Abstract
Leishmaniasis is one of the major health problems existing globally. The current chemotherapy for leishmaniasis presents several drawbacks like toxicity and increased resistance to existing drugs, and hence, there is a necessity to look out for the novel drug targets and new chemical entities. Current trend in drug discovery arena is the “repurposing” of old drugs for the treatment of diseases. In the present study, an antidepressant, ketanserin, was found lethal to both Leishmania donovani promastigotes and intracellular amastigotes with no apparent toxicity to the cells. Ketanserin killed promastigotes and amastigotes with an IC50 value of 37 μM and 28 μM respectively, in a dose-dependent manner. Ketanserin was found to inhibit L. donovani recombinant 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) enzyme with an IC50 value of 43 μM. Ketanserin treated promastigotes were exogenously supplemented with sterols like ergosterol and cholesterol to rescue cell death. Ergosterol could recover the inhibition partially, whereas cholesterol supplementation completely failed to rescue the inhibited parasites. Further, HMGR-overexpressing parasites were generated by transfecting Leishmania promastigotes with an episomal pspα hygroα-HMGR construct. Wild-type and HMGR overexpressors of L. donovani were used to study the effect and mode of action of this inhibitor. The HMGR overexpressors showed twofold resistance to ketanserin. These observations suggest that the lethal effect of ketanserin is due to inhibition of HMGR, the rate-limiting enzyme of the ergosterol biosynthetic pathway. Since targeting of the sterol biosynthetic pathway enzymes may be useful therapeutically, the present study may have implications in treatment of leishmaniasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is an infectious disease caused by the protozoan parasite of the genus Leishmania which manifests itself in several forms. All forms of the disease are transmitted to the human host by the bite of female sand fly of the genus Phlebotomus in the Old World and Lutzomyia in the New World. It is estimated that about half a million people die annually with visceral leishmaniasis and approximately 350 million people are at risk. The currently available drugs in the market have several limitations for the treatment of leishmaniasis due to toxicity, intravenous administration and the growing problem of drug resistance (Berman 2003). Pentavalent antimonial or SSG is no longer recommended for use as high levels of resistance in the Indian subcontinent has been reported (Sundar 2001). Other drugs like miltefosine and amphotericin B are in clinical use. Unfortunately, treatment failure cases to miltefosine (Pandey et al. 2009) and amphotericin B are emerging which raises serious concerns for their future use. Moreover, till now there is no vaccine available for treating leishmaniasis. Thus, the use of currently approved antimicrobials for use in leishmaniasis, development of new chemotherapeutic agents and identification of new drug targets are mandatory for the control of the disease.
3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase is an enzyme involved in the conversion of HMG-CoA to mevalonate, a precursor of cholesterol in humans, and ergosterol in plants, fungi and protozoa (Henriksen et al. 2006; Macreadie et al. 2006). Ergosterol is reported to be essential for growth and replication of Leishmania and fungi (Kulkarni et al. 2013). HMG-CoA reductase, the first rate-limiting enzyme of the sterol biosynthetic pathway is a potential drug target in several organisms. Statins interfere severely with the growth of protozoan parasites of the family Trypanosomatidae, such as Trypanosoma cruzi (Kessler et al. 2013), and various Leishmania species like Leishmania major, Leishmania mexicana (Ginger et al. 2001; Montalvetti et al. 2000), Leishmania donovani (Dinesh et al. 2014) and other parasites like Schistosoma mansoni (Chen et al. 1990), Plasmodium falciparum (Parquet et al. 2010) and Toxoplasma gondi (Cortez et al. 2009) by inhibiting HMGR. Recently, non-statin class of compound like resveratrol was also found to be effective against L. donovani (Dinesh et al. 2014).
Tricyclic drugs, antidepressants and antipsychotics are reported to be toxic to both promastigote and amastigote form of Leishmania (Zilberstein and Dwyer 1984). Tricyclic drugs exert its antileishmanial action by reducing proton motive force in L. donovani promastigotes, and antidepressants were reported to exert its effect by altering membrane function in Leishmania (Zilberstein et al. 1990). Recently, imipramine, a tricyclic antidepressant belonging to the class of cationic amphiphilic drugs, when administered orally was found to be active against both antimony-sensitive and antimony-resistant clinical isolates of L. donovani (Mukherjee et al. 2012). The drug acts by altering the proton motive force of parasite membrane, inhibiting trypanothione reductase and inducing the production of TNF-α for antileishmanial defence (Benson et al. 1992; Kubera et al. 2004; Zilberstein et al. 1990).
Ketanserin tartrate (herein after referred to as ketanserin) is a serotonin S2-receptor antagonist which is used as an antihypertensive agent and is also reported to inhibit HMG-CoA reductase (Suzukawa and Nakamura 1990a). It increases the release of pro-inflammatory cytokines in human bronchial epithelial cells and alveolar epithelial cells to possess anti-inflammatory properties in vivo (Lau et al. 2012). Serotonin increases the uptake of oxidized low-density lipoprotein (LDL) into macrophages which causes suppression of the immune system. Ketanserin, a serotonin antagonist, blocks the stimulatory effect of serotonin on oxidized LDL uptake (Aviram et al. 1992). It was recently proposed that the combination of ketanserin and propranolol could be a promising therapy for relieving inflammatory pain with minimal side effects (Wang et al. 2013). The antioxidant, anti-inflammatory and HMG-CoA reductase inhibitory properties of ketanserin suggested us to evaluate its efficacy in L. donovani.
In the present study, for the first time, the inhibitory effect of ketanserin on the in vitro viability of Leishmania promastigotes and amastigotes and its possible mode of action was investigated.
Materials and methods
Materials
Ketanserin tartrate 3-(2-[4-(4-Fluorobenzoyl)-1-piperidinyl] ethyl)-2,4(1H,3H)-quinazolinedione was purchased from Sigma (St. Louis, MO, USA). Ketanserin was prepared in 20 % ethanol, and the drug stocks were stored at −20 °C. pspα hygroα shuttle vector was a kind gift from Prof. R. Madhubala, School of Life Sciences, JNU, New Delhi, India. Polyclonal anti-rat HMGR antibody was a kind gift from Dr. Peter Edwards, UCLA Laboratory (Los Angeles, CA) (Garcia-Pelayo et al. 2004).
Parasite and cell culture
L. donovani wild-type (WT, MHOM/80/IN/Dd8) promastigotes were cultured at 24 °C in Rosewell Park Memorial Institute (RPMI)-1640 HEPES modified medium (Gibco/BRL, Life Technologies Scotland, UK) supplemented with 0.2 % sodium bicarbonate, 100 μg/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamycin and 10 % heat-inactivated foetal bovine serum (HI FBS) (Biological Industries). The medium was maintained at pH 7.2. THP-1 monocyte cell line was grown in RPMI-1640 medium containing 10 % HI FBS in a humidified atmosphere with 5 % CO2 at 37 °C.
Effect of ketanserin on L. donovani promastigotes
Logarithmic phase of L. donovani promastigotes were seeded in 96-well microtitre plates at a density of 2 × 105 parasites/200 μL/well. After 48 h of incubation, cells were treated with different concentrations of drug (10–100 μM) and were further kept for incubation at 24 °C for 48 h. The viability of parasites was assayed colorimetrically by MTT assay (Mosmann 1983).
Cytotoxicity effect of ketanserin
THP-1 monocyte cells were seeded at a density of 2 × 105 per well in a 96-well plate and incubated with 20 ng/mL of phorbol myristate acetate (PMA, Sigma). After 48 h of incubation, the unadhered cells were removed by washing with serum-free RPMI medium. Various concentrations of ketanserin were added (10, 20, 50 and 100 μM) to cells at 37 °C in a humidified atmosphere of 5 % CO2 for 48 h. MTT was added to each well and incubated for 4 h at 37 °C. The cells were centrifuged at 3,000g for 10 min and the supernatant was removed. The resultant purple formazan formed was dissolved in 100 μL DMSO and absorbance was read at 540 nm on a Tecan microplate reader.
In vitro screening against intracellular L. donovani amastigotes
Antileishmanial screening against intracellular L. donovani amastigotes in differentiated THP-1 monocytes was performed involving transformation and parasite rescue (Jain et al. 2012). THP-1 monocytes (2 × 105/well) were seeded on 96-well plate, and PMA (20 ng/mL) was added to differentiate THP-1 monocyte cells into macrophages. The cells were incubated at 37 °C with 5 % CO2 for 48 h for complete differentiation of the cells.
The differentiated cells were washed once with serum-free RPMI-1640 medium and were infected with L. donovani promastigotes in 1:10 ratio. The infected cells were washed with serum-free RPMI-1640 medium for complete removal of non-internalized promastigotes, and then, 200 μL of complete RPMI-1640 medium with 10 % FBS was added with different dilutions of ketanserin. Controlled lysis was performed by treating the cells with 20 μL of 0.05 % sodium dodecyl sulfate (SDS) (detergent) in RPMI-1640 medium for 30 s with shaking followed by addition of 180 μL of complete RPMI-1640 medium with 10 % FBS. The 96-well plate was incubated at 24 °C for 48 h for transformation of rescued amastigotes into promastigotes. After the 48 h incubation, MTT was performed as described earlier. The IC50 values of the treated leishmanial cells were calculated relative to the untreated control cells, and the results were expressed as the concentration of the compound inhibiting 50 % of the parasite growth. Miltefosine was used as the standard antileishmanial drug for data analysis.
Effect of ketanserin on recombinant LdHMGR
In order to express recombinant LdHMGR, Escherichia coli BL21(DE3) cells were transformed with pET30a-LdHMGR construct. Recombinant LdHMGR was then purified by nickel affinity chromatography to homogeneity as reported previously (Dinesh et al. 2014). In order to evaluate the effect of ketanserin on recombinant LdHMGR enzyme activity, purified enzyme was assayed in the presence of different concentrations of ketanserin. HMGR activity was based on the spectrophotometric measurement of the decrease in absorbance at 340 nm. The HMG-CoA-dependent oxidation of NADPH was monitored at 340 nm. Briefly, the reaction mixture contained 50 mM KH2PO4, 50 mM KCl, 5 mM DTT, 1 mM EDTA, 0.27 mM NADPH, 0.27 mM HMG-CoA and enzyme in a final volume of 200 μL at pH 7.2 (Hurtado-Guerrrero et al. 2002). Reactions were read for 300 s at 37 °C. One unit (U) of HMGR was defined as the amount of enzyme that catalyses the oxidation of 1 μmol of NADPH per min.
Rescue of L. donovani growth inhibition with sterols
To evaluate whether the inhibition of Leishmania growth is due to depletion of ergosterol levels, the drug-treated cells were exogenously supplemented with a range of ergosterol (50, 100 and 200 mmol/L) and cholesterol (50, 100 and 200 mmol/L) concentrations. The viability of the parasites upon rescue by ergosterol and cholesterol were analysed by MTT assay.
DNA constructs and transfection
The LdHMGR gene was cloned in pspα hygroα shuttle vector. LdHMGR gene was amplified by PCR using pET30a-LdHMGR as the template and forward sense primer 5′-TGCTCTAGAATGCGTCGCTCTCTGCTGCT-3′ flanked by XbaI restriction site and reverse primer 5′-CCCAAGCTTTTACTGAGTCGGAGGCTTGCG-3′ flanked by HindIII restriction site. The PCR product and vector were double-digested with XbaI and HindIII restriction enzymes and ligated. The ligation product was then transformed into E. coli DH5α cells, and colonies were screened by colony PCR. The positive clones with pspα hygroα-LdHMGR were checked by double digestion using XbaI and HindIII enzymes and further given for automated sequencing for confirmation of clones. Approximately, 10 μg of positive clone was transfected into 4 × 107 exponential-grown Leishmania promastigotes. The transfectants with hygromycin-resistance gene were selected after 14–20 days of transfection (Papadopoulou et al. 1992).
Protein immunoblotting
Protein samples were resolved on 10 % SDS-PAGE and transferred onto nitrocellulose membrane using electrophoresis transfer cell (Biorad). Anti-rat HMGR polyclonal antibody was used in 1:500 dilutions followed by anti-rabbit IgG conjugated with alkaline phosphatase as secondary antibody (Sigma, 1:10,000). The respective protein bands were visualized by incubating with nitro blue tetrazolium (NBT) and 5-bromo-4-chloro3-indolyl phosphate disodium salt (BCIP) as substrates.
Results
Effect of ketanserin on the growth of L. donovani promastigotes, amastigotes and macrophages
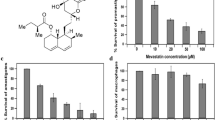
The structure of ketanserin is shown in Fig. 1. Different concentrations of ketanserin were added to L. donovani promastigotes which resulted in the retardation of parasite growth in a dose-dependent manner. The IC50 value for promastigote was 36 μM after 48 h of incubation period. Ketanserin resulted in ∼80 % inhibition of parasite growth at 100 μM concentration (Fig. 2a). Miltefosine was taken as the reference drug, and its IC50 value was 14.6 μM which correlated with the previously published results (Table 1) (Corral et al. 2014). To further evaluate its effect on amastigotes, its sensitivity was checked in an amastigote-macrophage model where ketanserin was found to kill the amastigotes in a dose-dependent manner exhibiting an IC50 value of 28 μM and 80 % inhibition at 100 μM (Fig. 2b). The amastigotes were ∼1.3-fold more sensitive to ketanserin than promastigotes. Miltefosine inhibited amastigote growth with an IC50 value of 3.4 μM which correlated with the previously reported data (Table 1) (Corral et al. 2014). Cytotoxicity of ketanserin was evaluated on THP-1 cells which were differentiated by treatment with 20 ng/mL of PMA for 48 h followed by exposure to various concentrations (10, 20, 50 and 100 μM) of drugs. Concentrations as high as 100 μM of ketanserin failed to cause any inhibition even after 48 h of drug addition. However, the standard drug killed the macrophage cells at an IC50 value of 43 μM. This data coincides with the already published results on the effect of miltefosine on THP-1 and J774A.1 cell line (Calogeropoulou et al. 2008; Dube et al. 2007).
These results showed that ketanserin displayed antileishmanial activity at noncytotoxic concentrations. The IC50 values of ketanserin and miltefosine as positive control are demonstrated in Table 1.
Effect of ketanserin on recombinant LdHMGR enzyme
LdHMGR enzyme was expressed and purified previously in our laboratory (Dinesh et al. 2014). We evaluated the effect of ketanserin on recombinant LdHMGR and found that 10 μM of ketanserin caused 20 % reduction in HMGR activity, whereas 100 μM could cause ∼90 % reduction in HMGR enzyme activity. Its IC50 value was found to be 43 ± 2.5 μM (Fig. 3). This data showed that ketanserin binds to the LdHMGR enzyme active site and inhibits its activity.
Rescue of ketanserin-mediated growth inhibition by sterols
To evaluate whether the antiproliferative effect of ketanserin was due to depletion of sterols, the drug-treated cells were exogenously supplemented with ergosterol and cholesterol. It was observed that while 200 mmol/L of ergosterol concentration could reverse the growth partially, the addition of cholesterol failed to overcome the inhibitory effect (Fig. 4). This shows that although HMGR is one of the targets for ketanserin, it may have other modes of action also. The present result where ketanserin-treated promastigotes were refractory to cholesterol supplementation coincides with our previous reports where cholesterol failed to rescue statin-induced Leishmania cell death (Dinesh et al. 2014).
Characterization of L. donovani transfectants overexpressing HMGR
Transgenic strains of L. donovani Dd8 promastigotes were created after transfection with episomal HMGR construct as discussed in the “Materials and methods”. The confirmation of overexpression was done by Western blot and estimation of HMGR enzyme activity. The crude cell lysate of wild-type and LdHMGR overexpressors were isolated as explained in the methods. When the lysate was tested for HMGR activity, overexpressors exhibited marked increase (∼1.75 fold) in the activity compared to the lysate of the wild-type promastigotes (Fig. 5a). This correlated well with the Western blot result where it showed twofold increased expression of HMGR enzyme in the overexpressing cell line. The 45 kDa band in the blot corresponds to LdHMGR (Fig. 5b, c). Both the activity and Western blot results confirm the overexpression of HMGR enzyme in L. donovani overexpressors.
Confirmation of LdHMGR overexpression in L. donovani promastigotes. a HMGR activity measured in wild-type (WT) and LdHMGR overexpressors; b Western blot analysis of wild-type (WT) and LdHMGR overexpressing parasites. Lane 1, WT crude lysate; lane 2, LdHMGR overexpressors and lane 3, rLdHMGR used as a control. c Densitometric scanning of the western blot in (b)
Next, the effect of ketanserin was evaluated on HMGR overexpressors taking wild type as control and found that the overexpressors exhibited twofold resistance to ketanserin. The IC50 of ketanserin in HMGR overexpressing strain was 75.1 ± 4.8 μM which was twofold higher than that found in wild-type promastigotes (Fig. 6). These results indicate that HMGR overexpression confers resistance to ketanserin, suggesting that the toxicity of ketanserin could be mediated primarily via sterol biosynthetic pathway HMGR enzyme.
Discussion
Leishmaniasis treatment is based on parenteral administration of highly toxic drugs including pentavalent antimonials, amphotericin B in its liposomal formulation and pentamidine (Baiocco et al. 2009; Croft et al. 2006; Murray et al. 2005; Palumbo 2009). Usage of miltefosine, an oral drug has become limited by its extremely long half-life and low therapeutic index. In view of these facts, there is an urgent need for the development of antileishmanial agents with enhanced efficacy and no cytotoxicity.
Sterol biosynthetic pathway enzymes are promising antifungal and antiprotozoan drug targets, and HMGR is one of them (Wanderley and Rodrigues 2009). Statins are specific inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase enzyme and is a blockbuster for the major pharmaceutical companies for control of cardiovascular diseases (Barrios-Gonzalez and Miranda 2010). Statins like atorvastatin was earlier reported to be an efficient inhibitor of L. donovani HMGR enzyme by altering the levels of ergosterol, the main membrane sterol (Dinesh et al. 2014). Non-statin class of compounds has also been evaluated for its efficacy as potent inhibitor of HMGR enzyme (Rozman and Monostory 2010). In the present study, we have evaluated the antileishmanial potential of ketanserin, an antidepressant. Ketanserin is a potent serotonin (5-hydroxytryptamine [5-HT2]) receptor antagonist with moderate affinity for histamine H1, α 1-adrenergic and (5-HT1c) receptors (Wenting et al. 1984). Ketanserin induces upregulation of LDL receptor activity by direct suppression of HMG-CoA reductase in human skin fibroblasts, and this may be one mechanism by which plasma LDL cholesterol is reduced by ketanserin (Suzukawa and Nakamura 1990b). General dosage of ketanserin is 20–40 mg per day to reduce the hypertension (Woittiez et al. 1986).
Several antidepressants have been earlier investigated for its antileishmanial potential. Sertraline, an antidepressant, killed L. donovani promastigotes and intracellular amastigotes at IC50 values of 2.2 and 2.3 mg/L, respectively. The drug was also effective in eliminating splenic (72 %) and liver (70 %) parasite loads in infected BALB/c mice through oral therapy (Palit and Ali 2008). Tricyclic antidepressants like amitryptyline and chlorprothixene are efficient in causing cell death of Leishmania parasites by decreasing proline transport with an IC50 value of 5 μM (Zilberstein et al. 1990). Imipramine, clomipramine and desipramine, tricyclic neuroleptics, have significant inhibitory effects on the growth of protozoan parasites like Crithidia luciliae and Trichomonas vaginalis (Hegenscheid and Presber 1990).
This work deals with ketanserin, a registered pharmaceutical drug to treat hypertension. We, for the first time, report the effect and mode of action of ketanserin on L. donovani. Ketanserin inhibited the growth of both extracellular promastigote and intracellular amastigote form of the parasite, whereas the macrophage cell line THP-1 was found to be refractory to ketanserin even till 100 μM concentration. The fact that it is parasite selective, and not host, qualifies it as potential candidate for future in vivo trials.
Since, ketanserin was earlier reported to inhibit HMGR enzyme, we incubated recombinant LdHMGR with various concentrations of ketanserin and found that it binds to the LdHMGR and inhibits with an IC50 value of 43 μM which is close to the IC50 value of 36 μM obtained from anti-promastigote assay. This indicates that ketanserin has specific affinity for the LdHMGR enzyme.
Further, to prove that ketanserin affects the sterol biosynthetic pathway, we exogenously added various concentrations of ergosterol and surprisingly found that 200 g/mol of ergosterol could partially reverse the ketanserin-mediated growth inhibition, suggesting that altering the ergosterol pool may not be the only mechanism by which it exerts its antileishmanial action. Cholesterol on the other hand, could not rescue the parasites from growth inhibition as reported in the previous studies where it failed to reverse atorvastatin-mediated growth inhibition (Dinesh et al. 2014). To further prove that one of the targets for ketanserin is HMGR, we generated HMGR-overexpressing cell lines which were confirmed by western blot analysis and specific activity. The HMGR-overexpressing cell line was found to be ∼twofold resistant to ketanserin than wild type, suggesting that ketanserin suppresses HMGR enzyme, and overexpression of this enzyme increases the antiproliferative potential of ketanserin.
Our results demonstrate that ketanserin is a potential antileishmanial compound and possibly inhibits Leishmania by targeting the HMGR enzyme though other modes of action are not ruled out. The present study demonstrated the new use of ketanserin for killing of Leishmania parasites. This finding can be referred to as “old drug but new use”. Further evaluation of ketanserin on sodium antimony gluconate (SAG) resistant parasites and its effect in in vivo model can be studied.
References
Aviram M, Fuhrman B, Maor I, Brook GJ (1992) Serotonin increases macrophage uptake of oxidized low density lipoprotein. Eur J Clin Chem Clin Biochem 30:55–61
Baiocco P, Colotti G, Franceschini S, Ilari A (2009) Molecular basis of antimony treatment in leishmaniasis. J Med Chem 52(8):2603–2612
Barrios-Gonzalez J, Miranda RU (2010) Biotechnological production and applications of statins. Appl Microbiol Biotechnol 85(4):869–883
Benson TJ, McKie JH, Garforth J, Borges A, Fairlamb AH, Douglas KT (1992) Rationally designed selective inhibitors of trypanothione reductase. Phenothiazines and related tricyclics as lead structures. Biochem J 286(Pt 1):9–11
Berman J (2003) Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 16(5):397–401
Calogeropoulou T, Angelou P, Detsi A, Fragiadaki I, Scoulica E (2008) Design and synthesis of potent antileishmanial cycloalkylidene-substituted ether phospholipid derivatives. J Med Chem 51(4):897–908
Chen GZ, Foster L, Bennett JL (1990) Antischistosomal action of mevinolin: evidence that 3-hydroxy-methylglutaryl-coenzyme a reductase activity in Schistosoma mansoni is vital for parasite survival. Naunyn Schmiedebergs Arch Pharmacol 342:477–482
Corral MJ, Gonzalez-Sanchez E, Cuquerella M, Alunda JM (2014) In vitro synergistic effect of amphotericin B and allicin on Leishmania donovani and L. infantum. Antimicrob Agents Chemother 58(3):1596–1602
Cortez E, Stumbo AC, Oliveira M, Barbosa HS, Carvalho L (2009) Statins inhibit Toxoplasma gondii multiplication in macrophages in vitro. Int J Antimicrob Agents 33(2):185–186
Croft SL, Seifert K, Yardley V (2006) Current scenario of drug development for leishmaniasis. Indian J Med Res 123(3):399–410
Dinesh N, Pallerla DS, Kaur PK, Kishore Babu N, Singh S (2014) Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb Pathog 66:14–23
Dube A, Singh N, Saxena A, Lakshmi V (2007) Antileishmanial potential of a marine sponge, Haliclona exigua (Kirkpatrick) against experimental visceral leishmaniasis. Parasitol Res 101(2):317–324
Garcia-Pelayo MC, Garcia-Peregrin E, Martinez-Cayuela M (2004) Differential translational effects of myristic acid and eicosapentaenoic acid on 3-hydroxy-3-methylglutaryl-CoA reductase from Reuber H35 hepatoma cells. Exp Biol Med (Maywood) 229(8):781–786
Ginger ML, Chance ML, Sadler IH, Goad LJ (2001) The biosynthetic incorporation of the intact leucine skeleton into sterol by the trypanosomatid Leishmania mexicana. J Biol Chem 276:11674–11682
Hegenscheid B, Presber HW (1990) Antiprotozoal effects of benzodiazepine derivatives. Angew Parasitol 31(4):231–237
Henriksen J, Rowat AC, Brief E, Hsueh YW, Thewalt JL, Zuckermann MJ, Ipsen JH (2006) Universal behavior of membranes with sterols. Biophys J 90:1639–1649
Hurtado-Guerrrero R, Pena-Diaz J, Montalvetti A, Ruiz-Perez LM, Gonzalez-Pacanowska D (2002) Kinetic properties and inhibition of Trypanosoma cruzi 3-hydroxy-3-methylglutaryl CoA reductase. FEBS Lett 510(3):141–144
Jain SK, Sahu R, Walker LA, Tekwani BL (2012) A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J Vis Exp 70:e4054
Kessler RL, Soares MJ, Probst CM, Krieger MA (2013) Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death. PLoS One 8(1):e55497
Kubera M, Kenis G, Bosmans E, Kajta M, Basta-Kaim A, Scharpe S, Budziszewska B, Maes M (2004) Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol 4(2):185–192
Kulkarni MM, Reddy N, Gude T, McGwire BS (2013) Voriconazole suppresses the growth of Leishmania species in vitro. Parasitol Res 112(5):2095–2099
Lau WK, Chan SC, Law AC, Ip MS, Mak JC (2012) The role of MAPK and Nrf2 pathways in ketanserin-elicited attenuation of cigarette smoke-induced IL-8 production in human bronchial epithelial cells. Toxicol Sci 125(2):569–577
Macreadie IG, Johnson G, Schlosser T, Macreadie PI (2006) Growth inhibition of Candida species and Aspergillus fumigatus by statins. FEMS Microbiol Lett 262(1):9–13
Montalvetti A, Pena-Diaz J, Hurtado R, Ruiz-Perez LM, Gonzalez-Pacanowska D (2000) Characterization and regulation of Leishmania major 3-hydroxy-3-methylglutaryl-CoA reductase. Biochem J 349:27–34
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Mukherjee S, Mukherjee B, Mukhopadhyay R, Naskar K, Sundar S, Dujardin JC, Das AK, Roy S (2012) Imipramine is an orally active drug against both antimony sensitive and resistant Leishmania donovani clinical isolates in experimental infection. PLoS Negl Trop Dis 6(12):e1987
Murray HW, Berman JD, Davies CR, Saravia NG (2005) Advances in leishmaniasis. Lancet 366(9496):1561–1577
Palit P, Ali N (2008) Oral therapy with sertraline, a selective serotonin reuptake inhibitor, shows activity against Leishmania donovani. J Antimicrob Chemother 61(5):1120–1124
Palumbo E (2009) Current treatment for cutaneous leishmaniasis: a review. Am J Ther 16(2):178–182
Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K (2009) Relapse of visceral leishmaniasis after miltefosine treatment in a Nepalese patient. Am J Trop Med Hyg 80(4):580–582
Papadopoulou B, Roy G, Ouellette M (1992) A novel antifolate resistance gene on the amplified H circle of Leishmania. EMBO J 11(10):3601–3618
Parquet V, Henry M, Wurtz N, Dormoi J, Briolant S, Gil M, Baret E, Amalvict R, Rogier C, Pradines B (2010) Atorvastatin as a potential anti-malarial drug: in vitro synergy in combinational therapy with quinine against Plasmodium falciparum. Malar J 9:139
Rozman D, Monostory K (2010) Perspectives of the non-statin hypolipidemic agents. Pharmacol Ther 127(1):19–40
Sundar S (2001) Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health 6(11):849–854
Suzukawa M, Nakamura H (1990a) Effect of ketanserin tartrate on HMG CoA reductase and LDL receptor activity in cultured human skin fibroblasts. Eur J Clin Pharmacol 39:217–220
Suzukawa M, Nakamura H (1990b) Effects of ketanserin tartrate on 3-hydroxy, 3-methylglutaryl coenzyme A reductase activity in cultured human skin fibroblasts. Cardiovasc Drugs Ther 4(1):69–72
Wanderley DS, Rodrigues JCF (2009) Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip Perspect Infect Dis 2009:1–19
Wang D, Zhou X, Hong Y (2013) Effects of a combination of ketanserin and propranolol on inflammatory hyperalgesia in rats. Eur J Pharmacol 721(1–3):126–132
Wenting GJ, Woittiez AJ, Man in’t Veld AJ, Schalekamp MA (1984) 5-HT, alpha-adrenoceptors, and blood pressure. Effects of ketanserin in essential hypertension and autonomic insufficiency. Hypertension 6(1):100–109
Woittiez AJ, Wenting GJ, van den Meiracker AH, Ritsema van Eck HJ, Man in’t Veld AJ, Zantvoort FA, Schalekamp MA (1986) Chronic effect of ketanserin in mild to moderate essential hypertension. Hypertension 8(2):167–173
Zilberstein D, Dwyer DM (1984) Antidepressants cause lethal disruption of membrane function in the human protozoan parasite Leishmania. Science 226:977–979
Zilberstein D, Liveanu V, Gepstein A (1990) Tricyclic drugs reduce proton motive force in Leishmania donovani promastigotes. Biochem Pharmacol 39:935–940
Acknowledgments
The authors are grateful for the financial support provided by the Ministry of Chemicals and Fertilizers, India. Special thanks to the Director of NIPER for the financial support. The authors thank Dr. Peter Edwards, UCLA Laboratory (Los Angeles, CA) for providing anti-rat HMGR antibody and Prof. R. Madhubala, School of Life Sciences, JNU, India for providing the pspα hygroα construct. Special thanks to Mr. Neerupudi Kishor Babu for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Dinesh, N., Kaur, P.K. et al. Ketanserin, an antidepressant, exerts its antileishmanial action via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) enzyme of Leishmania donovani . Parasitol Res 113, 2161–2168 (2014). https://doi.org/10.1007/s00436-014-3868-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3868-y