Abstract
The present paper describes the field evaluation of local gametocyte vaccine and its comparative efficacy with commercial anticoccidial vaccine, LivaCox®, used in breeder and broiler flocks in Pakistan. Humoral immune response in vaccinated and control chickens was monitored by enzyme-linked immunosorbent assay. Results demonstrated significantly elevated antibody titres in vaccinated groups as compared to control groups conducted both in Laboratory and field experiments. Significantly (P < 0.01) higher antibody titres in local gametocyte-vaccinated group as compared to LivaCox®-vaccinated chickens were recorded. Splenic cell migration inhibition assay was used to detect the cell-mediated immune (CMI) response, and results were expressed in terms of per cent migration index. Lower per cent migration index in LivaCox®-vaccinated chickens indicated the higher CMI response, as compared to local gametocyte-vaccinated chickens, although the difference was statistically non-significant (P > 0.05). Results of the challenge studies in laboratory experiments revealed significantly higher (P < 0.05) oocyst count in LivaCox®-vaccinated group as compared to local gametocyte-vaccinated chickens.Maximum protection (75%) against mixed species of genus Eimeria was recorded in chickens vaccinated with gametocyte vaccines as compared to LivaCox®-vaccinated group. The mean body weight gains in chickens vaccinated with local gametocyte vaccine were significantly better (P < 0.05) than in chickens vaccinated with LivaCox® vaccine, both in laboratory and field experiments. Majority of the chickens (70–72%) in control group demonstrated severe lesions (3.0–4.0), while 20–26% chickens showed moderate lesions (2.0). On the other hand, local gametocyte- and LivaCox®-immunized chickens developed 78% and 85% mild to moderated lesions (1.0–2.0), respectively. Results of the present study provide a probable explanation for cross-protection induced by Eimeria tenella gametocyte vaccines against other species of genus Eimeria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among avian diseases, coccidiosis is one of the most economically devastating diseases worldwide (Williams 1999; Shirley et al. 2004). Traditionally, the disease is controlled with chemical feed additives that can inhibit the life cycle stages of the parasites (Calnek et al. 1997). Although continuous medication has proven to be highly effective in controlling coccidiosis in modern intensive poultry production (Chapman 1999; Allen and Fetterer 2002), yet there are several disadvantages related to this strategy including withdrawal periods and development of drug resistance (Dalloul and Lillehoj 2005). Further, the possible restraints by the concerned regulatory authority on medicated feed to animals used for human consumption could also hinder controlling the disease through medication (McEvoy 2001). Thus, vaccination seems to be an effective and safe alternate to control coccidiosis.
Several commercial vaccines are being used to control coccidiosis in a number of countries of the world. There has been a general limitation to use these vaccines in broiler and high roster birds because of reduced weight gain and feed conversion ratios as compared to those prophylactically medicated chickens (Danforth 1998; Shapiro 2001; Crouch et al. 2003). Moreover, there is a risk of introducing unwanted Eimeria (E.) species into the environment as there is a regional variation in the antigenicity of coccidial strains (Fitz-Coy and Edger 1992; Martin et al. 1997). Also, certain strains of Eimeria exhibit immunological variation (Lee 1993), and the presence of such strains in the live vaccines could affect their efficacy. So a better understanding of strain variation is needed for any vaccine to give promising results against local field strains of Eimeria.
In our previous studies, gametocyte vaccine prepared from local isolates of Eimeria tenella gave protection against mixed species (mainly E. tenella, Eimeria acrervulina, Eimeria maxima and Eimeria necatrix) of coccidia in chicken in laboratory experiments (Ayaz 2003; Hafeez et al. 2006). The present paper describes the evaluation of gametocytes (E. tenella; local isolates) vaccine under field conditions and its comparative efficacy with imported live vaccine, LivaCox®.
Materials and methods
Gametocytes
Sporulated oocysts of E. tenella (local isolates) maintained in Immunoparasitology Laboratory, University of Agriculture, Faisalabad-Pakistan were used in the present study after excystation followed by ex-soprocystation to release sporozoites (Speer et al. 1973). The sporozoites were given two washings with phosphate-buffered saline (PBS), and their concentration was adjusted to 1.8 × 103 to 2 × 103 per 0.1 ml and stored at 4°C till further use.
Chicken embryos (9 days old; five batches of 150 each) procured from local hatchery were maintained at 39°C and 70% humidity in an incubator. Candling was performed to confirm the viability of the embryos. At 12 days of age, 0.1 ml suspension of sporozoites (from each isolate) was inoculated into the chicken embryos through chorio-allantoic membrane (five batches) along with penicillin and streptomycin using a sterile 25-guage needle and maintained at 39°C and 70% humidity for 5–7 days (Akhtar et al. 2002). Candling was performed daily to know the status of embryos.
On days 5–7 post-inoculation, chorio-allantoic fluid was collected from dead embryos to harvest the macro- and micro-gametocytes; gametocytes entangled with the embryo mass (urates or blood) were separated by treating with 1% trypsin at 39°C for 2 h (Hafeez 2005). Gametocytes were purified by centrifugation (14,500×g/5 min), washed twice with PBS and stored at 4°C till further use.
Gametocytes antigen
Gametocytes antigen was prepared following the method of Hafeez (2005). Briefly, gametocytes were homogenized by sonication (1 × 3 min; Nissei, Model US 330, Japan) in a jacketed vessel at 4–8°C. Supernatant collected after centrifugation (9,500×g/15 min) was used as antigen. Protein concentration was measured (Bradford 1976) and adjusted to 500 μg/0.2 ml with PBS to be used as vaccines (Hafeez et al. 2006).
Experimental and control groups were inoculated orally with vaccine and PBS, respectively. All the groups were boosted by the respective vaccine and PBS 15 days after the primary immunization.
Experimental design
The efficacy of gametocyte vaccine in comparison with LivaCox® (Biopharm, Czech Republic), an imported commercial vaccine, was evaluated in the field trials.
Experiment I
Five hundred day-old broiler chicks (at each location) were selected and purchased at three different localities for field trials. Chickens were fed with drawl feed and water ad libitum. At 5 days of age, chickens were divided into three groups (A, B and C) at each locality and were inoculated orally with local gametocyte and LivaCox® vaccines in comparison with the control at 10 days of age. All the groups were boosted by the respective vaccine on day 15 after the primary immunization.
On days 5 and 14 post-vaccination (pv), 50 chickens from each group were sacrificed; spleen and serum were collected for cellular and humoral responses, respectively. Spleen was used immediately; and sera were stored at −20°C for further use.
On the 21st day, 50 chickens from each group were sacrificed. Spleen was used immediately, and serum were separated and stored at −20°C for further use.
Experiment II
Laboratory trials were conducted at Experimental Station, Department of Veterinary Parasitology, University of Agriculture, Faisalabad-Pakistan. For this purpose, 600 day-old broiler chicks (Hubbard; three different batches) purchased from local market were raised under standard managemental conditions. At 5 days of age, chickens were divided into three groups A, B and C. Groups A and B were inoculated orally with experimental gametocyte and LivaCox® vaccines, respectively, in comparison with control group C (given PBS orally) at 10 days of age. On day 5 post-vaccination, ten chickens from each group were sacrificed, and spleen and serum were collected for cellular and humoral response.
On day 14 post-vaccination, blood and spleen were collected from ten of the chickens from each group. Spleen was used immediately; and serum was separated and stored at −20°C for further use. On the 15th day post-vaccination, the remaining chickens were given the booster dose of the respective vaccine. On day 21 post-vaccination, ten chickens from each group were sacrificed. Spleen was used immediately, and serum was separated and stored at −20°C for further use. The remaining chicken from each group were challenged with 65,000 sporulated oocysts of mixed species (mainly E. tenella, E. acrervulina, E. maxima and E. necatrix; local isolates). Faecal examination was conducted daily up to 10 days post-challenge, and number of oocyst per gram of droppings was calculated by using the McMaster counting technique (Ryley et al. 1976). Clinical symptoms and mortality in each group were recorded during the experiment. Per cent protection in each group was calculated from the survived chicken in each group (Hafeez et al. 2006). The lesions on the caeca of dead and survived chickens were enumerated (Johnson and Reid 1970) and were scored from 0 to 4.
Effect of vaccines on weight gain
Live weight gain in each group was recorded from day 1 to 21 post-vaccination, on every third day. Before each slaughtering on days 5, 14 and 21 post-vaccination, chickens were weighed.
Immunological studies
Enzyme-linked immunosorbent assay
Indirect enzyme-linked immunosorbent assay (ELISA) was performed to determine the antibody titer in vaccinated and control chickens according to the method of Smith et al. (1994) with minor modifications as described by Hafeez et al.( 2006). Briefly, microtitration plates (flat bottom, medium binding, polystyrene) coated with gametocyte antigen were washed and incubated at 37°C for 2 h with 10% fetal calf serum in washing buffer (PBS containing 0.01% Tween-20) to block any non-specific protein binding. After washing, test sera, diluted in PBS (1:10), was added and incubated at 37°C for 2 h. Plates were washed with washing buffer and probed with horseradish peroxidase-conjugated rabbit anti-chicken IgG diluted (1:400) in PBS (100 μl/well) and incubated at 37°C for 1 h. After final washing, freshly prepared orthophenylene diamine (OPD) (100 μl/well) was added and incubated at 37°C for 20 min. The reaction was stopped with 1.5 M H2SO4 (50 μl/well), and plates were read in ELISA reader (Bio Tek Elx 808) at 492 nm.
Each test serum was run in duplicate along with the positive and negative controls.
Modified splenic cell migration inhibition assay
Spleens collected at days 5, 15 and 21 pv were used in modified splenic cell migration inhibition test to detect the cellular immune response following the method of Morita et al. (1973) with modifications as described by Akhtar et al. (1999). Briefly, spleens were immersed immediately in the PBS after removal from the chicken and were labeled separately. They were minced into small pieces (0.3–0.5 mm) in a sterilized Petri-dish containing Hank’s balanced salt solution (HBSS). One medium size drop of 0.2% agarose prepared in double-distilled water was dispensed into the middle of each well of the microtitration plate (flat bottom) with the help of a single channel micropipette. One fragment of spleen was placed in each well with the help of fine sterilized forceps and pressed gently so that the splenic fragment adheres to the agar drop firmly. Fifty microlitres of fresh plasma collected from control chickens and 100 μl of HBSS was dispensed into each well of the plate. Fifty microlitres of the antigen was added in all wells of the alternative rows. Plates were covered with aluminum foil and incubated at 37°C for 24 h. Per cent migration index (μm) of each group was calculated from the mean migration distance by the following formula:
Statistical analysis
Data was analysed by complete randomized design, and means were compared by Duncan’s multiple-range test by using statistical software MINITABR.
Results and discussion
The potential of gametocyte stages of Eimeria to elicit an immune response in chickens was questioned for many years (Rose and Hesketh 1976), thought to be non-immunogenic and therefore could not provide protection to the host upon infection. In 1989, native gametocytes or their detergent extracts were found to be immunogenic in mice, rabbits and chickens (Pugatsch et al. 1989). Meanwhile, it was reported that gametogonic stages of E. maxima are highly immunogenic. Antigens isolated from gametocytes, 56 and 82 kDa, were immunogenic and possibly important to the induction of protective immunity (Wallach et al. 1989). Several other immunodominant antigens including 225, 100, 95, 65 and 50 kDa have also been identified (Mencher et al. 1989). It was further suggested by these authors that the antigens were likely glycosyated surfce proteins. It was also demonstrated that early endogenous stages are the most immunogenic, and asexual stage induced protective immunity which is important for disease prevention. Immunity induced by sexual stages does not apparently reduce disease but does limit oocyst output (Shirley 1992).
In year 2002, a commercial vaccine “CoxAbic®” based on native gametocytes of E. maxima was prepared. It is a novel vaccine based on the concept of “transmission blocking immunity” (Wallach et al. 1995; Wallach 1997, 2002). In laboratory and floor pen studies, CoxAbic® reduced oocyst shedding of the three major species of Eimeria (E. maxima, E. tenella and Eimeria acervulina) that cause coccidiosis in broiler chickens by 50–80% (Wallach 2002). In a multinational field trial involving four countries from three different continents, it was observed that chicken vaccinated with CoxAbic® performed at least as well as the coccidiostat-fed broiler controls (Michael 2002). The production of CoxAbic® is expensive, time-consuming and laborious because it relies on the affinity purification of the native gametocyte antigens from parasites isolated from their intracellular location within the intestines of chickens (Belli et al. 2004), so it does not gain the popularity worldwide. Keeping this in view, E. tenella (local isolate) sporozoites were adapted on the chorioallantoic membrane of chicken embryos and obtained uncountable number of gametocytes (Akhtar et al. 2002). In our previous studies, egg-propagated gametocyte antigen protected the broiler chickens against mixed species of genus Eimeria (Ayaz 2003; Ayaz et al. 2002, 2004). Further, efficacy of the adjuvanted and non-adjuvanted gametocytes vaccine was evaluated by immunizing chicken through oral and subcutaneous route; cellular, humoral and challenge responses were parameters to evaluate the vaccine (Hafeez et al. 2006).
The present paper describes the field evaluation of local gametocyte vaccine and its comparative efficacy with commercial vaccine, livaCocx; used in breeder flocks in Pakistan. Criteria for evaluation include the cellular and humoral responses; live weight gains; per cent mortality after challenge, number of oocysts shed in the litter and lesion scoring after challenge.
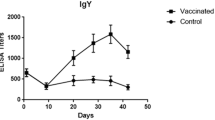
In the current study, ELISA was conducted to monitor the humoral response in vaccinated and control chickens, and results were expressed in terms of optical density (OD) values. Results demonstrated significantly elevated antibody titres (IgG) in vaccinated groups as compared to control (Tables 1 and 2), both in Laboratory and field experiments. Higher antibody titres in local gametocyte-vaccinated group as compared to LivaCox®-vaccinated chickens may be due to the nature of the antigenic material used in the vaccine. Antibody titres detected in LivaCox®-vaccinated group indicated somehow cross-antigenicity with the local isolates of E. tenella; as ELISA plates were coated with E. tenella gametocyte (local isolates) antigen. In our previous studies, higher IgA, IgG and IgM secreting splenic cells were detected by enzyme-linked immunospot assay in chicken immunized with gametocyte vaccine (Ayaz et al. 2008). In the present study, gametocytes of E. tenella used as antigen contained proteins of high molecular weights including 49.23 and 27.65 kDa along with 24.90, 22.75 and 13.20 kDa (Anwar 2008). Previously, proteins of molecular weights gam56 and gam82 kDa have been purified from the gametocytes of E. maxima. These gametocyte proteins, found in the wall forming bodies of the macrogametes (WFBs), play a crucial role in the development of Eimeria parasites. So vaccines containing gametocytes cause the production of antibodies that somehow prevent the formation of dityrosine bonds and their associated protein matrices, thereby inhibiting formation of the oocyst wall and, therefore, transmission of Eimeria parasites between chickens (Mello et al. 2006). Similar effects might also be observed against other species, including E. tenella and E. acervulina, due to the apparent conservation of gam82 and gam56 kDa protein in various species. From the results of the present study, it can be assumed that the antibodies produced in gametocyte-vaccinated chickens might interfere with the wall formation of the oocyst (Belli et al. 2006) or may limit the development of disease through inhibition of the growth, development and/or fertilization of gametes (Wallach et al. 1992; Smith et al. 1994). Further, Lillehoj and Lillehoj (2000) pointed out the potential role of Eimeria-specific antibodies to inhibit indirectly the parasite invasiveness and/or development. In the present study, splenic cell migration inhibition assay was used to detect the cell-mediated immune (CMI) response in chickens vaccinated with local gametocytes and LivaCox® vaccine in comparison with the control. Migration of sensitized splenic T cells inhibited more with antigen that is due to the fact that these sensitized T cells are re-sensitized with the test antigen in vitro. Sensitized splenic Tdth cells release interleukine-I, interleukine-2, interleukine-4 and other cytokines including migration inhibition factor, which inhibit the migration (Barrett 1988). This indicates that the test antigen triggered the T cells to initiate the cell-mediated immune response. In most parasitic infections, protection can be conferred experimentally on normal chicken by the transfer of spleen cells, especially T cells, from the immune chicken. This is because that these T cells secrete interleukine-10, which inhibits the production and activity of the interferon-gamma required to activate macrophages and eliminate the parasitic infection, probably by forming cytotoxic T cells and natural killer cells (Roitt et al. 1998). Results of the present study, both in laboratory and field experiments, demonstrated the lower per cent migration index in chickens vaccinated with LivaCox® vaccine, indicating the higher CMI response, as compared to local gametocyte-vaccinated chickens, although the difference was statistically non-significant (P > 0.05; Tables 3 and 4). The higher CMI response in LivaCox®-vaccinated chickens may be due to the fact that LivaCox® contains attenuated lines of the coccidian parasite (E. acervulina, E. maxima and E. tenella) that trigger the immune cells to induce immunity which is then maintained through continuous revaccination with oocyst present in the environment/litter (Anonymous 2002). Further, oocyst of the attenuated Eimeria lines of LivaCox® present in the litter in high numbers provides continuous stimulation of the immune system (especially T and B cells of chickens) that may lead to higher cell-mediated immune responses.
Results of the challenge studies in laboratory experiments revealed significantly higher (P < 0.05) oocyst count in LivaCox®-vaccinated group as compared to local gametocyte-vaccinated chickens (Fig. 1). In live vaccines like LivaCox®, immunity against coccidiosis usually develops within 14 days following vaccination. In commercial production, coccidiosis usually occurs in 21- to 28-day-old chickens, as coccidians need two to three developmental cycles to make sufficient number of oocysts to cause the disease. By this time, immunized chickens with live vaccines make high oocyst yield of attenuated lines of coccidian parasites including E. acervulina, E. maxima and E. tenella, due to which higher oocyst count was recorded in LivaCox®-vaccinated chickens in the present study. Although it is claimed that oocyst excreted in the litter, due to live vaccination, are desirable for keeping antigen stimulation to maintain sufficient immunity (Annonymous 2002), reduced oocyst count in gametocytes vaccinated chickens could be due to the fact that immunity induced by sexual stages limit the oocyst output (Shirley 1992). The reduced numbers of oocysts in the environment nevertheless elicit active immune response while reducing the severity of disease (Smith et al. 1994; Wallach et al. 1995).
In laboratory trials, immunized and un-immunized chickens were challenge on day 21 post-vaccination with sporulated oocysts of mixed species of genus Eimeria, mainly E. tenella, E. maxima, E. acervulina and E. necatrix. Results revealed maximum protection (74.22%) against mixed species of genus Eimeria in chickens vaccinated with gametocyte vaccines as compared to LivaCox®-vaccinated group (Fig. 2). These results are in contradiction to many other studies showing the inability of chickens to protect against hetrologous infection (Shirley 1989; Lillehoj 1998), though there are a few reports that indicate the cross-protection may exist within Eimeria species (Ayaz et al. 2008; Hafeez et al. 2006; Wallach et al. 1995). Results of the present study provide a probable explanation for cross-protection induced by E. tenella gametocyte vaccines against other species of genus Eimeria as the gametocytes of Eimerian parasites have the ability to induce cross-species protection due to the existence of conserved epitopes in different species (Wallach et al. 1995). It is worth mentioning that major protein band (49.23 kDa) observed in the E. tenella gametocytes (results not given) had almost similar size to the major band 56 kDa seen in E. maxima gametocytes (Wallach et al. 1989). It has been also demonstrated that maternal antibodies induced by infection with E. maxima are able to partially protect hatchlings against infection with E. tenella (Wallach 1997).
Significantly lower oocyst count in gametocyte-vaccinated group as compared to LivaCox®-vaccinated chickens was recorded. Higher oocyst count in LivaCox®-vaccinated chickens is due to the live oocyst of attenuated lines of Eimeria contained in the vaccine. Due to this, oocyst count in the faeces/litter is not considered an appropriate monitoring tool to ascertain the efficacy of live vaccines against coccidiosis (Anonymous 2002). Further, vaccinated chickens of both the groups were active, had normal feed and water intake and showed no typical signs of clinical coccidisosis, while chickens in control groups were dull and depressed with ruffled feather and had decreased feed and water intake.
Effect of vaccines on live body weight gains was recoded, from day 1 to 21 post-vaccination, on every third day. The mean body weight gains in chickens given with local gametocyte vaccine were significantly better (P < 0.05) than in chickens given the LivaCox® vaccine, both in laboratory and field experiments (Figs. 3 and 4). The lower weight gain in LivaCox®-vaccinated chickens may be due to the presence of three different antigens which may impose a stress on the growing birds (Bedrink and Kucera 1988). Further, it has been observed that use of live oocyst vaccines resulted in some loss of body weight gain and, consequently, reduced feed conversion (Kopko 1998) which is unacceptable in the broiler industry, limiting the use of these vaccines to replacement birds, a significantly smaller market in comparison.
To assess biological protection in laboratory experiments, lesion scores were determined in immunized and control chickens after challenge with Eimeria species. Surviving and dead chickens (during experiment after challenge) were monitored for lesion scoring using the method of Johnson and Reid (1970) on a scale of 0 to 4. In the present study, majority of the chickens (70–72%) in the control group demonstrated severe lesions (3.0–4.0), while 20–26% chickens showed moderate lesions (2.0). On the other hand, local gametocyte- and LivaCox®-immunized chickens developed 78% and 85% mild to moderated lesions (1.0–2.0), respectively. Decreased damage to the caecal mucosa in immunized chickens suggests the involvement of immune effector mechanism that may have resulted in attrition or inhibition of development of early stages of the parasite’s life cycle such as sporozoites or first-generation merogonic stage (Kopko 1998). The inability of gametocyte vaccine to decrease lesions in 100% of the vaccinated chickens may be attributed to variability in expression both among chickens and between experiments. Similarly, LivaCox® contained attenuated lines of Eimerian parasites that may have produced lesions in vaccinated chickens and unable to decrease lesions in 100% of the immunized chickens. These results demonstrated that mild to moderate lesions were produced despite immunity conferred due to LivaCox® vaccination which reflects that the coccidian parasites multiplied but may not lead to fatality to cause the disease (Conway et al. 1990, Conway et al. 1993 and Conway et al. 1999).
References
Akhtar M, Hayat CS, Ashfaque M, Hussain I, Khan MA, Ayaz S (1999) Modified splenic cells migration inhibition test for the detection of cell mediated immunity against coccidiosis in chickens. Pak J Biol Sci 2:419–421
Akhtar M, Ayaz MM, Hayat CS, Ashfaque M, Hussain I (2002) Adaptation of Eimeria tenella sporozoites (local isolate) in the embryonated hen’s eggs. Pak Vet J 22:40–41
Allen PC, Fetterer RH (2002) Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev 15:58–65
Annonymous (2002) LivaCoxÒ: live attenuated vaccines against coccidiosis in domestic poultry. Biopharm Research Institute of Biopharmacy and Veterinary Drugs. Czech Republic
Anwar MI (2008) Field trials of gametocytes vaccine (Local Isolate) and its comparative Efficacy with imported vaccine against coccidiosis in poultry. PhD thesis, University of Agriculture, Faisalabad, Pakistan
Ayaz MM (2003) Development of egg adapted vaccine (local isolate) against coccidiosis in poultry. Ph.D thesis, University of Agriculture, Faisalabad, Pakistan
Ayaz MM, Akhtar M, Hayat CS, Hussain I, Iqbal S (2002) Cellular immune response against coccidiosis in poultry through gametocytes. Proc. The 10th Intl. Congress of Parasitology Vancouver, Canada, pp 4–9
Ayaz MM, Akhtar M, Hayat S, Hussain I (2004) Control of coccidiosis in poultry through egg-adapted gametocytes. Proc. 1st Asean Congress on Parasitology and Tropical Medicine, Kuala lumpur, Malaysia, pp 23–25
Ayaz MM, Akhtar M, Hussain I, Muhammad F, Haq AU (2008) Immunoglobulin producing cells in chickens immunized with egg propagated Eimeria tenella gametocyte vaccines. Vet Med 53:210–216
Barrett JT (1988) Text book of immunology: an introduction to imunochemistry and immunobiology 5th edn. Mosby, Washington DC
Bedrink P, Kucera J (1988) Development of immunity against coccidiosis in broiler following administration of oocysts by different routes. Biologizae-a-chemizac-ivocissne vyroby Veternaria 24:507–514
Belli SI, Mai K, Skene CD, Gleeson MT, Witcomb DM, Katrib M, Finger A, Wallach G, Smith NC (2004) Characterization of antigenic and immunogenic properties of bacterially expressed, sexual stage antigens of coccidian parasite Eimeria maxima. Vaccine 22:4316–25
Belli SI, Smith NC, Ferguson DJ (2006) The coccidian oocyst: a tough nut to crack. Trends Parasitol 22:416–423
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (1997) Diseases of poultry, 10th edn. Mosby Woife, United States of America
Chapman HD (1999) Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol 28:521–535
Conway DP, Mckenzie ME, Dayton AD (1990) Relationship of coccidial lesion scores and weight gain in infections of E. acervulina, E.maxima and E. tenella in broilers. Avian Pathol 19:489–496
Conway DP, Sasai KS, Gaffar M, Smothers CD (1993) Effects of different levels of Oocyst inocula of Eimeria acervulina, Eimeria tenella and E. maxima on plasma constituents, packed cell volume, lesion scores and performance in chickens. Avian Dis 37:118–123
Conway DP, Dayton AD, Mckenzie ME (1999) Comparative testing of anticoocidials in broiler chickens; the role of coccidial lesion scores. Poult Sci 78:529–535
Crouch CF, Andrews SJ, Ward RG, Francis MJ (2003) Protective efficacy of a live attenuated anticoccidial vaccine administered to 1-day-old chickens. Avian Pathol 32:297–304
Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis 49:1–8
Danforth HD (1998) Use of live oocyst vaccines in the control of avian coccidiosis: experimental studies and field trials. Int J Parasitol 28:1099–1109
Fitz-Coy H, Edger SA (1992) Pathogenicity and control of E. mitis infection in broiler chickens. Avian Dis 36:44–48
Hafeez MA (2005) Immunogenic characterization of Eimeria tenella gametocyte antigen as vaccine against coccidiosis in poultry. PhD Thesis, University of Agriculture, Faisalabad, Pakistan
Hafeez MA, Akhtar M, Hussain I (2006) Protective effect of egg-propagated Eimeria tenella (local isolates) gametocytes as vaccine(s) against mixed species of coccidia in chickens. Parasitol Res 98:539–544
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor pen experiments with chickens. Exp Parasitol 28:30–36
Kopko SH (1998) Responses of chickens to different immunizing strategies using a refractile body antigen of Eimeria tenella. PhD thesis,University of Gulph, Canada
Lee EH (1993) Immune variants in live coccidiosis vaccines. In: Barta JR, Fernando MA (eds) Proceedings of the VIth International Coccidiosis Conference. Univeristy of Guelph, Guelph, pp 118–121
Lillehoj HS (1998) Role of T lymphocytes and cytokines in coccidiosis. Int J Parasitol 28:1071–1081
Lillehoj HS, Lillehoj E (2000) Avian coccidiosis: a review of acquired intestinal immunity and vaccination strategies. Avian Dis 44:408–4025
Martin AG, Danforth HD, Brita JR, Fernando MA (1997) Analysis of immunological cross protection and sensitivities to anticoccidial drugs among five geographical and temporal strains of E. maxima. Int J Parasitol 5:527–533
McEvoy J (2001) Safe limits for veterinary drug residues: what do they mean? Northern Ireland Veterinary Today 37–40
Mello F, Paulo S, Alvarez R, Smith N (2006) A novel approach to coccidiosis control. Int Hatchery Prac 20:7–13
Mencher D, Pugatsch T, Wallach M (1989) Antigenic proteins of Eimeria maxima: cell free translation and detection with recovered chicken serum. Exp Parasitol 68:40–48
Michael A (2002) The practical use of a maternal vaccine against coccidiosis. World Poult 18:2–4
Morita C, Tzutsyn YY, Soekawa M (1973) Migration inhibition test of splenic ells of chickens infected with E. tenella. J Parasitol 59:199–200
Pugatsch T, Mencher D, Wallach M (1989) E. maxima: isolation of gametocytes and their immunogenicity in mice, rabbits and chickens. Exp Parasitol 68:127–134
Roitt I, Brostoff J, Male D (1998) Immunology, 4th edn. Mosby, London
Rose ME, Hesketh P (1976) Immunity to coccidiosis: stages of the life cycle of Eimeria maxima, which induce and are affected by the response of the host. Parasitol 73:25–37
Ryley JF, Meade R, Ifazalburst J, Robinson TE (1976) Methods in coccidiosis research: separation of oocyst from faeces. Parasitol 73:311–326
Shapiro D (2001) Coccidiosis control in replacement pullets. Int Hatch Pract 15:13–17
Shirley MW (1989) Development of a live attenuated vaccine against coccidiosis of poultry. Parasite Immunol 11:117–124
Shirley MW (1992) Research on avian coccidia: an update. Br Vet J 148:479–499
Shirley MW, Ivens A, Gruber A, Madeira AMBN, Wan KL, Dear PH, Tomley FM (2004) The Eimeria genome projects: a sequence of events. Trends Parasitol 20:199–201
Smith NC, Wallach M, Miller CM, Morgenstern R, Braun R, Eckert J (1994) Maternal transmission of immunity to Eimeria maxima: Enzyme linked immunosorbant assay analysis of protective antibodies induced by infection. Infect Immun 62:1348–1357
Speer CA, Hammond DM, Mahrt JL, Roberts WL (1973) Structure of the oocysts and sporocysts walls and excystation of sporozoites of Isospora canis. J Parasitol 59:35–40
Wallach MG (1997) The importance of transmission-blocking immunity in the control of infection by apicompexan parasites. Int J Parasitol 27:1159–1167
Wallach M (2002) The development of CoxAbicÒ a novel vaccine against coccidiosis. World Poult 18:2–4
Wallach M, Halabi A, Pillemer G, Sar-Shalom O, Mencher D, Gilad M, Bendheim U, Danforth HD, Augustine PC (1992) Maternal imununization with gametocyte antigens as a means of providing protective immunity against Eimeria maxima in chickens. Infect Immun 60:2036–2039
Wallach M, Smith NC, Braun R, Eckert J (1995) Potential control of chicken coccidiosis by maternal immunization. Parasitol Today 11:262–265
Wallach MG, Mencher D, Yarus S, Pillmemmer G, Halabi A, Pugatsch T (1989) Eimeria maxima: Identification of gametocyte antigens. Exp Parasitol 68:49–56
Williams RB (1999) A compartmentalized model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol 29:1209–1229
Acknowledgements
The funds for this project were sponsored by Pakistan Science Foundation, Islamabad, Pakistan (project no. P-PU/Bio/347).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anwar, M.I., Akhtar, M., Hussain, I. et al. Field evaluation of Eimeria tenella (local isolates) gametocytes vaccine and its comparative efficacy with imported live vaccine, LivaCox®. Parasitol Res 104, 135–143 (2008). https://doi.org/10.1007/s00436-008-1171-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1171-5