sperm cells. With respect to the genus Taenia, all the studied species show a single crested body (see Table 1) with a quite homogeneous thickness (between 50 and 80 nm) except for T. taeniaeformis that presents a 140 nm thick crested body (Miquel et al. 2009b).
These anterior structures may play a role during the fertilisation process between sperm cells and oocytes. The existing studies on fertilisation in several Platyhelminthes (monogeneans, digeneans and cestodes) show a similar pattern consisting in the spiral coiling of spermatozoa around the oocyte, followed by fusion of the respective plasma membranes (Justine and Mattei 1984, 1986; Świderski et al. 2004). Moreover, in some studies, it has been demonstrated that the posterior region of the sperm cell, containing the nucleus, is the last part to enter the oocyte (Justine and Mattei 1984). Thus, considering the anterior location of the apical cone and the crested body, these structures probably play an important role during the fusion of spermatozoon and oocyte membranes as occurs with other anteriorly located structures in digeneans, e.g. external ornamentations of the plasma membrane and lateral expansions (see Justine and Mattei 1982).
Twisted pattern of cortical microtubules
The presence of twisted cortical microtubules has been considered a synapomorphic character for the Tetrabothriidea and Cyclophyllidea (Justine 1998, 2001). Except for the Mesocestoididae (Mesocestoides litteratus and M. lineatus—Miquel et al. 1999, 2007), which exhibit a parallel arrangement of cortical microtubules, all cyclophyllideans studied so far present a submembranous layer of spirally coiled cortical microtubules. Considering both morphology and life cycle, but also according to molecular studies, the systematic position of mesocestoidids has been a subject of controversy among parasitologists (see Rausch 1994; Mariaux 1998). In this sense, because of the plesiomorphic condition of cortical microtubules in Mesocestoides, and based on sperm ultrastructure alone, Justine (2001) places the family Mesocestoididae out of the cyclophyllideans and basal to the Tetraborhriidea. The mature spermatozoon of T. hydatigena, as occurs in the remaining Taenia species, presents twisted cortical microtubules at an angle of around 50° (see Table 1). For the genus Echinococcus, in E. granulosus, a 35° angle of twisting was obtained from the observation and analysis of TEM micrographs published by Morseth (1969). In most cyclophyllideans, this angle of twisting is between 30 and 60°, and only in hymenolepidids and in the anoplocephalid Aporina delafondi this angle is comprised between 15 and 30° (for a review, see Marigo 2011). In this sense, it seems clear the existence of an inverse relation between the number of crested bodies and the angle of twisting of both cortical microtubules and crested bodies because A. delafondi and hymenolepidids exhibit the higher number of crested bodies (5 to 12).
Nucleus
In Taenia species, the nucleus is spiralled around the axoneme as in most cyclophyllideans. In fact, in species presenting a single axoneme in their spermatozoa, the nucleus is usually spiralled, whereas in other orders with biflagellate sperm, the nucleus shows a parallel arrangement throughout the spermatozoon. The unique exception refers to members of the Caryophyllidea, a basal order of tapeworms with spermatozoa containing a single axoneme and a parallel nucleus (Yoneva et al. 2011, 2012a, b). Thus, among the Eucestoda, the caryophyllideans are the sole group that presents the type III spermatozoon (Levron et al. 2010).
In the present paper, we demonstrate for the first time the location of the nucleus between the axoneme and the periaxonemal sheath. This location was clearly visible when longitudinal sections of the transitional areas between pre-nuclear and nuclear regions were observed.
Concerning the shape of the nucleus, in most species with a spiralled nucleus, cross-sections show a horseshoe shaped nucleus. In the case of Taenia species, the nucleus sometimes encircles totally the axoneme, particularly when the remaining structures (periaxonemal sheath, intracytoplasmic walls and cortical microtubules) are replaced by the nucleus. This occurs in the posterior areas of the nuclear region.
Periaxonemal sheath
The presence of a periaxonemal sheath in the spermatozoon has been considered a synapomorphy for the Tetrabothriidea and Cyclophyllidea, with a probable reversal in some cyclophyllideans (see Justine 1998, 2001). To date, this structure has been shown in the tetrabothriidean Tetrabothrius erostris (Stoitsova et al. 1995) and in several cyclophyllideans belonging to different families namely Anoplocephalidae, Catenotaeniidae, Davaineidae, Dilepididae, Dipylidiidae, Gryporhynchidae, Metadilepididae and Taeniidae (see Levron et al. 2010). Concerning taeniids, a periaxonemal sheath surrounding the axoneme was reported in the spermatozoon of T. crassiceps, T. hydatigena, T. mustelae, T. parva, T. saginata, T. solium and T. taeniaeformis (see Table 1). This structure was also observed in Echinococcus multilocularis (Shi et al. 1994).
Intracytoplasmic walls
The presence of intracytoplasmic walls connecting the periaxonemal sheath and the peripheral microtubules has been described in numerous species. In fact, according to Justine (1998), periaxonemal sheath and transverse intracytoplasmic walls are related characters and the presence of both characters is frequent. This is the case of Taenia species, other cyclophyllideans such as Davaineidae, Metadilepididae, Paruterinidae and certain Anoplocephalidae, and also tetrabothriideans. In this sense, the simultaneous presence of these two structures characterises the type VII spermatozoon of tapeworms according to Levron et al. (2010). However, there are some exceptions such as catenotaeniids, dilepidids, dipylidiids, gryporhynchids and some anoplocephalids (see Levron et al. 2010), which lack transverse intracytoplasmic walls and thus, they present type VI spermatozoa.
Posterior spermatozoon extremity
The general pattern of the posterior spermatozoon extremity for the species of the genus Taenia is the sole presence of the axoneme. Thus, most of the studied species exhibit a relatively long posterior region characterised by the absence of cortical microtubules and nucleus and presence of the axoneme surrounded by the plasma membrane. The unique exception is T. saginata (see Bâ et al. 2011), which exhibits an extremely short posterior region where the nucleus practically reaches the posterior spermatozoon tip (see Table 1).
Concluding remarks
A comparison of all the available results from ultrastructural studies on taeniids allows us to establish the main characteristics of the mature spermatozoon for the species of the genus Taenia. These characters are the presence of (1) a long electron-dense apical cone, (2) a single helical crested body, (3) a periaxonemal sheath, (4) transverse intracytoplasmic walls, (5) a spiralled nucleus sometimes surrounding totally the axoneme, (6) a submembranous layer of twisted cortical microtubules and (7) a posterior extremity without cortical microtubules.
To date, ultrastructural characters described in the genus Echinococcus concern E. granulosus and E. multilocularis (Morseth 1969, Shi et al. 1994). However, these results are insufficient to make a comparative analysis with those of Taenia. In E. granulosus, most published TEM micrographs correspond to late spermatids, and the spermiogenesis process seems to include the growth of a cytoplasmic extension and an external developing flagellum. This is consistent with the Bâ and Marchand’s type 3 spermiogenesis pattern, which has already been observed in some Taenia species. With respect to the mature spermatozoon, it contains a single axoneme, spiralled cortical microtubules and nucleus. No data concerning other important characteristics such as crested bodies, periaxonemal sheath or intracytoplasmic walls are available. In the case of E. multilocularis, a periaxonemal sheath is present in the spermatozoon but other characteristics are unknown. On the other hand, the scarcity of data on the genus Echinococcus emphasises the necessity to perform complete analyses of species included in this genus in order to establish a general pattern for the genus and also for comparison with Taenia species.
References
Bâ A, Bâ CT, Quilichini Y, Dieng T, Marchand B (2011) Ultrastructure of the spermatozoon of Taeniarhynchus saginatus (syn. Taenia saginata) (Goeze, 1782) Weinland, 1858 (Cestoda, Taeniidae) an intestinal parasite of human. Parasitol Res 108:831–836. doi:10.1007/s00436-010-2125-2
Bâ CT, Marchand B (1995) Spermiogenesis, spermatozoa and phyletic affinities in the Cestoda. Mém Mus Natn Hist Nat Paris 166:87–95
Barrett NJ, Smyth JD (1983) Observations on the structure and ultrastructure of sperm development in Echinococcus multilocularis, both in vitro and in vivo. Parasitology 87:li.
Featherston DW (1971) Taenia hydatigena. III. Light and electron microscope study of spermatogenesis. Z Parasitenkd 37:148–168. doi:10.1007/BF00259555
Hoberg EP, Mariaux J, Justine J-L, Brooks DR, Weekes PJ (1997) Phylogeny of the orders of the Eucestoda (Cercomeromorphae) based on comparative morphology: historical perspectives and a new working hypothesis. J Parasitol 83:1128–1147
Justine J-L (1991a) Phylogeny of parasitic Platyhelminthes: a critical study of synapomorphies proposed on the basis of the ultrastructure of spermiogenesis and spermatozoa. Can J Zool 69:1421–1440. doi:10.1139/z91-203
Justine J-L (1991b) Cladistic study in the Monogenea (Platyhelminthes), based upon a parsimony analysis of spermiogenetic and spermatozoal ultrastructural characters. Int J Parasitol 21:821–838. doi:10.1016/0020-7519(91)90151-V
Justine J-L (1998) Spermatozoa as phylogenetic characters for the Eucestoda. J Parasitol 84:385–408. doi:10.2307/3284502
Justine J-L (2001) Spermatozoa as phylogenetic characters for the Platyhelminthes. In: Littlewood DTJ, Bray RA (eds) Interrelationships of the Platyhelminthes. Taylor and Francis, London, pp 231–238
Justine J-L, Mattei X (1982) Réinvestigation de l’ultrastructure du spermatozoïde d’Haematoloechus (Trematoda: Haematoloechidae). J Ultrastruct Res 81:322–332. doi:10.1016/S0022-5320(82)90060-0
Justine J-L, Mattei X (1984) Ultrastructural observations on the spermatozoon, ovocyte and fertilization process in Gonapodasmius, a gonochoristic Trematode (Trematoda: Digenea: Didymozoidae). Acta Zool (Stockh) 65:171–177. doi:10.1111/j.1463-6395.1984.tb00822.x
Justine J-L, Mattei X (1986) Ultrastructural observations on fertilization in Dionchus remorae (Platyhelminthes, Monogenea, Dionchidae). Acta Zool (Stockh) 67:97–101. doi:10.1111/j.1463-6395.1986.tb00853.x
Levron C, Miquel J, Oros M, Scholz T (2010) Spermatozoa of tapeworms (Platyhelminthes, Eucestoda): advances in ultrastructural and phylogenetic studies. Biol Rev 85:523–543. doi:10.1111/j.1469-185X.2009.00114.x
Littlewood DTJ, Bray RA, Clough KA (1998) A phylogeny of the Platyhelminthes: towards a total-evidence solution. Hydrobiologia 383:155–160
Loos-Frank B (2000) An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Taenia Linnaeus’ (Cestoda) in table format. Syst Parasitol 45:155–183. doi:10.1023/A:1006219625792
Mariaux J (1998) A molecular phylogeny of the Eucestoda. J Parasitol 84:114–124
Marigo AM (2011) Étude ultrastructurale de la spermiogenèse et du spermatozoïde chez les cestodes. Apports en Taxonomie et Phylogénie. PhD Thesis, University of Barcelona. http://www.tdx.cat/handle/10803/109219
Miquel J, Eira C, Świderski Z, Conn DB (2007) Mesocestoides lineatus (Goeze, 1782) (Mesocestoididae): new data on sperm ultrastructure. J Parasitol 93:545–552. doi:10.1645/GE-1008R.1
Miquel J, Feliu C, Marchand B (1999) Ultrastructure of spermiogenesis and the spermatozoon of Mesocestoides litteratus (Cestoda, Mesocestoididae). Int J Parasitol 29:499–510. doi:10.1016/S0020-7519(98)00202-1
Miquel J, Foronda P, Torres J, Świderski Z, Feliu C (2009a) Ultrastructural study of the spermatozoon of Taenia taeniaeformis (Batsch, 1786) (Cestoda, Cyclophyllidea, Taeniidae), an intestinal parasite of Felis catus from La Palma (Canary Islands, Spain). Parasitol Res 104:1477–1483. doi:10.1007/s00436-009-1351-y
Miquel J, Hidalgo C, Feliu C, Marchand B (2000) Sperm ultrastructure of Taenia mustelae (Cestoda, Taeniidae), an intestinal parasite of the weasel, Mustela nivalis (Carnivora). Invert Reprod Dev 38:43–51. doi:10.1080/07924259.2000.9652435
Miquel J, Świderski Z, Foronda P, Torres J, Feliu C (2009b) Ultrastructure of spermatogenesis of Taenia taeniaeformis (Batsch, 1786) (Cestoda, Cyclophyllidea, Taeniidae) and comparison of spermatological characters in the family Taeniidae Ludwig, 1886. Acta Parasitol 54:230–243. doi:10.2478/s11686-009-0040-4
Morseth DJ (1969) Spermtail finestructure of Echinococcus granulosus and Dicrocoelium dendriticum. Exp Parasitol 24:47–53. doi:10.1016/0014-4894(69)90220-3
Ndiaye PI, Miquel J, Marchand B (2003) Ultrastructure of spermiogenesis and spermatozoa of Taenia parva Baer, 1926 (Cestoda, Cyclophyllidea, Taeniidae), a parasite of the common genet (Genetta genetta). Parasitol Res 89:34–43. doi:10.1007/s00436-002-0702-8
Olson PD, Littlewood DTJ, Bray RA, Mariaux J (2001) Interrelationships and evolution of the tapeworms (Platyhelminthes: Cestoda). Mol Phylogenet Evol 19:443–467. doi:10.1006/mpev.2001.0930
Rausch RL (1994) Family Mesocestoididae Fuhrmann, 1907. In: Khalil KF, Jones A, Bray RA (eds) Keys to the cestode parasites of vertebrates. CAB International, Wallingford, pp 309–314
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Shi DZ, Liu DS, Wang SK, Craig PS (1994) The ultrastructure of Echinococcus multilocularis. Chin J Paras Dis Contr 7:40–41
Świderski Z, Conn DB, Miquel J, Mlocicki D (2004) Fertilization in the cestode Gallegoides arfaai (Mobedi and Ghadirian, 1977) Tenora and Mas-Coma, 1978 (Cyclophyllidea, Anoplocephalidae). Acta Parasitol 49:108–115
Tian X, Yuan L, Huo X, Han X, Li Y, Xu M, Lu M, Dai J, Dong L (1998a) Ultrastructural observations on the transformation of the spermatozoon in spermatogenesis of taeniid cestodes. Chin J Parasitol Paras Dis 16:269–273
Tian X, Yuan L, Li Y, Huo X, Han X, Xu M, Lu M, Dai J, Dong L (1998b) Ultrastructural observation on spermatocytogenesis in taeniid cestode. Chin J Parasitol Paras Dis 16:209–212
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1987) Veterinary Parasitology. Longman Scientific & Technical, Essex
Verster A (1969) A taxonomic revision of the genus Taenia Linnaeus, 1758 s. str. Onderstepoort J Vet Res 36:3–58
Waeschenbach A, Webster BL, Bray RA, Littlewood DTJ (2007) Added resolution among ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with complete small and large subunit nuclear ribosomal RNA genes. Mol Phylogenet Evol 45:311–325. doi:10.1016/j.ympev.2007.03.019
Waeschenbach A, Webster BL, Littlewood DTJ (2012) Adding resolution to ordinal level relationships of tapeworms (Platyhelminthes: Cestoda) with large fragments of mtDNA. Mol Phylogenet Evol 63:834–847. doi:10.1016/j.ympev.2012.02.020
Willms K, Robert L (2007) Ultrastructure of a spermatid transport system in the mature proglottids of experimental Taenia crassiceps (WFU strain). Parasitol Res 101:967–973. doi:10.1007/s00436-007-0570-3
Willms K, Caro JA, Robert L (2003) Ultrastructure of spermatogonia and spermatocyte lobules in Taenia solium strobilae (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 90:479–488. doi:10.1007/s00436-003-0897-3
Willms K, Robert L, Jiménez JA, Everhart M, Kuhn RE (2004) Ultrastructure of spermiogenesis and the spermatozoon in Taenia crassiceps strobilae WFU strain (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 93:262–267. doi:10.1007/s00436-004-1125-5
Yoneva A, Levron C, Ash A, Scholz T (2012a) Spermatological characters of monozoic tapeworms (Cestoda: Caryophyllidea), including first data on a species from the Indomalayan catfish. J Parasitol 98:423–430
Yoneva A, Levron C, Oros M, Orosová M, Scholz T (2011) Ultrastructure of spermiogenesis and mature spermatozoon of Breviscolex orientalis (Cestoda: Caryophyllidea). Parasitol Res 108:997–1005. doi:10.1007/s00436-010-2144-z
Yoneva A, Levron C, Oros M, Orosová M, Scholz T (2012b) Spermiogenesis and spermatozoon ultrastructure of Hunterella nodulosa (Cestoda: Caryophyllidea), a monozoic parasite of suckers (Catostomidae) in North America. Folia Parasitol 59:179–186. doi:10.14411/fp.2012.025
Acknowledgments
This study was financially supported by the European Commission Contract KBBE 2010 1.3-01 265862 (PARAVAC). The authors are grateful to Almudena García from the “Centres Científics i Tecnològics de la Universitat de Barcelona (CCiTUB)” for her assistance in the preparation of samples. JM is a member of the AGAUR group (2014 SGR 1241).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miquel, J., Khallaayoune, K., Azzouz-Maache, S. et al. Spermatological characteristics of the genus Taenia inferred from the ultrastructural study on Taenia hydatigena . Parasitol Res 114, 201–208 (2015). https://doi.org/10.1007/s00436-014-4179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4179-z