Abstract
Green synthesis technology is one of the rapid, reliable and best routes for the synthesis of silver nanoparticles (AgNPs). There are bioactive compounds with enormous potential in Azadirachta indica (Neem). The extraordinary mosquitoes warrant nanotechnology to integrate with novel molecules. This will be sustainable technology for future. Here, we synthesized AgNPs using aqueous extracts of leaves and bark of Az. indica (Neem). We tested AgNPs as larvicides, pupicides and adulticides against the malaria vector Anopheles stephensi and filariasis vector Culex quinquefasciatus. The results were obtained using UV-visible spectrophotometer and the images were recorded with a transmission electron microscope (TEM). The efficacy tests were then performed at different concentrations varying many hours by probit analysis. The synthesized AgNPs were spherical in shape and with varied sizes (10.47-nm leaf and 19.22-nm bark). The larvae, pupae and adults of filariasis vector C. quinquefasciatus were found to be more susceptible to our AgNPs than the malaria vector An. stephensi. The first and the second instar larvae of C. quinquefasciatus show a mortality rate of 100 % after 30 min of exposure. The results against the pupa of C. quinquefasciatus were recorded as LC50 4 ppm, LC90 11 ppm and LC99 13 ppm after 3 h of exposure. In the case of adult mosquitoes, LC50 1.06 μL/cm2, LC90 2.13 μL/cm2 and LC99 2.4 μL/cm2 were obtained after 4 h of exposure. These results suggest that our AgNPs are environment-friendly for controlling malarial and filarial vectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Azadirachta indica (Neem) belongs to the family of Meliaceae. It is a medicinal plant regarded as a scared tree and a panacea for all diseases. Neem extracts have shown to have insecticidal effects. Mosquitoes are vectors of many diseases, including malaria, filariasis, dengue and Japanese encephalitis. Among these malaria, spread by the bite of female Anopheles mosquito and filariasis and spread by Culex mosquito are the two vector borne diseases of the tropical region and are considered as major public health concerns.
According to WHO, there were about 219 million cases of malaria in 2010 (with an uncertainty range of 154 million to 289 million) and an estimated 660,000 deaths (with an uncertainty range of 490,000 to 836,000). Malaria mortality rate has fallen by more than 25 % globally since 2000 and by 33 % in the WHO African region. Most deaths occur among children living in Africa, where malaria claims the life of a child every minute. Country-level burden estimates available for 2010 show that an estimated 80 % of malaria deaths occur in just 14 countries and about 80 % of cases occur in 17 countries. Together, the Democratic Republic of the Congo and Nigeria account for over 40 % of the total estimated malaria deaths globally (World Health Organization 2013a).
On the other hand, nearly 1.4 billion people in 73 countries worldwide are threatened by lymphatic filariasis, commonly known as elephantiasis. Over 120 million people are currently infected, with about 40 million disfigured and incapacitated by the disease (World Health Organization 2013b). Numerous products of plant origin have received considerable attention as potent bioactive compounds against various species of mosquitoes.
Previous studies have evaluated essential oil from the leaves of Feronia limonia for chemical constituents and insecticidal activity against larvae of Anopheles stephensi, Aedes aegypti and Culex. quinquefasciatus (Senthikumar et al. 2013). Another study evaluated essential oils extracted from fresh leaves of wild and cultivated plants of Ruta chalepensis L. (Rutaceae) for larvicidal and repellent properties against the Asian tiger mosquito, Aedes albopictus (Conti et al. 2013). In other works, larvicidal and repellent activities of essential oil from Artemisia (Zhu and Tian 2013), Toddalia (Liu et al. 2013) and Coriandrum (Benelli et al. 2013) were evaluated against the adult and larvae of An. stephensi, Ae. aegypti and C. quinquefasciatus. There is a crucial need to produce new insecticides due to resistance and high cost of organic insecticides which are more environment-friendly, safe and target specific.
Synthesizing nanoparticles using plants and microorganisms can eliminate this problem by making the nanoparticles more biocompatible. The use of Neem (Az. indica) leaf broth in the extracellular synthesis of pure metallic silver and gold nanoparticles and bimetallic Au/Ag nanoparticles has been reported previously (Shankar et al. 2004). Silver nanoparticles have been successfully synthesized using crude Neem leaf (Az. indica) extract at room temperature (Shukla et al. 2009). The effect of process variables like reductant concentrations, reaction pH, mixing ratio of the reactants and interaction time on the morphology and size of silver nanoparticles synthesized using aqueous extract of Az. indica (Neem) leaves has been investigated (Tripathy et al. 2010). The plant synthesis of cadmium oxide nanoparticles using flowers extract of Achillea wilhelmsii as the reducing agent has been studied (Andeani and Mohsenzadeh 2013). Studies on antimicrobial activity of nanoparticles of Neem extract on groundwater collected from four river samples in India have been carried out (Yadav and Mathur 2013). A simple and eco-friendly method for the synthesis of silver NPs using an aqueous solution of Pulicaria glutinosa plant extract as a bioreductant has recently been reported (Khan et al. 2013).
The larvicidal activity of biosynthesized nanoparticles against filariasis and malaria causing Culex and Anopheles mosquito vector has been evaluated (Dhanasekaran and Thangaraj 2013; Subarani et al. 2013). The activities of silver nanoparticles (AgNPs) synthesized using Murraya koenigii plant leaf extract against first to fourth instars larvae and pupae of An. stephensi and Ae. aegypti have been determined (Suganya et al. 2013).
In this study, we have successfully synthesized the AgNPs from extracts of leaves and bark of Az. indica. Furthermore, these biologically synthesized AgNPs were found to have a high larvicidal, pupicidal and adulticidal activities. The Environmental Protection Agency (EPA) in 2009 has cleared like nanosilver, nanogold and other nanometals to be used for public health. So, this could be a potential rapid green technology for control of mosquitoes.
Materials and methods
Plant materials
Fresh leaves and bark of Az. indica were collected from the campus and the botanical garden of Dayalbagh Educational Institute in India. The voucher specimen is maintained in our laboratory for future use.
Extracts preparation
Leaves and bark of Az. indica were washed with distilled water for removing the dust particles. A plant leaf broth was prepared by placing 10 g of leaves (finely cut) in a 250-mL flask with 100 mL of deionized water. The bark was air dried and converted into powder, and a bark broth was prepared by placing 10 g of bark powder in a 250 mL of deionized water. These mixtures were boiled at 60 °C, for 5 min, decanted or filtered through Whatman-1 filter paper.
AgNP synthesis
After the extract preparation, the AgNPs were synthesized by using the following method with some modifications (Subarani et al. 2013). After obtaining the aqueous extract of leaves and bark, the filtrates were treated with aqueous 1 mM AgNO3 solution in an Erlenmeyer flask and incubated at room temperature. Formation of AgNPs was indicated the dark brown colouration of the solutions.
AgNPs characterization
Synthesis of AgNPs was confirmed by sampling the reaction mixture at regular intervals, and the absorption maxima was scanned by UV–vis spectra, at the wavelength of 350–750 nm in a UV-3600 Shimadzu spectrophotometer at 1-nm resolution. The micrographs of AgNPs were obtained by TECHNAI 200 Kv transmission electron microscope (TEM) (Fei, Electron Optics). For transmission electron microscopy analysis, samples were prepared on carbon-coated copper TEM grids.
Rearing of mosquito vectors
The larvae of C. quinquefasciatus and An. stephensi (these are commonly found in Agra, India, and are not listed as endangered and protected species) were collected from various localities including urban, rural and semi-urban regions of Agra (27°, 10′ N, 78° 05′ E), India. The areas of collection were public areas, and no permissions were required. The larvae were reared in deionized water containing glucose and yeast powder. The colonies of C. quinquefasciatus and An. stephensi were maintained in the laboratory at a temperature of 25 °C with a relative humidity of 75 ± 5 % and 14 h of photoperiod. The larvae of C. quinquefasciatus and An. stephensi were maintained in separate enamel containers as per the standard method (Gerberg et al. 1994). The pupae were collected from the culture tray and transferred to petri dishes containing 50 mL of water. The petri dishes were placed inside a screened cage (25 cm in length × 15 cm in width × 5 cm in depth) to retain emerging adults, for which 5 % sucrose solution in water provided to the adults.
Bioassays, data management and statistical analysis
AgNPs synthesized from Az. indica were tested for their killing activities against the larvae, pupae and adults of C. quinquefasciatus and An. stephensi and were assessed by using the standard method (World Health Organization 2005). All larvae and pupae of C. quinquefasciatus and An. stephensi were separated and placed in a container in microbe-free deionized water. After that, different test concentrations of AgNPs in 100 mL deionized water were prepared in 250-mL beakers. Bioassays were conducted separately for each instar and pupae at five different concentrations of aqueous AgNPs (2, 4, 6, 8, 10 ppm for larvae and 20, 40, 60, 80, 100 ppm for pupa). To test the larvicidal and pupicidal activity of our AgNPs, 20 pupae and larvae of each stage were separately exposed to 100 mL of test concentrations. Thereafter, we examined their mortality after different time of treatment during the experimental periods.
The adulticidal bioassays were carried out with laboratory-reared C. quinquefasciatus and An. stephensi as per standard procedures recommended by the World Health Organization with some modifications (World Health Organization 2006). The freshly emerged 3-day-old sugar-fed adults were used for the assay. The five different volumes 2.13, 2.66, 3.2, 3.73 and 4.26 μL/cm2 of aqueous AgNPs of leaves and bark synthesized were sprayed in a cage (25 cm long × 15 cm wide × 5 cm deep) containing 100 mosquitoes. The exposed mosquitoes were kept under observation, and the dead mosquitoes were discarded every day. Each bioassay including the control was conducted in triplicates on different days. Similarly, the control (aqueous extract of leaf and bark without AgNO3) was run to test the natural mortality against the each larval stage of C. quinquefasciatus and An. stephensi. The data on the efficacy were subjected to probit analysis (Finney 1971). The control mortality was corrected by Abbott’s formula (Abbott 1925).
Results
Analysis of UV–vis spectra of synthesized AgNPs
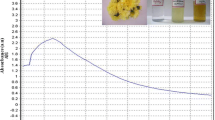
The aqueous extracts of leaf and bark of Az. indica were light yellow and brown in colour before immersion in AgNO3 solution. The colour of leaf and bark aqueous extracts changed to dark red after immersing in AgNO3 solution after 72 h of incubation. The change in colour is a signal for the formation of AgNPs. Figure 1a, b shows the UV–vis spectra of AgNPs synthesized by using the leaf and bark of Az. indica recorded from reaction medium before (1) and after immersion of AgNO3 (2) after 72 h. Absorption spectra of AgNPs formed in the reaction medium has a broad absorption band centred at 480 nm c.a.
Analysis of TEM of synthesized AgNPs
Figure 2a, b shows the TEM micrographs of leaf and bark of Az. indica-synthesized AgNPs. The 10.47 and 19.22 nm sized and spherical-shaped AgNPs were obtained.
Study on the efficacy of AgNPs synthesized using the aqueous extract of leaves of Az. indica against the C. quinquefasciatus
The efficacy of the AgNPs by using the aqueous extract of leaves of Az. indica was tested against the larvae, pupae and adults of C. quinquefasciatus. Different concentrations of aqueous AgNPs (2, 4, 6, 8, 10 ppm; 20, 40, 60, 80, 100 ppm; and 2.13, 2.66, 3.2, 3.73, 4.26 μL/cm2) were tested against the larvae, pupae and adults of C. quinquefasciatus.
The larvae of C. quinquefasciatus were found more susceptible to the AgNPs than the pupae and adults. The first and the fourth instar larvae of C. quinquefasciatus showed 100 % mortality in response to the AgNPs after 15 min and 1 h 30 min of exposure. While the second instar larvae (LC50 6, LC90 12, LC99 14 ppm) and the third instar larvae (LC50 10, LC90 18, LC99 20 ppm) showed the efficacy after 1 h 30 min of exposure with their probit equations, 95 % confidential limits, chi-square and r values (Table 1). All these chi-square values at 3 degrees of freedom (df) for the second and the third instars of C. quinquefasciatus were found higher than the critical value of chi-square at 0.05 significance level. In the control group, no mortality was observed. The observed LC values have shown the degree of susceptibility of AgNPs synthesized by using aqueous extract of leaves of Az. indica during the four larval stages of C. quinquefasciatus in order of the first instar > the second instar > the third instar < the fourth instar.
The efficacy against the pupae of C. quinquefasciatus (LC50 1, LC90 8, LC99 10 ppm) after 3 h and adults (LC50 0.53, LC90 2.66, LC99 4.26 μL/cm2) after 4 h was recorded with their probit equations, 95 % confidential limits, chi-square and r values (Table 1). All these chi-square values at 3 df for pupae and adults of C. quinquefasciatus were found higher than the critical value of chi-square at 0.05 significance level. In the control group, no mortality could be observed.
Study on the efficacy of AgNPs synthesized using the aqueous extract of bark of Az. indica against the C. quinquefasciatus
The AgNPs synthesized using the aqueous extract of bark of Az. indica were tested against the larvae, pupae and adults of C. quinquefasciatus. Different concentrations of aqueous AgNPs (2, 4, 6, 8, 10 ppm; 20, 40, 60, 80, 100 ppm; and 2.13, 2.66, 3.2, 3.73, 4.26 μL/cm2) were tested against the larvae, pupae and adults of C. quinquefasciatus.
The larvae of C. quinquefasciatus were found to be more susceptible to AgNPs than the pupae and adults. The first, the second and the third instar larvae of C. quinquefasciatus showed 100 % mortality to the AgNPs after 30 min and 1 h of exposure. The fourth instar larvae (LC50 2, LC90 12, LC99 14 ppm) showed the efficacy after 1 h of exposure with their probit equations, 95 % confidential limits, chi-square and r values (Table 1). All these chi-square values at 3 df for the fourth instars of C. quinquefasciatus were found higher than the critical value of chi-square at 0.05 significance level. In the control group, no mortality was observed. The observed LC values show the degree of susceptibility of AgNPs synthesized using aqueous extract of leaves of Az. indica during the four larval stages of C. quinquefasciatus in the order of the first instar > the second instar > the third instar > the fourth instar.
The efficacy against the pupae of C. quinquefasciatus (LC50 4, LC90 11, LC99 13 ppm) after 3 h and adults (LC50 1.06, LC90 2.13, LC99 2.4 μL/cm2) after 4 h was recorded with their probit equations, 95 % confidential limits, chi-square and r values (Table 1). All these chi-square values at 3 df for pupae and adults of C. quinquefasciatus were found to be higher than the critical value of chi-square at 0.05 significance level. In the control group, no mortality was observed.
Study on the efficacy of AgNPs synthesized using the aqueous extract of leaves of Az. indica against the An. stephensi
The efficacy of AgNPs synthesized using the aqueous extract of leaves of Az. indica was tested against the larvae, pupae and adults of C. quinquefasciatus. The different concentrations of aqueous AgNPs (2, 4, 6, 8, 10 ppm; 20, 40, 60, 80, 100 ppm; and 2.13, 2.66, 3.2, 3.73, 4.26 μL/cm2) were tested against the larvae, pupa and adult of An. stephensi.
The larvae of An. stephensi were found to be more susceptible to the AgNPs than the pupae and adults. The first instar larvae (LC50 2, LC90 10, LC99 11 ppm) after 4 h, the second instar larvae (LC50 2, LC90 9, LC99 11 ppm) after 5 h, the third instar larvae (LC50 2, LC90 10, LC99 12 ppm) after 12 h and the fourth instar larvae (LC50 1, LC90 8, LC99 11 ppm) after 17 h show the efficacy with their probit equations, 95 % confidential limits, chi-square and r values (Table 2). All these chi-square values at 3 df for the first, the second, the third and the fourth instars of C. quinquefasciatus were found to be higher than the critical value of chi-square at 0.05 significance level. In the control group, no mortality was observed. The observed LC values showed the degree of susceptibility of AgNPs synthesized using aqueous extract of leaves of Az. indica among the four larval stages of An. stephensi in the order of the first instar > the second instar > the third instar > the fourth instar. No significant mortality was observed against pupae, adults and in the control group.
Study on the efficacy of AgNPs synthesized using the aqueous extract of bark of Az. indica against the An. stephensi
The AgNPs synthesized using the aqueous extract of bark of the Az. indica were tested against the larvae, pupa and adult of An. stephensi. Different concentrations of aqueous AgNPs (2, 4, 6, 8, 10 ppm; 20, 40, 60, 80, 100 ppm; and 2.13, 2.66, 3.2, 3.73, 4.26 μL/cm2) were tested against the larvae, pupa and adult of An. stephensi.
The first and the second instar larvae of An. stephensi show 100 % mortality after 1 h of exposure to AgNPs, whereas no mortality was observed among the third and the fourth instar larvae after 1 h. However, the pupae and adults did not exhibit mortality against the AgNPs after 24 h of exposure.
Discussion
In this study, we successfully synthesized AgNPs of spherical shape and varying sizes using the aqueous extracts of the leaves and the bark of Az. indica. Further, these AgNPs were tested as insecticides against the larvae, pupae and adults of C. quinquefasciatus and An. stephensi. The leaf and neem bark have not been tested against the mosquito control, previously. In our study, the good results were observed at very low concentrations and very short of time also. The results were different at various life stages in C. quinquefasciatus and An. stephensi because these nanoparticles work on cuticle of mosquitoes which under metamorphosis during in life cycle; therefore, these could differ in working except Culex species and Anopheles species, depending upon the cuticle formation during the process which has already been accepted as the case of different in metabolically.
An economically viable and “green chemistry” approach for biological synthesis of AgNPs using aqueous leaf extract of P. dulce have been reported as larvicidal activity against the C. quinquefasciatus previously (Raman et al. 2012). The larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector has also been evaluated before (Dhanasekaran and Thangaraj 2013).
Larvicidal activities of synthesized AgNPs using aqueous leaf extract of Vinca rosea (L.) (Apocynaceae) against the larvae of malaria vector An. stephensi Liston and filariasis vector C. quinquefasciatus Say (Diptera: Culicidae) were also determined (Subarani et al. 2013). In larvicidal activity, their results showed the maximum efficacy in AgNPs against fourth instar larvae of An. stephensi (LC50 12.47 and 16.84 mg/mL and LC90 36.33 and 68.62 mg/mL) on 48 and 72 h of exposure. The efficacy against C. quinquefasciatus (LC50 43.80 mg/mL and LC90 120.54 mg/mL) was on 72-h exposure, and aqueous extract showed 100 % mortality against An. stephensi and C. quinquefasciatus (LC50 78.62 and 55.21 mg/mL and LC90 184.85 and 112.72 mg/mL) on 72-h exposure at concentrations of 50 mg/mL, respectively.
Larvicidal activity of AgNPs using leaf extract of Nerium oleander (Apocynaceae) against the first to the fourth instar larvae and pupae of malaria vector, An. stephensi (Diptera: Culicidae) was carried out in an earlier study (Roni et al. 2013). The fabrication, characterization and mosquito larvicidal bioassay of AgNPs synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii was observed (Haldar et al. 2013). Moreover, the activity of AgNPs synthesized using Murraya koenigii plant leaf extract against the first to the fourth instars larvae and pupae of An. stephensi and Ae. aegypti was determined too (Suganya et al. 2013). Among these previous studies, the AgNPs were synthesized by using the aqueous extract of leaf and nanoparticles tested against the first and the fourth instar larvae and pupae of mosquitoes. However, in the present study, we have synthesized AgNPs by using aqueous extracts of leaf and bark of Az. indica. These AgNPs were tested as larvicidal, pupicidal and adulticidal activities against the larval stages and pupae of An. stephensi and C. quinquefasciatus.
The AgNPs were synthesized using the extract of Neem leaf and Triphala for evaluating their antimicrobial activities (Gavhane et al. 2012). Further, Coleus forskohlii root-synthesized AgNPs were tested as antimicrobial activity against bacteria and fungus (Baskaran and Ratha bai 2013). However, the antimicrobial activity of AgNPs synthesized using fresh flower of Myosotidium hortensia was evaluated on gram positive (Bacillus substilis) and gram negative (Klebsiella planticola) bacteria (Gnanajobitha et al. 2013). These studies have shown the anti-microbial activity of nanoparticles synthesized from plants. Whereas in our study, we have evaluated the larvicidal, pupicidal and adulticidal activities of AgNPs form plant (Neem) extracts.
Antibacterial applications of AgNPs synthesized from the aqueous extract of Az. indica (Neem) leaves were also evaluated (Tripathi et al. 2009). Recently, green synthesizes of AgNPs reduced and stabilized by exuded gum from Anacardium occidentale has been developed for evaluating in vitro antibacterial and cytotoxic activities (Quelemes et al. 2013). The above results show the antibacterial activities of AgNPs synthesized using plant extracts. Whereas in our study, we synthesized AgNPs using the extracts from the leaf and the bark of Az. indica. Further, these synthesized AgNPs have been tested against the larvae, the pupae and the adults of malaria vector An. stephensi and filariasis vector C. quinquefasciatus.
The efficacy of the fungus-mediated silver and gold nanoparticles has been tested against the Ae. aegypti larvae (Soni and Prakash 2012a). The fungus Aspergillus niger has been selected for the synthesis of gold nanoparticles (AuNPs). Additionally, the larvicidal efficacy of these AuNPs has been tested using the larvae of three mosquito species: An. stephensi, C. quinquefasciatus and Ae. aegypti (Soni and Prakash 2012b). The effect of AgNPs synthesized with Chrysosporium keratinophilum, Verticillium lecanii and Fusarium oxysporum f. sp. pisi has been evaluated against the adult mosquito of filariasis vector C. quinquefasciatus (Soni and Prakash 2012c). The efficacy of silver and gold generated larvicide with the help of entomopathogenic fungus Chrysosporium tropicum has been evaluated against the larvae of An. stephensi and C. quinquefasciatus (Soni and Prakash 2012d). Recently, the AgNPs by using the soil fungus As. niger 2587 have been synthesized and tested against the larvae and pupae of An. stephensi, C. quinquefasciatus and Ae. aegypti (Soni and Prakash 2013). The above results show the efficacy of silver and gold nanoparticles synthesized by using the fungi against the larvae and adult mosquitoes. However, in the present study, we have tested AgNPs from plants against the larvae, pupae and adults of mosquitoes.
Conclusions
The objective of this study was successfully accomplished with the synthesis of AgNPs by using aqueous extracts of medicinal plant Az. indica Neem and testing them against major vector species. The AgNPs were spherical in shape with varying sizes. The AgNPs were successfully tested against the larvae, pupae and adults of malaria vector An. stephensi and filariasis vector C. quinquefasciatus. The AgNPs synthesized by using the extract of leaves of Az. indica were found highly effective against the C. quinquefasciatus than the An. stephensi. This rapid green method would be useful for development of clean, non-toxic and environmentally acceptable metal nanoparticles. The rapid green AgNPs have the potential to be used as a novel economical and indigenous environment-friendly technology for controlling larvae, pupae and adults of mosquito vectors.
References
Abbott SW (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265
Andeani JK, Mohsenzadeh S (2013) Phytosynthesis of cadmium oxide nanoparticles from Achillea wilhelmsii flowers. J Chem. doi:10.1155/2013/147613
Baskaran C, Ratha bai V (2013) Green synthesis of silver nanoparticles using Coleus forskohlii roots extract and its antimicrobial activity against bacteria and fungus. Int J Drug Dev Res 5:114–119
Benelli G, Flamini G, Fiore G, Cioni PL, Conti B (2013) Larvicidal repellent activity of the essential oil of Coriandrum sativum L. (Apiaceae) fruits against the filariasis vector Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 112:1155–1161
Conti B, Leonardi M, Pistelli L, Profeti R, Ouerghemmi I, Benelli G (2013) Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis L. (Rutaceae) against Aedes albopictus Skuse (Diptera: Culicidae), an arvovirus vector. Parasitol Res 112:991–999
Dhanasekaran D, Thangaraj R (2013) Evaluation of larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector. Asian Pac J Trop Dis 3:174–179
Finney DJ (1971) Probit analysis, 3rd edn. Cambridge University Press, Cambridge
Gavhane AJ, Padmanabhan P, Kamble SP, Jangle SN (2012) Synthesis of silver nanoparticles using extract of neem leaf and triphala and evaluation of their antimicrobial activities. Int J Pharma Bio Sci 3:88–100
Gerberg EJ, Barnard DR, Ward RA (1994) Manual for mosquito rearing and experimental techniques. J Am Mosq Control Assoc 5:98
Gnanajobitha G, Vanaja M, Paulkumar K, Rajeshkumar S, Malarkodi C, Annadurai G, Kannan C (2013) Green synthesis of silver nanoparticles using Millingtonia hortensis and evaluation of their antimicrobial efficacy. Int J Nanomater Biostr 3:21–25
Haldar KM, Haldar B, Chandra G (2013) Fabrication, characterization and mosquito larvicidal bioassay of silver nanoparticles synthesized from aqueous fruit extract of putranjiva, Drypetes roxburghii (Wall.). Parasitol Res 112:1451–1459
Khan M, Khan M, Adil SF, Tahir MN, Tremel W, Alkhathlan HZ, Al-Warthan A, Siddiqui MRH (2013) Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomed 8:1507–1516
Liu XC, Dong HW, Zhou L, Du SS, Liu ZL (2013) Essential oil composition and larvicidal activity of Toddalia asiatica roots against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol Res 112:1197–1203
Quelemes PV, Araruna FB, Faria BFED, Kuckelhaus SAS, Silva DAD, Mendonça RZ, Eiras C, Soares MJDS, Leite JRSA (2013) Development and antibacterial activity of cashew gum based silver nanoparticles. Int J Mol Sci 14:4969–4981
Raman N, Sudharsan S, Veerakumar V, Pravin N, Vithiya N (2012) Pithecellobium dulce mediated extra-cellular green synthesis of larvicidal silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 96:1031–1037
Roni M, Murugan K, Panneerselvam C, Subramaniam J, Hwang JS (2013) Evaluation of leaf aqueous extract and synthesized silver nanoparticles using Nerium oleander against Anopheles stephensi (Diptera: Culicidae). Parasitol Res 112:981–990
Senthikumar A, Jayaraman M, Venkatesalu V (2013) Chemical constituents and larvicidal potential of Feronia limonia leaf essential oil against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Parasitol Res 112:1337–1342
Shankar SS, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci 15:496–502
Shukla VK, Pandey S, Pandey AC (2009) Green synthesis of silver nanoparticles using neem leaf (Azadirachta indica) extract. Int Conf AdvNanomater Nanotechnol 1276:43
Soni N, Prakash S (2012a) Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res 110:175–184
Soni N, Prakash S (2012b) Synthesis of gold nanoparticles by the fungus Aspergillus niger and its efficacy against mosquito larvae. Reports Parasitol 2:1–7
Soni N, Prakash S (2012c) Fungal-mediated nano silver: an effective adulticide against mosquito. Parasitol Res 111:2091–2098
Soni N, Prakash S (2012d) Entomopathogenic fungus generated nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac J Trop Dis 2:S356–S361
Soni N, Prakash S (2013) Possible mosquito control by silver nanoparticles synthesized by soil fungus (Aspergillus niger 2587). Adv Nanoparticles 2:125–132
Subarani S, Sabhanayakam S, Kamaraj C (2013) Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control against Anopheles stephensi liston and Culex quinquefasciatus say (Diptera: Culicidae). Parasitol Res 112:487–499
Suganya A, Murugan K, Kovendan K, Kumar PM, Hwang JS (2013) Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res 112:1385–1397
Tripathi A, Chandrasekaran N, Raichur AM, Mukherjee A (2009) Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. J Biomed Nanotechnol 5:93–98
Tripathy A, Raichur AM, Chandrasekaran N, Prathna TC, Mukherjee A (2010) Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanoparticle Res 12:237–246
World Health Organization (2005) Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/13
World Health Organization (2006) Vector borne diseases in India. Report of a brainstorming session, 9 November 2006
World Health Organization (2013a) Malaria. http://www.who.int/mediacentre/factsheets/fs094/en/index.html
World Health Organization (2013b) Lymphatic filariasis. http://www.who.int/mediacentre/factsheets/fs102/en/
Yadav A, Mathur P (2013) Studies on antimicrobial activity of nanoparticles of neem extract on groundwater collected from four river samples of India. Int J Adv Pharmaceut Res 4:1676
Zhu L, Tian Y (2013) Chemical composition and larvicidal activity of essential oil of Artemisia gilvescens against Anopheles anthropophagus. Parasitol Res 112:1137–1142
Acknowledgments
We sincerely thank Prof P. S. Satsangi Sahab, Chairman, Advisory Committee on Education, Dayalbagh Educational Institute, and Prof Prem K. Kalra, Director, Dayalbagh Educational Institute, for their support and encouragement. We also thank the UGC, New Delhi, Major Research Project (39-599/2010) for financial support. We also thank Dr. Shashi Wadhawa for access to TEM (AIIMS, New Delhi).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soni, N., Prakash, S. Silver nanoparticles: a possibility for malarial and filarial vector control technology. Parasitol Res 113, 4015–4022 (2014). https://doi.org/10.1007/s00436-014-4069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4069-4