Abstract
Rhomboid protein in Apicomplexa was associated with the process of host cell invasion. To evaluate the potential of the protein in eliciting protective immunity against challenge, a DNA vaccine pVAX1-Rho encoding Eimeria tenella rhomboid was constructed. Recombinant protein was expressed in Hela cells and verified by indirect immunofluorescence and western blotting analysis. In vivo experiments, 1-week-old chickens were randomly divided into three groups. Experimental group of chickens were immunized with DNA vaccines while control group of chickens were injected with pVAX1 plasmid alone or sterile water. Two weeks following the booster dose, all chickens were inoculated orally with 5 × 104 sporulated oocysts of E. tenella. The host immunity and protective efficacy of this vaccine against E. tenella challenge in broilers were evaluated. Results showed that specific antibody, the levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), and the percentages of CD4+ and CD8+ T lymphocyte cells were significantly increased in the pVAX1-Rho group. Challenge experiments demonstrated that pVAX1-Rho vaccination could reduce oocyst excretion, decrease cecal lesion, increase bodyweight gains and provide chickens with oocysts decrease ratio around 75.8 %. These results suggest that the pVAX1-Rho was able to induce humoral and cellular responses and generate protective immunity against E. tenella infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eimeria species cause coccidiosis in poultry, resulting in severe economic losses. Currently, drug resistance against most anticoccidials has been observed due to long-term drug use. Moreover, public concerns increase on chemical residues in food and this makes the development of an effective vaccine strategy more urgent (Williams 1999; Allen and Fetterer 2002; Li et al. 2006; Sharman et al. 2010). Unattenuated live vaccines or attenuated live vaccines for coccidiosis control are available in the poultry industry (Irfan et al. 2008). However, these live vaccines are limited by the pathogenicity or the risk of reverting back to a pathogenic form (Sharman et al. 2010). Consequently, research efforts have been invested in the development of anticoccidial subunit or DNA vaccines as an alternative to the live vaccines. Although significant efforts have been made, there is no DNA vaccine available against Eimeria tenella. It has been a hot pursuit for a suitable candidate as target for E. tenella vaccine. Especially, proteins involved in host cell invasion, including rhomboid protein, are proposed as targets of drug- or vaccine-based control options (O’Donnell and Blackman 2005; Dowse et al. 2008; Muhammad et al. 2010). Experimental data from T. gondii have linked rhomboid protein with the invasion process. Rhomboid protease cleaved cell surface adhesins during invasion by Toxoplasma and Plasmodium (Brossier et al. 2005; Dowse and Soldati 2005; Baker et al. 2006; O’Donnell et al. 2006).
In prior works, we have cloned a rhomboid-like cDNA sequence from E. tenella (Li et al. 2006; Zheng et al. 2011). The recombinant rhomboid proteins of E. tenella expressed in E. coli, fowlpox virus, and Mycobacterium bovis BCG could elicit immune responses and provide partial protection against E. tenella challenge (Li et al. 2012; Yang et al. 2008; Wang et al. 2009).
In the present study, DNA vaccine pVAX1-Rho encoding E. tenella rhomboid gene was constructed. Specific antibody, the levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), and the percentages of CD4+ and CD8+ T lymphocyte cells were evaluated in chicken immunized with pVAX1-Rho. The protective efficacy of this DNA vaccine against E. tenella challenge was also examined.
Materials and methods
Construction of plasmid pVAX1- Rho
The open reading frame (ORF) of E. tenella rhomboid gene was amplified by PCR with genomic DNA as template, using the following primers:
-
(Forward primer):
5′-CGATATCATGTCGGACATCGAATCCCAGAGAGT-3′,
-
(Reverse primer):
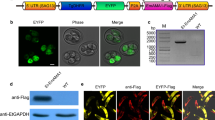
5′-CCGCTCGAGTTATGCGCATCCCATGGGCAAAGGA-3′, in which the EcoRv and XhoI restriction sites were introduced, respectively. The amplified DNA fragments were inserted into pMD18-T vector (TaKaRa, China) to form a recombinant plasmid pMD-Rho. The rhomboid ORF in pMD-Rho was subcloned into eukaryotic expression vector pVAX1 to form a plasmid pVAX1-Rho as shown in Fig. 1.
pVAX1-Rho expression in Hela cells
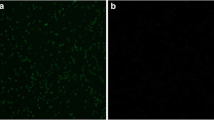
Recombinant plasmid pVAX1-Rho was transfected into Hela cells with lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were fixed with 100 % acetone for 30 min. After washing with PBS-0.1 % Triton-X-100 (PBST) for three times, the cells were blocked in 10 % bovine serum for 2 h and washed again. The cells were then incubated with mouse anti-sporozoites of E. tenella polyclonal antibody at 37 °C for 2 h. This polyclonal antibody was prepared as described previously (Zheng et al. 2011). After washing with PBST for three times, the plates were incubated with fluorescein isothiocyanate-labeled goat anti-mouse IgG antibody (1:2,000; Boster Biological Technology, Wuhan, China) at 37 °C for 2 h. After washing, fluorescences were examined under a fluorescence microscope (Carl Zeiss, Germany). Hela cells transfected with pVAX1 served as the negative control.
For western blotting, cells lysates were prepared and verified by SDS-PAGE and transferred to a polyvinylidene difluoride membrane as described previously (Yu et al. 2010). Then mouse anti-sporozoites E. tenella polyclonal antibody (1:500) and HRP-labeled goat anti-mouse IgG antibody (1:2,000) were used respectively.
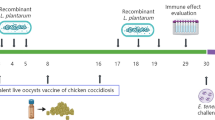
Vaccination and infection
One-day-old male Leghorn broilers obtained from a commercial breeder (Changchun, China) were reared in a coccidian-free environment. Food and water without anti-coccidia drugs were available ad libitum, and constant light was provided at night during the entire experimental period. At 7 days of age, the chickens were randomly assigned to three groups (20 birds in each group). Experimental group of chickens were intramuscularly injected with pVAX1-Rho (100 μg/each) while control group of chickens were injected with pVAX1 plasmid alone or PBS (100 μL/each) for three times at 7, 14, and 28 days of age. Two weeks after the final immunization, chickens were challenged with sporulated oocysts of E. tenella (Xinjiang strain, provided by Dr. Xun Suo, Chinese agricultural university) at a dose of 3 × 104 per bird.
Specific antibody responses
Serum samples were collected from 10 chickens randomly chosen in each group at days 7, 14, 28, and 42 and Rho-specific IgG antibody was examined by ELISA (Li et al. 2012). The optical density (OD) value was read at 490 nm in a microplate reader.
Serum levels of IL-2 and IFN-γ
Serum samples were collected randomly at days 7, 14, 28, and 42. IL-2 and IFN-γ were measured using a direct binding ELISA kit specifically designed for chicken (BlueGene Biotechnology Co., Shanghai, China) according to the manufacturer’s instructions. The results were described as picograms of IL-2 or IFN-γ per 100 μl of serum samples.
The percentages of CD4+ and CD8+ cells
Chickens selected randomly were killed at day 42 and their spleens were removed aseptically. The splenocytes were incubated with R-phycoerythrin-conjugated mouse anti-chickens CD4 antibody (0.1 mg/ml) and fluorescein-conjugated mouse anti-chickens CD8α antibody (0.5 mg/ml) (Southern Biotech Associates, Inc) for evaluation of the percentages of CD4+ and CD8+ cells (Li et al. 2012). The results were analyzed by flow cytometry.
Protective efficacy
The protective efficacy of pVAX1-Rho against E. tenella challenge was evaluated according to the cecal lesion scores, the numbers of oocysts, and bodyweight gains (BWG). Weight gain was determined by the body weight of the chickens at the end of the experiments subtracting the body weight at the time of challenge. Cecal lesion scores were determined according to the method of Johnson and Reid (1970). Oocyst counts were expressed by the number of oocysts from 1 g of the caecal content from each chicken using McMaster’s counting technique. Oocyst reduction ratio (ORR) was calculated as follows: ORR (percent) = OC − OV/OC × 100 %. OC = oocyst counts in controls, OV = oocyst counts in vaccinated birds.
Statistical analysis
Statistical analysis was performed using SPSS 14.0 software for variance and Duncan’s multiple ranges. P < 0.05 was considered statistically significant.
Results
Identification of recombinant plasmid pVAX1-Rho
The ORF fragment of E. tenella rhomboid gene was amplified by PCR and inserted into pVAX1 eukaryotic expression vector and the resultant plasmids were named pVAX1-Rho (Fig. 1).
Recombinant protein expression in Hela cells revealed by IFA and western blotting
Fluorescence could be seen in Hela cells transfected with plasmid pVAX1-Rho 48 h after transfection, but not in cells transfected with pVAX1 vector (Fig. 2). Western blotting analysis showed that Hela cells transfection with pVAX1-Rho plasmid resulted in the expression of the expected recombinant proteins (rhomboid, 28 kDa), whereas no band was detected from cells transfected with pVAX1 vector (Fig. 3).
Evaluation of specific antibody response
Two weeks after the third immunization, vaccination with plasmid pVAX1-Rho induced specific IgG antibody response. Compared with the PBS group, the differences in mean absorbance values were significant (Fig. 4).
Antibody responses induced by plasmids pVAX1-Rho. Chickens were immunized with plasmids pVAX1-Rho, while control group of chickens were injected with pVAX1 plasmid alone or PBS (100 μL/each) for three times at 7, 14, and 28 days of age. Sera were collected, diluted 1:200, and analyzed by ELISA. The asterisks represented significant increase of serum antibody when compared with those of pVAX1 or PBS control (**P < 0.01; *P < 0.05)
Evaluation of IL-2 and IFN-γ productions
The average expression level of IL-2 in pVAX1-Rho group was 271.97 ± 29.23 pg/100 μl, which was significantly higher when compared to the PBS group with 46.41 ± 18.17 pg/100 μl (Fig. 5). The average expression level of IFN-γ in pVAX1-Rho group was 316.42 ± 34.23 pg/100 μl, which was a significant increase compared to the PBS group (62.28 ± 23.17 pg/100 μl) Fig. 6.
Evaluation of the percentages of CD4+ and CD8+ lymphocytes
Two weeks after the third immunization, the percentages of CD4+ and CD8+ T lymphocytes in the pVAX1-Rho group increased significantly when compared with those in PBS group. The data was shown in Table 1.
Protective efficacy of pVAX1-Rho vaccination
The number of oocysts, BWG, cecal lesion scores, and oocyst decrease ratio were summarized in Table 2. After challenge, birds immunized with pVAX1-Rho gained bodyweight, had reduced numbers of oocysts, and had significantly decreased cecal lesion compared with chickens in the PBS group. The DNA vaccine pVAX1-Rho could provide chickens with an oocyst decrease ratio around 75.8 %.
Discussion
To evaluate the potential of E. tenella rhomboid as DNA vaccine, recombinant plasmid pVAX1-Rho was constructed and expressed in Hela cells. Compared to control group, pVAX1-Rho vaccination was able to elicit significant specific antibody and cellular immune responses. Birds in pVAX1-Rho group had reduced oocysts output, decreased cecal lesion, as well as increased bodyweight gain against E. tenella challenge.
In recent years, significant progress has been made in the identification of DNA vaccine candidates in E. tenella which could elicit a protective immune response. DNA pcDNA3-SO7 with a single SO7gene of E. tenella was the first naked DNA to be used to vaccinate chickens (Kopko et al. 2000). Since then, several genes were attempted as DNA vaccine candidates for E. tenella, i.e., E. tenella SO7 (Kopko et al. 2000; Klotz et al. 2007), E. tenella MZ5-7 (Geriletu and Xurihua 2011), E. tenella EtMIC2 (Ding et al. 2005), E. tenella TA4 (Xu et al. 2008; Song et al. 2009), E. tenella refractile body gene (Et1A; Wu et al. 2004), E. acervulina 3-1E (Song et al. 2000; Lillehoj et al. 2005; Ma et al. 2011), E. acervulina lactate dehydrogenase (LDH)(Song et al. 2010), and E. acervulina cSZ-2 (Shah et al. 2010a,b, 2011). Various eukaryotic expression vectors were used for Eimeria DNA vaccines construction, i.e., pVAX1 (Song et al. 2010; Shah et al. 2010a, 2011), pcDNA4.0 (Geriletu and Xurihua 2011), pcDNA3.1 (Wu et al. 2004; Ma et al. 2011), pcDNA3 (Kopko et al. 2000; Klotz et al. 2007), pVR1012 (Klotz et al. 2007), and pBK-CMV (Song et al. 2000). No significant differences were found concerning different vector systems for SO7 such as pcDNA3 and pVR1012 (Klotz et al. 2007). Protective efficacy of the DNA vaccines for E. tenella had been identified and could obviously alleviate cecal lesions, bodyweight loss, and reduce oocyst excretion. In the present study, a key host invasion molecule (E. tenella rhomboid) as vaccine target and pVAX1 as the eukaryotic expression vector were used. Immune mechanism and protective efficacy of this DNA vaccine pVAX1-Rho were evaluated.
Protective immunity against protozoa within the phylum Apicomplexa (e.g., Cryptosporidia, Eimeria, Neospora, Plasmodia, and Toxoplasma) is generally CD4+ T cell dependent and is elicited along the IL-12/IFN-gamma/iNOS effector axis (Brake 2002). In the present study, the percentages of CD4+ and CD8+ T lymphocytes, IFN-γ, and IL-2 cytokines were examined. The increases for the percentages of CD4+ and CD8+ T lymphocytes of the chickens immunized with pVAX1-Rho indicated that the plasmids could activate cell-mediated immunity. Cytokines synthesized and secreted by leukocytes play important regulatory roles during the immune response against infections. In avian coccidiosis, it was shown that IFN-γ is produced by the host mat sites of infection (Rothwell et al. 2000; Yun et al. 2000), and IFN-γ release has been used to screen for protective antigens against E. tenella infections (Breed et al. 1999). Co-expression of cytokines IL-2 and IFN-γ with Eimeria antigen could enhance the immune responses of DNA vaccines (Lillehoj et al. 2005; Xu et al. 2008; Song et al. 2009; Shah et al. 2010b; Shah et al. 2011). In this report, IFN-γ and IL-2 levels in the serum samples collected from vaccinated birds were significantly higher than those of the control group. However, under certain conditions, antibodies seem to be more important in protection against Eimeria infections (Constantinoiu et al. 2008). In the present study, specific IgG antibody against E. tenella was elicited in chickens immunized with recombinant plasmids. These results suggest that pVAX1-Rho was capable to elicit humoral and cell-mediated immunity in birds.
The protective efficacy of pVAX1-Rho against homologous challenge was also evaluated. As shown from the results, oocysts excretion, and cecal lesions were decreased significantly and the body weight also increased significantly in birds immunized with pVAX1-Rho compared with the control.
In summary, vaccination with an E. tenella rhomboid gene involved in host invasion process could be an alternative strategy in the development of an effective anti-E. tenella DNA vaccine. Homologues of E. tenella rhomboid protease have been identified in T. gondii, Plasmodium spp., and Cryptosporidium parvum (Baker et al. 2006; Trasarti et al. 2007; Sheiner et al. 2008), and results from the present study raises the possibility of using rhomboid protease as a vaccine candidate for apicomplexan parasites.
References
Allen PC, Fetterer RH (2002) Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol 15:58–65
Baker RP, Wijetilaka R, Urban S (2006) Two Plasmodium rhomboid proteases preferentially cleave different adhesions implicated in all invasive stages of malaria. PLoS Pathog 2:e113
Brake DA (2002) Vaccinology for control of apicomplexan parasites: a simplified language of immune programming and its use in vaccine design. Int J Parasitol 32:509–515
Breed DG, Schetters TP, Verhoeven NA, Boot-Groenink A, Dorrestein J, Vermeulen AN (1999) Vaccination against Eimeria tenella infection using a fraction of E. tenella sporozoites selected by the capacity to activate T cells. Int J Parasitol 29:1231–1240
Brossier F, Jewett TJ, Sibley LD, Urban S (2005) A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc Natl Acad Sci USA 102:4146–4151
Constantinoiu CC, Molloy JB, Jorgensen WK, Coleman GT (2008) Antibody response against endogenous stages of an attenuated strain of Eimeria tenella. Vet Parasitol 154:193–204
Ding X, Lillehoj HS, Dalloul RA, Min W, Sato T, Yasuda A, Lillehoj EP (2005) In ovo vaccination with the Eimeria tenella EtMIC2 gene induces protective immunity against coccidiosis. Vaccine 23:3733–3740
Dowse TJ, Soldati D (2005) Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parasitol 21:254–258
Dowse TJ, Koussis K, Blackman MJ, Soldati-Favre D (2008) Roles of proteases during invasion and egress by Plasmodium and Toxoplasma. Subcell Biochem 47:121–139
Geriletu XL, Xurihua LX (2011) Vaccination of chickens with DNA vaccine expressing Eimeria tenella MZ5-7 against coccidiosis. Vet Parasitol 177:1–2
Irfan AM, Akhtar M, Hussain I, Haq AU, Muhammad F, Abdul HM, Shahid MM, Bashir S (2008) Field evaluation of Eimeria tenella (local isolates) gametocytes vaccine and its comparative efficacy with imported live vaccine, LivaCox®. Parasitol Res 104:135–143
Johnson J, Reid WM (1970) Anticoccidial drug: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36
Klotz C, Gehre F, Lucius R, Pogonka T (2007) Identification of Eimeria tenella genes encoding for secretory proteins and evaluation of candidates by DNA immunisation studies in chickens. Vaccine 25:6625–6634
Kopko SH, Martin DS, Barta JR (2000) Responses of chickens to a recombinant refractile body antigen of Eimeria tenella administered using various immunizing strategies. Poult Sci 79:336–342
Li J, Zhang X, Liu Q, Yin J, Yang J (2006) Eimeria tenella: cloning of a novel Eimeria tenella cDNA encoding a protein related to rhomboid family from F2 hybrid strain. Exp Parasitol 113:215–220
Li J, Zheng J, Gong P, Zhang X (2012) Efficacy of Eimeria tenella rhomboid-like protein as a subunit vaccine in protective immunity against homologous challenge. Parasitol Res 110(3):1139–1145
Lillehoj HS, Ding X, Quiroz MA, Bevensee E, Lillehoj EP (2005) Resistance to intestinal coccidiosis following DNA immunization with the cloned 3-1E Eimeria gene plus IL-2, IL-15, and IFN-gamma. Avian Dis 49:112–117
Ma D, Ma C, Pan L, Li G, Yang J, Hong J, Cai H, Ren X (2011) Vaccination of chickens with DNA vaccine encoding Eimeria acervulina 3-1E and chicken IL-15 offers protection against homologous challenge. Exp Parasitol 127:208–214
Muhammad AA, Shah XS, Li XX, Yan R, Song H, Rui RZ, Cheng YL, Li X (2010) The DNA-induced protective immunity with chicken interferon gamma against poultry coccidiosis. Parasitol Res 107:747–750
O’Donnell RA, Blackman MJ (2005) The role of malaria merozoite proteases in red blood cell invasion. Curr Opin Microbiol 8:422–427
O’Donnell RA, Hackett F, Howell SA, Treeck M, Struck N, Krnajski Z, Withers-Martinez C, Gilberger TW, Blackman MJ (2006) Intramembrane proteolysis mediates shedding of a key adhesin during erythrocyte invasion by the malaria parasite. J Cell Biol 174:1023–1033
Rothwell L, Muir W, Kaiser P (2000) Interferon-gamma is expressed in both gut and spleen during Eimeria tenella infection. Avian Pathol 29:333–342
Shah MA, Yan R, Xu L, Song X, Li X (2010a) A recombinant DNA vaccine encoding Eimeria acervulina cSZ-2 induces immunity against experimental E. tenella infection. Vet Parasitol 169:185–189
Shah MA, Song X, Xu L, Yan R, Song H, Rui RZ, Cheng YL, Li X (2010b) The DNA-induced protective immunity with chicken interferon gamma against poultry coccidiosis. Parasitol Res 107:747–750
Shah MA, Song X, Xu L, Yan R, Li X (2011) Construction of DNA vaccines encoding Eimeria acervulina cSZ-2 with chicken IL-2 and IFN-γ and their efficacy against poultry coccidiosis. Res Vet Sci 90:72–77
Sharman PA, Smith NC, Wallach MG, Katrib M (2010) Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol 32:590–598
Sheiner L, Dowse TJ, Soldati-Favre D (2008) Identification of trafficking determinants for polytopic rhomboid proteases in Toxoplasma gondii. Traffic 9:665–677
Song KD, Lillehoj HS, Choi KD, Yun CH, Parcells MS, Huynh JT, Han JY (2000) A DNA vaccine encoding a conserved Eimeria protein induces protective immunity against live Eimeria acervulina challenge. Vaccine 19:243–252
Song X, Xu L, Yan R, Huang X, Shah MA, Li X (2009) The optimal immunization procedure of DNA vaccine pcDNA-TA4-IL-2 of Eimeria tenella and its cross-immunity to Eimeria necatrix and Eimeria acervulina. Vet Parasitol 159:30–36
Song H, Song X, Xu L, Yan R, Shah MA, Li X (2010) Changes of cytokines and IgG antibody in chickens vaccinated with DNA vaccines encoding Eimeria acervulina lactate dehydrogenase. Vet Parasitol 173:219–227
Trasarti E, Pizzi E, Pozio E, Tosini F (2007) The immunological selection of recombinant peptides from Cryptosporidium parvum reveals 14 proteins expressed at the sporozoite stage, 7 of which are conserved in other apicomplexa. Mol Biochem Parasitol 152:159–169
Wang Q, Li J, Zhang X, Liu Q, Liu C, Ma G, Cao L, Gong P, Cai Y, Zhang G (2009) Protective immunity of recombinant Mycobacterium bovis BCG expressing rhomboid gene against Eimeria tenella challenge. Vet Parasitol 160:198–203
Williams RB (1999) A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 29:1209–1229
Wu SQ, Wang M, Liu Q, Zhu YJ, Suo X, Jiang JS (2004) Construction of DNA vaccines and their induced protective immunity against experimental Eimeria tenella infection. Parasitol Res 94:332–336
Xu Q, Song X, Xu L, Yan R, Shah MA, Li X (2008) Vaccination of chickens with a chimeric DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 induces protective immunity against coccidiosis. Vet Parasitol 156:319–323
Yang G, Li J, Zhang X, Zhao Q, Liu Q, Gong P (2008) Eimeria tenella: construction of a recombinant fowlpox virus expressing rhomboid gene and its protective efficacy against homologous infection. Exp Parasitol 119:30–36
Yu Q, Li J, Zhang X, Gong P, Zhang G, Li S, Wang H (2010) Induction of immune responses in mice by a DNA vaccine encoding Cryptosporidium parvum Cp12 and Cp21 and its effect against homologous oocyst challenge. Vet Parasitol 172:1–7
Yun CH, Lillehoj HS, Zhu J, Min W (2000) Kinetic differences in intestinal and systemic interferon-gamma and antigen-specific antibodies in chickens experimentally infected with Eimeria maxima. Avian Dis 44:305–312
Zheng J, Li J, Wang Q, Xiang X, Gong P, Cao L, Cai Y, Zhang G, Zhang X (2011) Sequence analysis and verification of Eimeria tenella rhomboid bait plasmid suitability for CytoTrap yeast two-hybrid system. Parasitol Res 108:253–259
Acknowledgments
This project was supported by the National Natural Science Foundation of China (nos. 31072123 and 30500370), Program for New Century Excellent Talents in University and Jilin Provincial Department of Science and Technology (no. 20070135).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Y., Zheng, J., Li, J. et al. Protective immunity induced by a DNA vaccine encoding Eimeria tenella rhomboid against homologous challenge. Parasitol Res 112, 251–257 (2013). https://doi.org/10.1007/s00436-012-3132-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-3132-2