Abstract
The goal of this study was to evaluate the relative influence of terrestrial habits, season, and host body size on the species richness and abundance of helminth parasites in the toad Melanophryniscus klappenbachi, for which a greater abundance of nematode parasites was expected. A total of 90 toads were collected in the Chaco Province, Argentina. The helminth community found in infected toads included 17 taxa and was dominated particularly by larval parasites. Contrary to our expectations, nematode species showed lower values of infection parameters. Infected toads harbored a maximum of seven species, and the mean helminth richness was 3.16 ± 1.66 species per infected toads. Season played a significant effect on determining the species richness and abundance of the parasite infracommunity. Similarly, the prevalence of infection of several helminth species (8/47 %) varied greatly over time. Host body size was the main factor in determining the infrapopulation structure of helminth parasites. Species richness was significantly and negatively correlated with host body size. Strong associations were observed mainly between larvae of some species. The transmission strategies of parasites suggest that this bufonid acquires infections through direct contact with larval parasites from aquatic and terrestrial habitats and by ingestion of infective larvae. The characteristic of the host tegument, such as the presence of alkaloids, could significantly contribute to the low occurrence of infection by skin-penetrating nematodes. Results also showed that diets and mobility of the host and the local microhabitat conditions play an important role in parasitic infections of toads.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the study of the helminth community, no single factor is generally responsible for the level of parasite in a community. One of the most important considerations is the range of life cycles involved: some parasites lack intermediate hosts, others move sequentially among different host species to complete their life cycles, and several larval stages develop in different aquatic and terrestrial microhabitats. Thus, the helminth community depends a lot more on transmission, i.e., parasites vary in how they are acquired by the host, penetrate, or get eaten. This would suggest that the life history of the host would determine where and how it picks up its helminths through its diet or spending time in the water or on land (Esch 1971). A small change in the parasite transmission level causes great changes in the number of adults as well as changes in the parasite community structure (Marcogliese and Cone 1997; Esch et al. 2002). Kennedy (1975) emphasized the importance of the host diet in the control of infection level, but its seasonal changes, which are indirectly influenced by temperature, and the seasonal maturation cycle among parasites induce seasonal changes in the levels of infection. Chubb (1979) also indicated that the development of helminth parasites can show a marked seasonality in mid-latitude climates, i.e., the cold season causes changes in acquisition of the parasite by the host. However, these results may also vary among regions with subtropical climates, where the temperature is not a significant factor to determine strong seasonal patterns of helminth development. For instance, ecological studies regarding helminth parasites in Argentinean vertebrate hosts from subtropical environments have shown that there is more time for amphibian hosts to become infected during the year because there is no or little freezing temperature unlike the temperate region of the world (Hamann 1999, 2006).
Other important factors in the helminth community structure are species richness and their relative abundance, which are specifically related to the interactions between helminth parasites at the infracommunity level. In this sense, Holmes and Price (1986) considered that interactive communities are species-rich, with species regularly cooccurring at densities such that there is often evidence of interactions between the species, and that isolationist communities are species-poor, with species being independent of each other. Thus, communities may be located anywhere along this continuum, and those that parasitize amphibians tend to be found towards the isolationist, i.e., show little evidence of parasitic interactions (Aho 1990; Muzzall 1991; Bolek and Coggins 2003; Luque et al. 2005; Yoder and Coggins 2007; Ibrahim 2008; Hamann et al. 2006a, b, 2010, 2012, 2013a). Notably, the relationships between the richness species and host body size and between host mobility and ability to be in different microhabitats, together with the ability to feed on different intermediate host species (which leads to a greater likelihood of acquiring food-borne parasites), provide a particular structure to parasite species communities.
In recent decades, the study of communities of toad amphibian parasites and host-parasite relationships has gained interest in diverse regions of South America (Bursey et al. 2001; Goldberg and Bursey 2003; Iannacone 2003; Luque et al. 2005; Lux Hoppe et al. 2008; Pinhão et al. 2009; Santos et al. 2013). Hamann et al. (2013a) have previously reported the first study of the ecology of helminth parasite communities in the Argentinean toad amphibian Rhinella fernandezae (Gallardo, 1957). The present study was also performed in bufonids but with different habitat use and different host behavior (e.g., adult toads have diurnal habits and live on dry land with a predominance of terrestrial bromeliads in the Chaco region), which consequently provide the parasite communities and indicate the diversity of intermediate and definitive hosts in this local environment (Poulin and Morand 2004). No taxonomic and ecological studies have been carried out on helminth infections in this bufonid toad.
Based on certain assumptions, the approach used in this current study predicts (1) that the terrestrial habit of toads determines greater abundance of nematode fauna, (2) that the short winters allow the development of helminths most of the year, and (3) that the helminth community is influenced by the diet and size of the host. These premises were tested by analyzing the definitive host Melanophryniscus klappenbachi Prigioni & Langone (2000), a member of the family Bufonidae. M. klappenbachi is a typical amphibian species of the Chaco region, and its distribution in Argentina covers Chaco, Formosa, Santa Fe, and Santiago del Estero provinces (Frost 2014). This species has typically diurnal habits and breeds in temporary aquatic environments. Several breeding periods occur during the rainy season. After heavy rainfall, there is an explosive breeding, and a large number of individuals congregate in these sites. The couples perform multiple ovipositions, depositing eggs in small groups that are attached to submerged vegetation by the male. These toads are small to medium in size (22 to 34 mm) and have aposematic coloration type, with patches of yellow, red, and orange on dark backgrounds. The behavior associated with this type of color is called “unken-reflex.” When facing potential predators or capture, the specimens have members upwards, bending the body and exposing the ventral spots (see Manzano et al. 2004). A large number of lipophilic alkaloids (termed “dendrobatid alkaloids”) with pharmacological potential have been isolated from the skin glands of these amphibians. These substances, which are either synthesized de novo or obtained from their diet (see Daly et al. 2008), are considered to provide a defense against predation during the mainly diurnal breeding episodes of toads (Santos and Grant 2011). Feeding studies indicate that adults feed mainly on ant and scarce acari (unidentified mites) (Daly et al. 2008).
The objectives of this study were (1) to determine the richness and diversity of helminth parasites at the component and infracommunity levels, (2) to analyze the helminth cycles of transmission, (3) to examine the species affinities (covariation and association) of helminth infracommunities, and (4) to evaluate the influence of season on the abundance and number of parasitic helminth species in the toad M. klappenbachi.
Materials and methods
Study area
The study area (50 ha) is located within the Chaco biogeographic province, Chaco Province, Argentina (27° 23′ S, 58° 58′ W). The habitat is characterized by deciduous dry woodland with herbaceous grasses and numerous cacti and terrestrial bromeliads. The river bank is dominated by alder forests (palo bobo) or creole sauce or gallery forest logging. The forest vegetation consists of quebracho (Schinopsis balansae), urunday (Astronium balansae), and ñandubay (Prosopis affinis), with herbaceous strata composed of areas that include gramineous (Elionurus muticus) and terrestrial bromeliads (Aechmea distichantha, Bromelia serra). The mean annual temperature is between 20 and 23 °C and the mean annual precipitation between 500 and 1,200 mm (Cabrera and Willink 1980). Table 1 shows the values of temperature and precipitation by seasons during sampling period, in the study area.
Collection and examination of host and parasites
Adult specimens of M. klappenbachi were collected in autumn 2011 (n = 21) and in winter (n = 22), spring (n = 26), and summer (n = 21) 2012–2013. Specimens were hand-captured always by two people between 1400 and 1700 hours. Toads were transported live to the laboratory and killed in a chloroform (CHCL3) solution. Their snout-vent length (SVL) and body weight were recorded. At necropsy, hosts were sexed and the esophagus, stomach, gut, lungs, liver, urinary bladder, kidneys, body cavity, musculature, skin, and brain examined for parasites. Helminths were observed in vivo, counted, and killed in hot distilled water and preserved in 70 % ethyl alcohol. For identification, the digeneans, cestodes, and acanthocephalans were stained with hydrochloric carmine, cleared in creosote, and mounted in Canada balsam. Nematodes were cleared in glycerine or lactophenol and examined as temporary mounts. Helminths were deposited in the Helminthological Collection of Centro de Ecología Aplicada del Litoral (CECOAL) Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Corrientes city, Corrientes, Argentina [accession numbers CECOAL, 12082218, Glypthelmins palmipedis (Lutz, 1928) ; 12082217, Catadiscus longicoecalis (Caubisens Poumarau, 1965); 12082208, Styphlodora sp.; 13031117, Bursotrema tetracotyloides (Szidat, 1960); 12082201, Travtrema aff. stenocotyle (Cohn, 1902); 12082215, Opisthogonimus sp.; 13031107, Cylindrotaenia sp.; 12082207, Mesocestoides sp.; 11051215 and 12082204, Cosmocerca sp. 1; 12082202, Cosmocerca sp. 2; 11051202, Physaloptera sp.; 13031118, Seuratoidea gen sp.; 13031103, Spiroxys sp.; 13031116 Rhabdochonidae gen. sp.; 12113009, Centrorhynchus sp.]. Toads were deposited in the Herpetology Collection of CECOAL-CONICET, with accession number LHC, 5120. The approval for using animals in this research was given by the Secretary of Natural Resources, Department of Fauna and Protected Areas from Production Ministry, Chaco Province, Argentina.
Statistical analysis
The infection prevalence, intensity, and abundance were calculated for helminths according to Bush et al. (1997). The measures of community richness and diversity employed included the following: the total number of helminth species (=richness), Shannon index (H′) (Shannon and Weaver 1949), and evenness (J′) as H′/H′ maximum (Zar 2010). The Brillouin index (BH) and evenness (E) were used at the infracommunity level (Pielou 1966). The diversity index was used with decimal logarithms (log10). Species richness is the number of helminth species, and mean helminth species richness is the sum of helminth species, per individual toads, divided by the total sample size. A distribution of infracommunity richness values was tested to see if they indicate some sort of structure between the numbers of helminth species vs. number of toads. Berger-Parker index of dominance (d) was used to determine the most abundant species (Magurran 2004). The Kruskal–Wallis test, and posteriori Dunn tests (Zar 2010), was used to compare richness of helminths between seasons. The probabilities were calculated according to the Bonferroni procedure (Hochberg 1988), because it provides great control over type I error.
Helminth communities have been classified at the infracommunity (all helminth infrapopulations within a single M. klappenbachi) and component community (all helminth infracommunities within a population of the M. klappenbachi) levels (Bush et al. 1997). Comparison of k proportion (χ 2) was used to test for differences in prevalence of helminths between seasons. Kruskal–Wallis test (H) was used to test for differences in size of toads between seasons. Spearman’s rank test (r s ) was used to indicate the relationship between host body size, parasitic abundance, and infracommunity descriptors. Spearman’s rank test (r s ) was used to calculate possible species covariation. The species associations was analyzed with a chi-square test (χ 2), with Yates correlation. The degree of aggregation of different species of parasites was calculated by the relation variance (s 2) and mean (\( \overline{x} \)). The helminth community structure was examined according to the methodology outlined by Thul et al. (1985), in which helminth species are classified into four groups (dominant, codominant, subordinate, and unsuccessful) by taking into account their prevalence, intensity, and maturity factor (equal to 1.0 if at least one mature specimen of species j is found and equal to 0 if otherwise), which is related to the degree of host specificity.
The non-metric multi-dimensional scaling (NMDS), an ordination technique, was used to study the pattern in helminth community structure based on abundance of parasite species composition. For that, a similarity matrix was constructed based on the Bray–Curtis measure. The abundance of infection of each parasite species in each host and its relation with season was analyzed through a one-way analysis by means of a non-parametrical permutational ANOVA (PERMANOVA) test (Anderson et al. 2008).
For abundance vs. host body size and covariation and associations of parasites, the species considered were those that had at least 8 % occurrences in the amphibian population (seven species). The softwares used were Xlstat 7.5 (Addinsoft 2004) and Primer v6 (Anderson et al. 2008).
Results
Helminth community characteristics
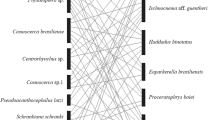
The helminth component community for this toad population consisted of 17 species of helminths (Table 2). The predominant groups of parasites were trematodes (8/47 %) followed by nematodes (6/35 %); the other groups of parasites were represented by very few species (two Cestoda and one Acanthocephala). Helminth diversity (H′) and evenness (J′) were 0.59 and 0.48, respectively. Centrorhynchus (L.) was the most abundant species (d = 0.57). Most (94 %) of the helminth parasite species of M. klappenbachi showed an aggregated pattern of distribution. Five species (29 %) were reported at adult stage and use M. klappenbachi as definitive host, and 12 species (71 %) were reported at larval stage and infect this bufonid toad as intermediate or paratenic hosts. Parasite transmission occurs to the toad host by skin penetration (53 %) and oral ingestion (47 %) (Table 2). Parasites were found in different sites of infection in the host (Fig. 1). The most parasitized organs in this host were the serous membrane of the stomach with Centrorhynchus sp. (12 %), followed by the kidneys with Styphlodora sp. (7 %), and the small intestine with G. palmipedis (7 %) (Fig. 1).
Relative frequency in percentage per infection site (Gm, gastric mucosa; Ss, serous of stomach; Bc, body cavity; Me, mesenteries; Ub, urinary bladder wall; Mu, muscles; Pz, pharyngeal zone; Liv, liver; Fb, body fat; Ki, kidney; Si, small intestine; Li, large intestine) of helminth species (total worm frequency, N = 538). Y-axis scales are different for each helminth species
At the infracommunity level, the mean helminth richness was 3.16 ± 1.66 (maximum = 7) species per infected toad. Multiple infections were observed in 79 % of toads, with 2, 3, 4, 5, 6, and 7 species occurring in 15, 9, 24, 19, 3, and 1 toads, respectively. The distribution of helminth infracommunity species richness observed did not show good fit to Poisson distribution (χ 2 = 21.21, df = 6, P = 0.002). Mean helminth value of diversity (HB) and evenness (E) were 0.25 ± 0.16 and 0.52 ± 0.32, respectively.
Interspecific relationships in the infracommunity
Five correlations between helminth species occurrence were significant (three were negative and two were positive) (Table 3). Three associations were found among the seven helminth species considered: Styphlodora sp./C. longicoecalis (χ 2 = 4.70 , df = 1, P < 0.05), Mesocestoides sp./Styphlodora sp. (χ 2 = 3.85, df = 1, P < 0.05), and Centrorhynchus sp./Styphlodora sp. (χ 2 = 21.26, df = 1, P < 0.05).
Parasitic infection related to size of host
The infection prevalence of the 90 toads examined was 97 %. Total length of the toads ranged from 22.00 to 34.50 mm (29.30 ± 2.37), and weight ranged from 1.00 to 4.80 g (2.12 ± 0.83). The abundances of helminth species and ecological descriptors were primarily not significantly correlated (P > 0.05) with host body size. Nevertheless, significant negative correlation was observed for Styphlodora sp. vs. length of the host (r s = −0.26, n = 86, P < 0.05).
There were no significant differences in the weight of frogs between season (Kruskal–Wallis H = 6.83, df = 3, P = 0.08), but the length of frogs were different between seasons (Kruskal–Wallis H = 9.04, df = 3, P = 0.03). In the last case, posteriori multiple pairwise comparisons using Dunn’s procedure were calculated. These data showed that the length of frogs was only different between winter and summer (P < 0.05) (Table 1). Infection related to host body size per season showed predominantly a negative correlation with host body size. Significant negative correlation was observed for species richness vs. host length (winter r s = −0.46, n = 22, P < 0.05; spring r s = −0.49, n = 26, P < 0.05), and a significant negative correlation was also observed for abundance of helminths vs. host length (Cosmocerca sp. 1/autumn r s = −0.54, n = 17, P < 0.05; C. longicoecalis/ winter r s = −0.47, n = 22, P < 0.05; Styphlodora sp./spring r s = −0.46, n = 26, P < 0.05). Nevertheless, significant positive correlation was observed for G. palmipedis vs. length of the host (summer r s = 0.53, n = 21, P < 0.05).
Importance of species within the community
Helminth species were classified according to community importance values (Table 2); two species were strongly characteristic of the community (e.g., C. longicoecalis), and two species contributed significantly to the community, although to a lesser extent (e.g., Cosmocerca sp. 1). One species occurred infrequently and did not contribute significantly to the community (e.g., Cylindrotaenia sp.), and 12 that are able to enter the host but not to attain maturity therein contributed little to the community and are characteristic of a different host (e.g., Mesocestoides sp.).
Influence of season in the helminth community
A non-parametric PERMANOVA test was performed using data from the season as classification factors and the abundance of all helminth parasites (17 species) as dependent variables. Results showed that season (ANOVA pseudo F = 13.44, P = 0.0001) indicated significant differences among the four groups of seasons in terms of their infracommunity species abundance. The factor interaction was not significant only between winter and spring (P < 0.05) (Table 4). The NMSD ordination of helminth communities, represented in two dimensions (stress = 0.18), indicated potential differences among the abundance of parasitic species composition among the four groups of seasons (Fig. 2).
The seasonal variations of helminth species in relation to parasitological descriptors are shown in Table 2. Results of comparison of k proportion in helminth prevalence infections showed that seasons had a significant effect on 47 % of the species (Table 5).
Species richness in component community showed no significant differences between seasons (autumn vs. winter x 2 = 0.94, P > 0.05; autumn vs. summer x 2 = 1.37, P > 0.05; autumn vs. spring x 2 = 1.37, P > 0.05) (Table 6); however, infracommunity species richness was related to seasons (Kruskal–Wallis H test = 38.4, df = 3, P < 0.05). In the last case, posteriori multiple pairwise comparisons using Dunn’s procedure were calculated. These data showed that the infracommunity species richness was significantly lower in autumn than in the other seasons and that the species richness in summer was higher than that in spring (P < 0.05).
Discussion
The results of this study indicate that M. klappenbachi hosts a high richness of helminth species (larvae + adults), with a maximum of seven species per toad. However, the richness of the adult parasite community was lower than that of other South American bufonid toads (Table 7). Particularly, the importance of the species in the community shows that C. longicoecalis and G. palmipedis would be favored by the transmission, because both trematodes release their infective stage in the water (Smyth and Smyth 1980), where the warm temperature and high rainfall have an important effect on the development of early stages (e.g., cercariae) as well as on intermediate hosts as snails and tadpoles (Kennedy 1975; Chubb 1979; Esch and Fernandez 1994; Hamann 2006; Hamann et al. 2010). Therefore, M. klappenbachi becomes infected either by ingestion of metacercariae with aquatic vegetation (e.g., C. longicoecalis) or by skin penetration of cercariae (e.g., G. palmipedis) either in tadpoles or adult frogs. In addition, three larval species (Styphlodora sp., Mesocestoides sp., and Centrorhynchus sp.) have a high prevalence of infection (>40 %) for which M. klappenbachi represents an intermediate host (e.g., Styphlodora sp.) and/or a paratenic host (Mesocestoides sp. and Centrorhynchus sp.) in the parasite life cycles. The potential definitive hosts for these larval stages are snakes, birds, and mammals, respectively (Grabda-Kazubska 1963; Smyth and Smyth 1980; Prudhoe and Bray 1982). The helminth infections in M. klappenbachi showed a typical aggregated pattern of distribution for many of the parasites (94 %). The aggregated distributions among these toads and heterogeneity in susceptibility to infection or in exposure to parasites are the main factors of aggregation and explained also by the clumped distributions of helminth species (see Poulin 2013).
Most authors consider that parasite communities in toad amphibians are depauperate and isolationist (Bolek and Coggins 2003; Yoder and Coggins 2007; Ibrahim 2008), while others consider no fixed pattern, i.e., communities could be arranged along an axis from isolationist communities to interactive communities (Luque et al. 2005; Hamann et al. 2013a). In this study, the helminth infracommunities of M. klappenbachi exhibited few abundant species and poor species richness. An individual toad generally harbors only three helminth species, and in most cases, the prevalence is low, so that opportunities for interspecific interactions are limited. For instance, the scarce species affinity was evident. On the one hand, we noted associations for those larval and adult species whose individuals were located in different toad organs (Styphlodora sp. + C. longicoecalis), and on the other hand, we found a negative correlation between two species at different infection sites (e.g., Mesocestoides sp./Cosmocerca sp. 1). Thus, M. klappenbachi infracommunities showed a proximity to the isolationist extreme of the continuum, as well as the presence of depauperate infracommunities when only the adult helminth species were considered. Finally, the cooccurrence of metacercariae observed in this amphibian host could affect the structure of gastrointestinal helminths in the definitive hosts and can also serve as indicators of the biodiversity surrounding the habitat, such as the abundance of several major animal groups as turtles, snakes, birds, and mammals (Marcogliese and Cone 1997).
With regard to amphibian body size, several authors suggest that this variable positively influences species richness and abundance of the helminth parasites (Muzzall 1991; McAlpine 1997; Bolek and Coggins 2000; Yoder and Coggins 2007; Ibrahim 2008; Hamann et al. 2012, 2013a). Indeed, it is important to point out that in the present study, the infection of helminth species decreased in larger amphibians. This result suggests that larger toads have less helminth parasites because they probably offer a large number of lipophilic alkaloids (termed “dendrobatid alkaloids”) with pharmacological potential in a body surface area that could avoid the parasitic attack, especially of infective larval stages that actively penetrate into the host through the skin (e.g., cosmocercids). Another possible reason of the limited penetration of parasites could be related to that adults have a thicker integument compared with juvenile toads and by the immune response of the host. On the other hand, the infrapopulations of Mesocestoides sp. and Centrorhynchus sp. that infect the toads by the foot web via predator–prey interactions also decrease in abundance in larger hosts. This can occur as the result of an increase in the amount of a single prey type consumed, e.g. mainly Formicidae. Additionally, the preference of this food item was reflected in the production of alkaloid profiles in the skin (Daly et al. 2008). Lastly, these infections are highly dependent on the diet of toads and hence on the nature of the microhabitat.
As demonstrated in a previous research (Hamann et al. 2013b) in amphibian hosts from a variety of habitats in Corrientes, Argentina, the diets and mobility of the host and the local microhabitat conditions may have considerable influence on the trematode parasite communities and are important factors in the development of the latter. The results of the present study also showed that the microhabitat preference plays an important role in parasitic infections. Specifically, adult M. klappenbachi toads live and feed almost exclusively on terrestrial habitats and reproduce and develop into tadpoles in temporary marshes in remnants of deciduous xerophilous forest with a predominance of grasses and terrestrial bromeliads. The parasite fauna is dominated by high richness of larval trematodes, particularly allogenic species whose definitive hosts (e.g., snakes, mammals, and birds) are temporary visitors to the aquatic microhabitat thereby introducing infective stage. Additionally, these larvae provide a discontinuous but seasonally predictable source of infective parasites to the habitat and therefore for the trematode community (Esch and Fernandez 1994). By contrast, adult trematodes are poorly represented regarding the species richness, which could be related to the toad diet type, which includes mainly ants and, to a lesser extent, oribatid mites and isopods (M.I. Duré per. obs.). The digeneans found are characterized by having heteroxenous life cycles that take place in aquatic environments, and their cercariae could infect the toad host in all life stage (Hamann and González 2009). On the other hand, the poorer nematological fauna and lower abundance of parasitic infections reflect the limited penetration of the infective forms of nematodes, e.g., the genus Cosmocerca, which can infect through skin penetration (direct life cycle) (Anderson 2000). This result is different from those found in bufonid amphibians in temperate region (Bolek and Coggins 2003; Yoder and Coggins 2007) and tropical region (Bursey et al. 2001; Goldberg and Bursey 2003; Luque et al. 2005; Ibrahim 2008; Lux Hoppe et al. 2008; Pinhão et al. 2009; Santos and Amato 2010; Hamann et al. 2013a), where nematode species dominate the community. Specifically, when compared to the helminth community of bufonids (e.g., R. fernandezae) from Corrientes, Argentina, the latter showed an equal richness of nematodes and trematodes but was better represented by skin-penetrating nematodes (e.g., Cosmocerca podicipinus Baker & Vaucher, 1984) as dominant species (Hamann et al. 2013a).
The temperature is likely to provide the vital synchronization of the life cycle of helminth parasites in their definitive and intermediate hosts and to affect the temporal and spatial distribution of helminth parasites (Chubb 1979). The results of the present study show that, in most cases, the prevalence of platyhelminths and acantocephalans showed a temporal variation. Similarly, species richness and abundance in the parasite infracommunities showed variation among seasons. These results could be related to colonization, i.e., temporal constraints on the activity and foraging patterns of the definitive hosts, as well as to the parasite transmission cycles. The availability of invertebrates may also be irregular during the seasons, and therefore, the helminth parameters are not similar (Esch et al. 2002). Specifically, the lowest infection parameters for many species in autumn compared with summer may be related to the low level of rainfalls. This dry season induces changes in the behavior of these amphibians, i.e., less frequency of the toads in the breeding site and consequently a decrease in the exposure time to helminth larvae. So, the temporal variations observed in the helminth infracommunities may be accounted for by the recruitment of immature parasites throughout the different seasons of the year, which coincides with the explosive breeding of M. klappenbachi after heavy rainfall and by the timing of the larval release by adult parasites throughout the year. In this regard, climatic factors such as warm temperature and high rainfall in summer are significant in determining the high level of parasitic infection of M. klappenbachi from Chaco, Argentina.
In conclusion, the features of the community of helminths in M. klappenbachi suggest that the characteristic of the tegument (e.g., the presence of alkaloids in the skin glands) could contribute significantly to the low occurrence of infection by skin-penetrating nematodes. In this case, more studies under laboratory conditions are needed to confirm this hypothesis. In contrast, the trematode fauna shows high infections resulting from acquisition of species by penetration of infective larvae in smaller amphibians since it spends more time in water and therefore have a higher probability of contact with these parasites. At the same time, the local host factors and their biology, i.e., terrestrial microhabitat preference, explosive breeding in ephemeral bodies of water, and the diet type intermediate between that of a generalist and a specialist play an important role in parasitic infections of these toads.
References
Addinsoft (2004) Xlstat for excel, version 7. 5. Addinsoft, London
Aho JM (1990) Helminth communities of amphibians and reptiles: comparative approaches to understanding patterns and processes. In: Esch GW, Bush AO, Aho JM (eds) Parasite communities: patterns and processes. Chapman & Hall, London, pp 157–196
Anderson RC (2000) Nematode parasites of vertebrates: their development and transmission, 2nd edn. CABI International, Oxford
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA + for PRIMER v6. Plymouth
Bolek MG, Coggins JR (2000) Seasonal occurrence and community structure of helminth parasites from the eastern American toad, Bufo americanus americanus, from southeastern Wisconsin, USA. Comp Parasitol 67:202–209
Bolek MG, Coggins JR (2003) Helminth community structure of sympatric eastern American toad, Bufo americanus americanus, northern leopard frog, Rana pipiens, and blue-spotted salamander, Ambystoma laterale, from southeastern Wisconsin. J Parasitol 89:673–680
Bursey CR, Goldberg SR, Parmelee JR (2001) Gastrointestinal helminths of 51 species of anurans from Reserva Cuzco Amazónico, Peru. Comp Parasitol 68:21–35
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. Revisited J Parasitol 83:575–583
Cabrera AL, Willink A (1980) Biogeografía de América Latina. Monografía 13, Organización de los Estados Americanos
Chubb JC (1979) Seasonal occurrence of helminths in freshwater fishes. Part II. Trematoda. Adv Parasitol 17:141–296
Daly JW, Garraffo HM, Spande TF, Yeh HJC, Peltzer PM, Cacivio PM, Baldo JD, Faivovich J (2008) Indolizidine 239Q and quinolizidine 275I. Major alkaloids in two Argentinian bufonid toads (Melanophryniscus). Toxicon 52:858–870
Esch GW (1971) Impact of ecological sucession on the parasite fauna in centrarchids from oligotrophic and eutrophic ecosystems. Am Midl Nat 86:160–168
Esch GW, Fernandez JC (1994) Snail-trematodes interactions and parasite community dynamics in aquatic systems: a review. Am Midl Nat 131:209–237
Esch GW, Barger MA, Fellis KJ (2002) The transmission of digenetic trematodes: style, elegance, complexity. Integr Comp Biol 42:304–312
Frost DR (2014) Amphibian species of the world: an online reference. Version 6.0 Electronic Database accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA. Accessed 9 March 2014
Goldberg SR, Bursey CR (2003) Helminths of two anuran species; Atelopus spurrelli (Bufonidae) and Dendrobates histrionicus (Dendrobatidae), from Colombia, South America. Parasitol Int 52:251–253
Grabda-Kazubska B (1963) The life cycle of Metalepthophallus gracillimus (Lühe, 1909) and some observations on the biology and morphology of developmental stages of Letophalus nigrovenosus (Bellingham, 1844). Acta Parasitol Pol 11:349–370
Hamann MI (1999) Population biology of Spirocamallanus inopinatus (Travassos, Artigas y Pereira, 1928) (Nematoda, Camallanidae) in Serrasalmus spilopleura Kner, 1860 (Pisces, Characidae) from Corrientes, Argentina. Res Rev Parasitol 59:1–6
Hamann MI (2006) Seasonal maturation of Glypthelmins vitellinophilum (Trematoda: Digenea) in Lysapsus limellus (Anura: Pseudidae) from an Argentinean subtropical permanent pond. Braz J Biol 66:85–93
Hamann MI, González CE (2009) Larval digenetic trematodes of tadpoles of six amphibian species from Northeastern Argentina. J Parasitol 95:623–628. doi:10.1645/GE-1738.1
Hamann MI, González CE, Kehr AI (2006a) Helminth community structure of the oven frog Leptodactylus latinasus (Anura, Leptodactylidae) from Corrientes, Argentina. Acta Parasitol 51:294–299. doi:10.2478/s11686-006-0045-1
Hamann MI, Kehr AI, González CE (2006b) Species affinity and infracommunity ordination of helminths of Leptodactylus chaquensis (Anura: Leptodactylidae) in two contrasting environments from northeastern Argentina. J Parasitol 92:1171–1179
Hamann MI, Kehr AI, González CE (2010) Helminth community structure of Scinax nasicus (Anura: Hylidae) from a South American subtropical area. Dis Aquat Org 93:71–82. doi:10.3354/dao02276
Hamann MI, Kehr AI, González CE (2012) Community structure of helminth parasites of Leptodactylus bufonius (Anura: Leptodactylidae) from northeastern Argentina. Zool Stud 51:1454–1463
Hamann MI, Kehr AI, González CE (2013a) Helminth communities in the burrowing toad, Rhinella fernandezae, from northeastern Argentina. Biologia 68:1155–1162. doi:10.2478/s11756-013-0272-5
Hamann MI, Kehr AI, González CE (2013b) Biodiversity of trematodes associated with amphibians from a variety of habitats in Corrientes, Argentina. J Helminthol 87: 286–300. doi: 10.1017/S0022149X12000302 DOI:10.1017/S0022149X12000302#_blank
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–808. doi:10.1093/biomet/75.4.800
Holmes JC, Price RD (1986) Communities of parasites. In: Anderson DJ, Kikkawa J (eds). Community ecology: patterns and processes, Oxford, pp187–213
Iannacone J (2003) Helmintos parásitos de Telmatobius jelskii (Peters) (Anura, Leptodactylidae) de Lima, Perú. Rev Bras Zool 20:131–134
Ibrahim MM (2008) Helminth infracommunities of the maculated toad Amietophrynus regularis (Anura: Bufonidae) from Ismailia, Egypt. Dis Aquat Organ 82:19–26
Kennedy CR (1975) Ecological animal parasitology. Blackwell, Oxford
Luque JL, Martins AA, Tavares LER (2005) Community structure of metazoan parasites of the yellow Cururu toad Bufo ictericus (Anura, Bufonidae) from Rio de Janeiro, Brazil. Acta Parasitol 50:215–220
Lux Hoppe EG, Pedrassani D, Hoffmann-Inocente AC, Tebaldi JH, Storti LF, Zanuzzo FS, Avancini N, Do Nascimento AA (2008) Estudos ecológicos em taxocenoses helminthcas de Chaunus ictericus (Spix, 1824) e C. schneideri (Werner, 1894) (Anura: Bufonidae) simpátricos, capturados no Distrito de São Cristovão, municipio de Trés Barras. Santa Catarina. Rev Bras Parasitol Vet 17:166–169
Magurran AE (2004) Measuring biological diversity. Blackwell, Oxford
Manzano AS, Baldo D, Barg GM (2004) Temas de la biodiversidad del litoral fluvial argentino INSUGEO. Miscelánea 12:271–290
Marcogliese DJ, Cone DK (1997) Foof webs: a plea for parasites. Trens Ecol Evol 12:320–325
McAlpine DF (1997) Helminth communities in bullfrogs (Rana catesbeiana) green frogs (Rana clamitans), and leopard frogs (Rana pipiens) from New Brunswick, Canada. Can J Zool 75:1883–1890
Muzzall PM (1991) Helminth infracommunties of the frogs Rana catesbeiana and Rana clamitans from Turkey Marsh, Michigan. J Parasitol 77:366–371
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Pinhão R, Wunderlich AC, Anjos LA, Silva RJ (2009) Helminths of toad Rhinella icterica (Bufonidae), from the municipality of Botucatu, São Paulo, Brazil. Neotrop Helminthol 3:35–40
Poulin R (2013) Explaining variability in parasite aggregation levels among host samples. Parasitology 140:541–546. doi:10.1017/S0031182012002053
Poulin R, Morand S (2004) Parasite biodiversity. Smithsonian Institution, Washington
Prudhoe S, Bray RA (1982) Platyhelmint parasites of the amphibian. British Museum (Natural History), Oxford University Press, London
Santos VGT, Amato SB (2010) Helminth fauna of Rhinella fernandezae (Anura: Bufonidae) from the Rio Grande do Sul Coastland, Brazil: analysis of the parasite community. J Parasitol 96:823–826. doi:10.1645/GE-2388.1
Santos RR, Grant T (2011) Diel pattern of migration in a poisonous toad from Brazil and the evolution of chemical defenses in diurnal amphibians. Evol Ecol 25:249–258. doi:10.1007/s10682-010-9407-0
Santos VGT, Amato SB, Borges-Martins M (2013) Community structure of helminth parasites of the “Cururu” toad, Rhinella icterica (Anura: Bufonidae) from southern Brazil. Parasitol Res 112:1097–1103. doi:10.1007/s00436-012-3236-8
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Illinois, p 117
Smyth JD, Smyth MM (1980) Frogs as host-parasite systems. I. An introduction to parasitology through the parasites of Rana temporaria, R. esculenta and R. pipiens. Macmillan Press, London
Thul JE, Forrester DJ, Abercrombie CL (1985) Ecology of parasitic helminths of wood ducks, Aix sponsa, in the Atlantic Flyway. Proc Helminthol Soc Wash 52:297–310
Yoder HR, Coggins JR (2007) Helminth communities in five species of sympatric amphibians from three adjacent ephemeral ponds in southeastern Wisconsin. J Parasitol 93:755–760
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice-Hall Inc, Upper Saddle River
Acknowledgments
Financial support was provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina, through grant PIP 11220090100345.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamann, M.I., Kehr, A.I. & González, C.E. Helminth community structure in the Argentinean bufonid Melanophryniscus klappenbachi: importance of habitat use and season. Parasitol Res 113, 3639–3649 (2014). https://doi.org/10.1007/s00436-014-4029-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4029-z