Abstract
In classical textbooks of parasitology, the mature proglottids of taeniids are depicted as structures in which the individual testis are connected to the vas deferens through the vas efferens system, usually depicted as a network of channels. From our morphological analyses of proglottids in the cestode Taenia crassiceps, we have been unable to identify this channel network. It is unclear how the spermatids are transported from the testes to the vas deferens, as is unresolved the location of the cells responsible for the production of testosterone (Leydig cells) or the possible equivalent of Sertoli cells, necessary for the differentiation process of these cells. In this experimental work, we have examined the ultrastructure of tissues in the vicinity of the vas deferens in mature proglottids obtained from the intestines of hamsters infected with cysticerci from the peritoneum of infected mice. Worm tissues were fixed, processed, and sectioned for transmission electron microscopy. Significant areas of the testis epithelia emitted cytoplasmic projections surrounded by extracellular matrix, where they appear as septated pockets enclosing free axonemes and spermatids. Vas efferens walls are made up of nucleated cells with cytoplasm annealing to each other through cell membrane junctions. Lodged between the junctions are membrane-bound pouches with dense granules found as aggregates or aligned in a semicircular array. The efferens wall exhibits numerous spermatids emerging into the lumen, an observation that suggests the epithelial wall may have the maturing functions of Sertoli cells of vertebrates. Large cells adjacent to the vas efferens contained prominent rough endoplasmic reticulum and large mitochondria, characteristics described for Leydig cells of vertebrates. Our observations suggest that taeniid spermatids are either transported from the testes to the vas system by epithelial pockets or that the epithelial pockets may be cross-sections of a highly coiled vas efferens system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several publications have described the process of spermiogenesis and spermatogenesis in cestode tapeworms (Miquel et al. 1998, 1999; Ndiaye et al. 2002, 2003; Willms et al. 2003b, 2004). In the Taeniidae, spermiogenesis begins with the accumulation of small aggregates of mitotic cells in the neck region, which undergo first meiosis in small clusters located along the inner border of the excretory ducts and eventually mature into numerous testis in the proglottids. In the mature and gravid proglottids, the testis exhibit spermatocytes in different stages of maturation, and mature spermatids are eventually found in the vas efferens system (Willms et al. 2003b).
In classical descriptions of adult tapeworm structure (Cheng 1973; Lumsden and Hildreth 1983), the mature proglottid is illustrated as a structure in which the individual testicular lobes are connected to the vas deferens by the vas efferens, made up by a network of channels. During the course of our morphological analyses in tissues of proglottids from two taeniid cestodes, Taenia solium and Taenia crassiceps, we were unable to identify such a channel network.
It is unclear, therefore, how the spermatids are transported from the testis lobes to the vas deferens, as is unresolved the location of the equivalent of Leydig cells (Payne et al. 1996) responsible for the production of testosterone, which is produced by metacestodes of T. solium and T. crassiceps (Valdez et al. 2006; Jiménez et al. 2006), or the equivalent structures for Sertoli cells described in vertebrate gonads, which are necessary for the differentiation of spermatogonia to mature spermatids (Russel and Griswold 1993).
In this experimental work, we have examined the ultrastructure of tissues in the vicinity of the vas deferens of mature proglottids, to study the pathway of spermatids from testes to vas deferens, as well as the ultrastructure of the vas efferens wall and the cell types surrounding them.
Materials and methods
Experimental model
T. crassiceps cysticerci (WFU strain) maintained in the peritoneal cavity of female Balb/c mice were recovered and rinsed in phosphate-buffered saline (PBS; Willms et al. 2004). Eight selected cysticerci with developed scolex were fed to golden hamsters pretreated for 5 days with 40 mg/kg of albendazole (Smith Kline Beecham, Mexico) and immune-suppressed beginning the day of infection and every 2 weeks for the duration of the experiment, by intramuscular injection of 2 mg of methyl-prednisolone acetate (Depo-medrol, Upjohn, Mexico; Willms et al. 2003b).
Tissue processing
Live tapeworms were recovered at different times after infection and processed according to methods described previously (Willms et al. 2003b, 2004).Worms were rinsed in PBS, immersed in Karnovsky fixative, processed, and embedded in Spurr.
Thick sections were prepared from four different experiments and photographed by light microscopy to aid in the selection of tissues to be examined by transmission electron microscopy. Electron micrographs were prepared from thin sections of mature proglottids, by selecting areas of the vas deferens and surrounding tissues and photographed in a JEOL 1200EM microscope.
Image processing
Electron micrographs were digitized in a scanner (HP ScanJet 6300) and prepared for publication by processing in power point mode.
Results
All electron micrographic images were obtained from thin sections of tissues in the vicinity of the vas deferens in mature proglottids recovered from hamster intestines 20–40 days postinfection. The images are representative of proglottids from three different experiments, chosen for excellent fixation properties.
Observations were carried out on the following tissues: testis epithelium, spermatic pockets or pouches emerging from testis epithelial wall, spermatic pouches surrounded by extracellular matrix, vas efferens wall and contents, and tissues and cells surrounding the vas efferens.
Testis epithelium
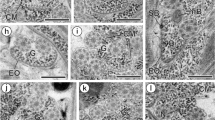
The characteristic epithelial envelope surrounding the testis in mature proglottids was observed in all lobes and included the microtubular organs described previously (Willms et al. 2003a, b, 2004). Along the luminal surface of the lobe, we observed some areas exhibiting specialized cells containing polysomes and structured macromolecules within the cytoplasm (Fig. 1a, b, double-headed arrows).
a–b Electron micrograph of a section from a mature proglottid of Taenia crassiceps illustrating the epithelial wall of a testis. a A large nucleated (Nu) cell in the lumen, closely attached to a microtubular organ (white thick arrow) with a number of prominent star-shaped macromolecules (double arrow) in the cytoplasm. At the lower end of the cell is a spermatid with cortical microtubules, which appear to merge with the cytoplasm (thin arrow). The cell is surrounded by a number of immature spermatids. Bm Basal matrix. b Luminal cell connected (white double arrow) to a microtubular organ (white arrow), containing bundles of longitudinal and cross-sectioned microtubules in the cytoplasm (black arrow)
Spermatid pouches connected to testis epithelium
Significant areas of testicular envelopes emitted cytoplasmic projections (Fig. 2a–d), forming pockets enveloping cross-sectioned axonemes/spermatids, which pierced the basal matrix of the epithelial wall and were surrounded by the extracellular matrix.
a–d Plate with sections from tissues adjacent to the testis, illustrating epithelial projections emerging from an epithelial wall (tes). a A low-power view of two testes containing a number of spermatids (double arrow) and septated (small white arrows) epithelial projections in the interstitial tissue, some of which enclose spermatids. mf Myofibers, sper spermatids. b Illustrates testis wall (tes) to which an adjacent septated epithelial pocket containing a spermatid axoneme (black arrow) is apposed. c The epithelial wall of a testis (black arrow) with a cytoplasmic projection containing spermatid axonemes (white arrow). d Testis wall with a large septated cytoplasmic projection connected to the wall (white arrows), with lumen showing a number of spermatids in cross-section. tes Lumen of testis
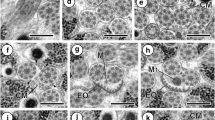
In the extracellular matrix, a number of epithelial pouches were observed (Fig. 3), all of which enclosed axoneme structures, both in the lumen as well as embedded in the epithelial cytoplasm of these pockets. Many of these structures were septated, exhibiting axonemes or parts of these in the lumen.
a–f Plate illustrating epithelial pockets with luminal spermatid structures surrounded by extracellular matrix. a Low-power image of a multiseptated epithelial pocket containing spermatid structures (white arrows). b Wall of a, showing an axoneme within the epithelial cytoplasm (white arrow) as well as a number of microvilli (black arrow). c Higher magnification of axonemes (double arrow) and microvilli in cross-section. d Low-power image of two epithelial pockets with spermatid structures in the lumen as well as in the cytoplasmic wall. e Higher magnification of d, illustrating a spermatid (black arrow) as well as microvilli in the lumen (white arrow). f Wall of epithelial pocket surrounding an axoneme (white arrow), with a second axoneme embedded in the cytoplasm
Vas efferens
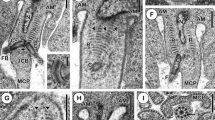
A number of small spermatid channels were identified in the vicinity of the vas deferens. In Fig. 4 is illustrated a mosaic of images from a small vas efferens (diameter = 17 μm). The wall of the vessel exhibits several nucleated cells, the cytoplasm’s of which anneal to each other through numerous junctions (thick black arrow). Lodged between the junctions are a number of circular or elongated membrane-bound pouches (0.8–1.4 μm in diameter) with large dense granules (230–270 nm in diameter), some with the granules aligned in a semicircular array, others in aggregates enclosed by a plasma membrane. These pouches are also observed in the extracellular matrix surrounding the vas, as well as abutting the luminal side of the vessel in areas covered with filamentous cytoplasmic processes or microvilli (Figs. 5b, 6g, and 7). When the epithelial wall is examined at a higher magnification, a number of axoneme-like structures can be found either embedded or emerging from the cytoplasmic wall into the lumen (Fig. 6a–f, white arrows). In Fig. 6d, a spermatid is situated halfway between the vessel epithelium (white downward arrow), and the lumen, with the helical microtubules, is clearly visible (white upward arrow).
Cross-section of a vas efferens from tissue in the vicinity of the vas deferens in a mature proglottid of Taenia crassiceps. The vessel depicts the syncytial wall made up of several nucleated (Nu) cells with a number of granular cytoplasmic sacs (white arrows) wedged between junctions (thick black arrow). A second type of structure with dense granular material arranged along the outer edge of a large vesicular organelle is also wedged between epithelial junctions (double black arrow). In the lumen are shown a number of mature spermatids, as well as empty membranes (black arrows). mf Myofibrils in cross section, lmf longitudinal myofibrils
a–d Plate showing sections of vas efferens wall structure. a Segment of a possible Leydig cell (Lc) in which two sacs with granules surrounded by plasma membrane are shown (black arrow). Two granular pouches are lodged between junctions (angled white arrow) of the efferens wall. b Vas efferens wall illustrating a dense granular cytoplasmic strand lodged between junctions (double white arrow). The short white arrow points to an epithelial junction. vew Vas efferens wall, mf myofibril, lu lumen. c Coiled cytoplasmic strand with dense granules situated outside of vew (white arrow). d Higher magnification of coiled vesicular organelles with dense granules (white arrows) shown in Fig. 4. vew Vas efferens wall
a–h Plate with images of spermatid structures embedded and/or emerging from the vas efferens wall into the lumen. a, b, c Axonemes partially embedded in the vas wall, with bulb-like ends protruding in the lumen (white arrows point to embedded axoneme). d Spiraled cortical microtubules of a whole spermatid with one end partially embedded in the vas wall (arrow pointing down) and the other end free in the lumen (arrow pointing up). e, f Two axoneme-like structures embedded in the vas wall (white arrows). g Higher magnification of granule sac (also shown in Fig. 7) lodged between junctions on the luminal side of the vessel, where a number of microvilli are shown (white arrow). h Longitudinal section of a spermatid, surrounded by loose segmented microvilli (white arrow pointing up), in which an open-ended length of segmented microvilli is positioned in the transverse intracytoplasmic wall space (white arrow pointing down)
Section of tissue adjacent to a vas efferens wall (vew), in which a dense granular cytoplasmic sac is lodged between two epithelial junctions (white arrow), with a number of microvilli projecting into the lumen (lu), which also contains a number of mature spermatids. A large interstitial cell with nucleus, abundant endoplasmic reticulum (white asterisk), and some dense granules are found outside of the vas efferens wall. Below is the cytoplasm of a cell with similar characteristics in which a number of long mitochondria are shown (white arrow). The vas efferens wall is surrounded by several bundles of longitudinal myofibres (mf)
In the lumen are found mature spermatids mostly in the cross-section and several long strands of empty membrane (black arrows), as well as numerous segmented microvilli (Figs. 4, 5, and 6) emerging from the luminal wall of the vessel. These segmented microvilli appear to become inserted onto the outer surface of the spermatids (Fig. 6h). The microvilli have an outer diameter of 50–55 nm, corresponding roughly to the dimensions of the transverse intracytoplasmic walls described for several other spermatids of the taeniid family (Ndiaye et al. 2003). The staining properties of the periaxonemal membrane and the spiral cortical microtubules make it difficult to carry out more precise measurements on these structures.
The efferens wall is surrounded by a basal matrix, strands of cytoplasmic glycogen, the granular pouches described above, longitudinal myofibers, as well as the specialized cells described below.
Cells surrounding the vas efferens
The most conspicuous cell types in the proximity of the vas efferens channels are large cells with layers of rough endoplasmic reticulum (white asterisk), numerous elongated mitochondria, and well-developed Golgi, as well as a number of dense granules occasionally enclosed by plasma membrane (Fig. 7). The granules are found either loose in the cytoplasm, appearing as round, club, or drop-shaped bodies, but also observed within semicircular bands around translucent areas with fine tubules barely visible at high magnification (not shown) in all, similar to the granules in the pouches described between the membrane junctions of the vas efferens wall (Fig. 5a, b).
The cells are situated outside the longitudinal myofibers that are closely apposed to the epithelial wall of the vessel.
Discussion
The analysis of electron microscopic images of the male reproductive tissue in the vicinity of the vas deferens of mature proglottids of T. crassiceps revealed the existence of several structures, which to our knowledge have not been previously described in taeniids:
-
(a)
The presence of cells on the luminal side of the testis epithelial wall, which appear to fuse with the epithelial plasma membrane, creating a visible gap through which cytoplasmic material or macromolecules could be transported. The presence of microtubular structures merged with one of these cells suggests the synthesis and/or assembly of macromolecules in these specialized cells
-
(b)
The ultrastructure of a vas efferens system, characterized by epithelial pockets of mature spermatids, emerging from testis epithelium and surrounded by extracellular matrix
-
(c)
The identification of large pouches of dense granules inserted between the junctions of the vas efferens walls
-
(d)
The observation of spermatid structures embedded or emerging from the vas efferens wall into the lumen
The epithelial pockets connected by internuncial processes to the testicular epithelium and further observed in the extracellular matrix suggest either a transport system for bundles of spermatids or transverse sections of a highly convoluted channel system, which in itself may be a developing structure.
Because these epithelial pockets were observed in the extracellular matrix close to the testis lobes, it may also be speculated that they are in fact developing vas efferens channels, a fact that would also explain the presence of axonemes and microvilli in their lumina. Such a developing route would also explain why these epithelial pockets are not found merging with the larger vas efferens channels. A continuous system of small channels differentiating into vas efferens would also be in agreement with the gradual maturation patterns of spermatids in the testis, where three stages of sperm aggregates are found namely, spermatogonia, spermatocytes, and spermatids (Willms et al. 2003b, 2004). The presence of disassembled spermatid structures in the epithelial pouches cannot be readily explained because many of the spermatid bundles within the testis appear completely formed. The highly interconnected tissues of proglottids also suggest the possibility that these pockets are joined to the testis lobes by convoluted/spiraled epithelial channels (as is the vas deferens itself), of which the pouches are only cross-sections.
The ultrastructure of larger vas efferens channels is characterized by vessels containing bundles of mature spermatids. The wall of these vessels is constructed of several nucleated cells interconnected by junctions. The cytoplasmic wall contained a large number of membrane bound vesicles, reminiscent of those seen in the surface tegument (Willms et al. 2003a), as well as mitochondria. The external surface is surrounded by a basal matrix, and on the inner luminal surface, a large number of microvilli project into the lumen and frequently appear segmented. Some similarities can be observed with ultrastructural descriptions of the uterine epithelium in Hymenolepis species (Lumsden and Hildreth 1983; Conn 1993a, b), in which the epithelium is a syncytium made up of nucleated cells and microlamellae projecting into the uterine lumen.
A distinctive feature of the efferens vessels is the presence of large (1 μm in diameter) membrane-bound granules lodged between the epithelial junctions. The granules are dense, round, elliptical, or club shaped, often enclosing a translucent area. It is not clear whether the sacs of enclosed granules are in fact compressed spermatids being transported through the vessel wall or sacs of granules possibly containing androgens or other substances required for spermatid or vessel maturation. The precise makeup or function of these electron-dense granules remains to be worked out.
In the lumen of the efferens channels, a number of axoneme structures can be observed projecting from the wall into the lumen. At high magnification, several of these were clearly identified as spermatids, suggesting that these cells are traversing the vas efferens wall. An additional observation was the insertion of microvillus segments being assembled into the outer wall of axonemes, suggesting that the final assembly of spermatids is an ongoing process in these vessels. It may also be speculated that these vessels in fact have the function of Sertoli cells described in mammalian tissues, required for the maturation of spermatids (Russel and Griswold 1993). Although the Sertoli cell is part of the meiotic cycle in mammalian spermiogenesis, meiosis in taeniids proceeds within the testis, and it is possible that the vas efferens syncytium observed here may have Sertoli-like functions, which would explain the spermatids emerging from the efferens wall into the lumen.
The large cells surrounding the vas efferens exhibit some of the organelles described for Leydig cells in mammalian gonads. These cells contain abundant endoplasmic reticulum, a well developed Golgi apparatus, and very long mitochondria, in addition to aggregates of granules, similar to those described in the epithelial wall pouches. Although these organelles are also found in cestode myoblasts (Conn 1993b), it may also be speculated that they are the equivalent of the testosterone-producing Leydig cells in mammalian tissues (Payne et al. 1996). Published observations (Valdez et al. 2006; Jiménez et al. 2006) have demonstrated that cysticerci of T. solium and T. crassiceps synthesize androgens and estrogens in vitro. It is not clear which cells are responsible for this synthesis, but ongoing experiments in our laboratory are examining these tissues by histochemical methods to test for the presence of testosterone or precursor cells. Preliminary results showed that the immature and mature proglottids of adult taeniids have significant amounts of a key enzyme for the testosterone pathway, 3 β-hydroxisteroid dehydrogenase (Fernandez Presas et al. 2006). More precise histochemical localization of the sites for synthesis is being analyzed.
Although the complete spermatid transport system cannot be worked out from the present observations, they do conform to the syncytial nature of cestode tissues and suggest that spermatids are transported from the testis to the vas efferens system by way of epithelial pockets or channels and aided by closely apposed myofibers, which are abundant and ubiquitous in the proglottids of taeniids (Willms et al. 2003a).
The present observations, taken in conjunction with our previous ultrastructural analyses, illustrate that the male reproductive tissues evolve from an undifferentiated epithelial syncytium in the immature proglottids to a highly organized, compartmentalized system in the mature and gravid proglottids and further suggest the possibility that parasitic flatworms may have primitive male gonad structures such as Leydig and Sertoli cells, similar to those found in higher vertebrates.
References
Cheng T (1973) Cestoda. The true tapeworms. In: Cheng T (ed) General parasitology. Academic, New York, pp 474–573
Conn DB (1993a) The biology of flatworms (Platyhelminthes): parenchyma cells and extracellular matrices. Trans Am Microsc Soc 11:241–261
Conn DB (1993b) Ultrastructure of the gravid uterus of Hymenolepis diminuta (Platyhelminthes: Cestoda). J Parasitol 79:583–590
Fernandez Presas AM, Robert L, Jimenez JA, Romano MR, Willms K (2006) Localization of 3-β-hydroxisteroid dehydrogenase in mature strobilae of Taenia solium. Poster a1610, XI International Congress of Parasitology, Glasgow, UK
Jiménez P, Váldez RA, Romano MR (2006) Metabolism of steroid hormones by Taenia solium and Taenia crassiceps cysticerci. J Steroid Biochem Mol Biol 99:203–208
Lumsden RD, Hildreth MB (1983)The fine structure of adult tapeworms. In: Arme C, Pappas WP (eds) Biology of the Eucestoda (Chapter 6, Vol 1). Academic, New York, pp 177–234
Miquel J, Tidiane Ba C, Marchand B (1998) Ultrastructure of spermiogenesis of Dipyllidium caninum (Cestoda, Cyclophyllidea, Dipylidiidae),an intestinal parasite. Int J Parasitol 28:1453–1458
Miquel J, Feliu C, Marchand B (1999) Ultrastructure of spermiogenesis and the spermatozoon of Mesocestoides litteratus (Cestoda, Mesocestoididae). Int J Parasitol 29:499–510
Ndiaye PI, Miquel J, Marchand B (2002) Ultrastructure of spermiogenesis and spermatozoa of Taenia parva Baer 1926 (Cestoda, Cyclophyllidea, Taeniidae), a parasite of the common genet (Genetta gennetta). Parasitol Res 89:34–43
Ndiaye PI, Agostini S, Miquel J, Marchand B (2003) Ultrastructure of spermiogenesis and the spermatozoon in the genus Joyeuxiella Fuhrmann, 1935 (Cestoda, Cyclophyllidea, Dipylidiidae): comparative analysis of J. echinorhynchoides (Sonsino, 1889) and J. pasqualei (Diamare, 1893). Parasitol Res 91:175–186
Payne AH, Hardy MP, Russell LD (eds) (1996) The leydig cell. Cache River, Vienna, IL [ISBN 0-9627422-7-9]
Russel LD, Griswold MD (eds) (1993) The sertoli cells. Cache River, Vienna, IL [ISBN 0-9627422-0-1-X]
Valdez RA, Jimenez AL, Cartas AL, Gomez Y, Romano MR (2006) Taenia solium cysticerci synthesize androgens and estrogens in vitro. Parasitol Res 98:472–476
Willms K, Robert L, Caro JA (2003a) Ultrastructure of smooth muscle, gap junctions and glycogen distribution in Taenia solium tapeworms from experimentally infected hamsters. Parasitol Res 89:308–331
Willms K, Caro JA, Robert L (2003b) Ultrastructure of spermatogonia and spermatocyte lobules in Taenia solium strobilae (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 90(6):479–488
Willms K, Robert L, Jimenez JA, Everhart M, Kuhn RE (2004) Ultrastructure of spermiogenesis and the spermatozoon in Taenia crassiceps strobilae WFU strain (Cestoda, Cyclophyllidea, Taeniidae) from golden hamsters. Parasitol Res 93:262–267
Acknowledgments
The authors wish to thank Dr. Marta Romano for her advice on male gonad structures and Jose Agustín Jimenez for his help in the infection of mice and hamsters with T. crassiceps WFU. This work was supported in part by grant IN238602 from Papiit, DGAPA, and funds from the Facultad de Medicina, UNAM. The experiments herein comply with the current laws of Science and Technology in Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willms, K., Robert, L. Ultrastructure of a spermatid transport system in the mature proglottids of experimental Taenia crassiceps (WFU strain). Parasitol Res 101, 967–973 (2007). https://doi.org/10.1007/s00436-007-0570-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0570-3