Abstract
There is a need to advance commercial poultry production to cater to the essential protein needs of an ever-increasing population, however, the rampant occurrence of coccidiosis infection poses a threat to this achievement. This study evaluated the in vivo anticoccidial activities of the extracts and fractions of Garcinia kola against experimental Eimeria tenella infection using broiler chickens as experimental subjects. A total of 40 broiler chicks were experimentally infected with E. tenella and assigned randomly into five groups consisting of eight chicks each. Three days post experimental infection groups I and II were administered orally with tween 80 (0.8%) and Amprolium (30 mg/kg) and served as untreated and treated control groups, respectively whereas Groups III, IV, and V were administered orally with crude methanol extract (CME) at doses of 200, 400 and 600 mg/kg, respectively, for five consecutive days. Daily weight gains were recorded and faecal oocysts per gram (OPG) counts were made by the McMaster Egg counting technique. Blood samples from each experimental group were collected on days 0, 3, 6, and 8 for haematological examination. In the acute toxicity studies, the CME of G. kola did not produce any toxic effect or mortality at doses between 10 and 5000 mg/kg. The CME G. kola was then considered safe and the LD50 was assumed to be > 5000 mg/kg. Graded doses of CME of G. kola considerably (P < 0.05) improved body weight gain and decreased OPG in a dose-depended manner. There was also significant improvement in the Packed Cell Volume (PCV), Red Blood Cell (RBC) and White Blood Cell (WBC) counts upon treatment with the graded doses of CME of G. kola. Besides, G. kola significantly decreased histopathological lesions in the caecum. The results of this study indicates that G. kola may provide beneficial effects against E. tenella-induced coccidiosis in broiler chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Commercial poultry farming is expanding every day and contributing to the provision of affordable and high-quality protein. However, this sector is confronted with many diseases of which coccidiosis is among the most important (Malik et al. 2016; Shetshak et al. 2021). Coccidiosis is caused by multiple species of the protozoan parasites belonging to the genus Eimeria (Mohiti-Asli and Ghanaatparast-Rashti 2015). Caecal coccidiosis, which is caused by E. tenella, is the most important parasitic disease that requires continuous prophylactic treatment in poultry production (Malik et al. 2016; Habibi et al. 2016). The disease mainly affects young chicks and spreads from one chick to another by contact with infected droppings (Mohiti-Asli and Ghanaatparast-Rashti 2015) resulting in very high infectivity in an intensive husbandry poultry practice (Bindari et al. 2021; Gabriel et al. 2020; Geng et al. 2021; Snyder et al. 2021; Zhou et al. 2020). Infected droppings may contain sporulated oocyst of about seven (7) different species of Eimeria; E. acervulina, E. tenella, E. maxima, E. necatrix, E. brunette, E. mitis, and E. praecox, that invades the cells of the intestinal tract of chickens (Pop et al. 2015). Eimeria parasite generally causes enteritis and diarrhoea resulting in an inability of the birds to absorb dietary nutrients in the gastrointestinal tract. This occurs due to the disruption of the integrity of the intestinal mucosa (Isaac et al. 2017a, b; Qaid et al. 2021; Yan et al. 2021; Yu et al. 2021) leading to low weight gain and feed consumption (Pop et al. 2015), morbidity (Oyegbemi and Adejinmi 2012; Malik et al. 2016), poor feed conversion efficiency and in severe cases, mortality (Pop et al. 2015). In broiler chickens, the most prevalent species of Eimeria are E. tenella, E. acervulina, and E. maxima of which E. tenella is highly pathogenic causing haemorrhagic diarrhoea, a severe reduction in weight, and considerable mortality. E. maxima cause moderate pathogenicity producing lesser economic losses and mortality, whilst E. acervulina is mildly pathogenic, but it is the most common species found in chickens and causes poor feed conversion efficiency and mortality only during heavy infection (McDougald and Fitz-Coy 2008). Coccidial oocysts are easily circulated in the poultry house environment and have such a great reproductive potential, it is not easy to keep chickens free of coccidia especially under the existing intensive rearing conditions (Pop et al. 2015). It has been shown that poultry fed diet containing plant-based bioactive compounds such as green tea extracts or grape seed (proanthocyanidins) have lower small intestinal lesion scores, oocyst output, and mortality as well as improved weight gains and feed conversion ratio than in birds infected with Eimeria infections (Allen and Fetterer 2002; Jang et al. 2007; Naidoo et al. 2008; Wang et al. 2008; Wallace et al. 2010). These plant-derived products exert their anticoccidial activity possibly by protecting infected intestinal tissues from oxidative damage, thereby reducing the severity of coccidiosis (Eckert et al. 2021; Gaboriaud et al. 2021; Hansen et al. 2021; Schneiders et al. 2020). Contemporary science has acknowledged the vast array of compounds produced by plants that possess pharmacological effects and are included in the modern pharmacotherapy (Chabra et al. 2019; Daryani et al. 2015; Yarahmadi et al. 2016). Several studies have been geared towards the evaluation of the anticoccidial activities of natural products such as essential oils and extracts of plants and have been shown to be effective against several species of Eimeria in chickens (Abbas et al. 2012; Zaman et al. 2012; Lamidi et al. 2020). Among these products is artemisinin, a sesquiterpene lactone produced by aerial parts of Artemisia annua (Pop et al. 2015), berberine—a natural plant obtained from the roots of Berberis lyceum (Malik et al. 2016), extracts obtained from the leaves of Vernonia amygdalina and Azadirachta indica (Oyegbemi and Adejinmi 2012) and oregano essential oil (Mohiti-Asli and Ghanaatparast-Rashti 2015) are efficacious against Eimeria parasite in chickens. Garcinia kola Heckel belongs to the Family Clusiaceae or Gluttiferae (Mackeen et al. 2000; Panthong et al. 2007; Okoko 2009; Adesuyi et al. 2012; Abu et al. 2013; Ikpesu 2014). Garcinia kola is an angiospermae that is known in commerce as “bitter kola” (Adesuyi et al. 2012).
The seeds of G. kola are highly valued ingredients in the African traditional medicine and have been used in many herbal preparations either singly or in combination with other plants (Okunji et al. 2007; Akefe et al. 2019; Isaac et al. 2017a, b). The seeds are consumed as stimulants because on chewing, they produce a bitter astringent and resinous taste, followed by a slight sweetness (Okoko 2009; Adesuyi et al. 2012). Studies have shown that the major phytochemical constituents of this plant include a complex mixture of bioflavonoids—kolaviron (Adedara et al. 2013; Ayepola et al. 2014; Ikpesu 2014), xanthones (Mackeen et al. 2000; Ogbadoyi et al. 2011), triterpenoids (Magadula and Mwanbo 2010), and benzophenones (Okunji et al. 2007; Ikpesu 2014). Other bioactive compounds reported in the plant are tannins, saponins, alkaloids and cardiac glycosides (Adesuyi et al. 2012). The biflavonoids are the most abundant compound in G. kola while kolaflavones are the major components of the kolavirons. In addition, two new chromanols; garcinoic acid and garcinal together with tocotrienol from G. kola have been isolated (Terashima et al. 2002; Ameh et al. 2020).
Extracts of the genus G. kola have been extensively reported to exhibit diverse biological activity like antioxidant, anticarcinogenic, antigenotoxic (Mackeen et al. 2000; Terashima et al. 2002; Adedara et al. 2013), antipyretic, analgesic, and anti-inflammatory (Panthong et al. 2007; Adedara et al. 2013) effects. Studies also revealed the potent antiparasitic activity of Garcinia plant against particularly Trypanosoma brucei (Kubata et al. 2005; Ogbadoyi et al., 2011); ; . Magadula and Mwambo (2010) reported the antimalarial activity of G. kola. Similarly, Ikpesu (2014) demonstrated the restoration of inhibited anticholinesterase activities of G. kola in Clarias gariepinus. However, there are very few or no reports on the effect of G. kola on Eimeria parasites.
Materials and methods
Experimental animals
Sixty day-old broiler chicks were purchased from a reputable commercial hatchery and housed on a deep litter pen. The birds were kept at room temperature with continuous lightning. Chicks were fed with standardized anticoccidial free commercial starter feed. Water and feed were provided ad libitum. Feeders and drinkers were washed on a daily basis to reduce the risk of contamination. Faecal droppings of the chicks were screened for the coccidian parasite using a simple flotation technique as described by (Dryden et al. 2005) before the commencement of experimental infection. Ethical approval for the use of animals for the experiment was obtained from the Ahmadu Bello University Committee on Animal Use and Care (ABUCAUC) Ahmadu Bello University Zaria by submitting an application and a copy of the research proposal (ABUCAUC/2018/009).
Plant materials
Collection and identification
Fresh seeds of G. kola were purchased from Samaru Market in Zaria, Kaduna State Nigeria, and a sample of the seed was sent to the Herbarium, Department of Botany, Faculty of Life Sciences, Ahmadu Bello University Zaria, Nigeria for authentification and identification. The plant was identified and a voucher specimen number 279 was allocated for reference purpose. The husks were removed from the seeds of G. kola and the seeds were grated with stainless steel grater to reduce the particle size. This was done to increase the surface area needed for the sample to dry quickly. The grated plant material was then dried under the shade in open air under room temperature and kept in an airtight polyethene bags until required.
Extraction and concentration
The dried plant material was made pulverized using a mortar and a pestle. One (1) kg of the powdered seeds was exhaustively extracted by hot continuous extraction in a soxhlet apparatus (Redfern et al. 2014) with three liters of methanol (Sigma-Aldrich) as solvent. The liquid extract was concentrated in vacuo at 40 °C in a rotary evaporator (BUCHI Rotavapor™ R-300; Fisher Scientifica).
The crude methanol extract (CME) was serially partitioned with an equal volume of n-hexane, ethyl acetate, and butanol. The fractions were then weighed, labeled, and stored at 4 °C until required.
Phytochemical screening
The extract and fractions of G. kola were evaluated for the presence of carbohydrates, tannins, alkaloids, flavonoids, anthraquinones, steroids/triterpenes, cardiac glycocides, and saponins using standard procedures (Senguttuvan et al. 2014).
Parasite procurement
E. tenella oocysts (Houghton strain) were obtained from the Royal Veterinary College, University London, UK, and maintained in 2.5% potassium dichromate in the refrigerator in the Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria until when required for the experiment. Oocyst collection, purification, and sporulation were done using the standard procedure as described by Sathyanarayanan and Ortega (2006).
Acute toxicity study
Oral acute toxicity (LD50) of the crude methanol extract (CME) in broiler chickens was performed by the method of Lorke (1983). The animals were deprived of food for 24 h and water for 12 h. The study was carried out in two phases. In the first phase of the trial, three groups of chickens each comprising of 3 animals were administered with 10, 100, and 1000 mg/kg of the extracts orally and examined for mortality within 24 h. In the second phase of the experiment, 4 birds were randomly allocated into 4 groups of 1 chick each and were administered with 2000, 3000, 4000, and 5000 mg/kg respectively. The birds were similarly examined as in the first phase.
Experimental infection
Forty broiler chicks aged 14 days old were infected with the E. tenella oocysts. The sporulated E. tenella oocysts were concentrated by sedimentation via centrifugation (Kumar et al. 2014) and each bird was infected with 0.6 ml of water containing 10,000 oocysts orally using a graduated syringe (Pop et al. 2015).
Experimental Design
Infected chicks were randomly allocated into five groups of eight chicks each.
-
Group 1 was given 2% tween 80 (a solvent used to dissolve the extract) and it served as the untreated control.
-
Group 2 was administered with amprolium 250® (30 mg/kg) and it served as treated (Standard) control group.
-
Groups 3, 4, and 5 were treated with the CME at 200, 400, and 600 mg/kg respectively.
All treatments were administered orally and for 5 consecutive days. The effect of the extract on weight, mortality rate, and infection rate (oocysts per gram of faeces) were evaluated daily throughout the treatment period.
Experimental trial
Droppings from each treatment group were collected and screened for the presence of Eimeria oocysts using a simple floatation technique (Dryden et al. 2005). The oocysts count was determined using the McMaster counting technique as described by Zajac and Conboy (2012).
Three days post-treatment, 2 ml of blood was collected from each chick through the wing vein into a heparinised sample bottle. Birds that survived the period of the experiment were euthanatized by injecting 3 ml of air into the heart (Jaksch 1981) and were grossly examined. Sample of the caecum from a chick in each group was taken and lesions were scored. The caecal samples were then preserved in 10% formalin and processed for histopathological examination (Luna 1968).
Lesion score
The lesions on the caecum were macroscopically evaluated by using the scoring method of Johnson and Reid (1970). Grades from 0 to 4 were allocated to the lesion observed depending on its severity. The scoring was carried out as follows:
-
Score of 0 = Absence of any visible gross lesion
-
Score of 1 = Few scattered lesions
-
Score of 2 = Greater number of discrete lesions involving many areas of the caecum
-
Score of 3 = Extensively developed lesions which coalesce and thickening of the intestinal walls
-
Score of 4 = Lesions that extensively coalesce with thickening of the intestinal walls and the presence of bloody caecal content.
Oocyst decrease ratio (ODR)
Two grams of caecal content of the birds in each group were collected separately and oocysts per gram of content (OPG) was determined using the McMaster’s counting technique as described by Rose and Mockett (1983); Talebi and Mulcahy (2005); Zajac and Conboy (2012). The Oocyst decrease ratio was calculated using the formula below:
where A = mean number of oocysts in chicks treated with 2% tween 80; B = mean number of oocysts in chicks treated with the extracts/fractions.
Determination of survivability
Determination of the survivability of chicks as an indication of the anticoccidial activity of the extract of G. kola was evaluated using the “Anticoccidial Index” (ACI) as described by Zhang et al. (2014). The ACI is a measure for assessing the protective effect of a substance against coccidia and was calculated using the formula below:
The survival rate was estimated by dividing the number of chicks that survived by the number of initial chicks at the start of the experiment. The relative rate of weight gain of the chicks in each group was determined by subtracting the bodyweight of the chicks at the time of challenge with the organism from the bodyweight of the chicks at the end of the experiment. The ACI usually ranges from 0 to 200 (Jeffers 1974; Chapman 1998).
ACI value of:
-
≥ 180 will be considered high performance
-
160–179 will be considered as effective and
-
< 160 will be considered as ineffective.
Haematology
The blood samples collected during the experimental trial were analysed for haematological parameters such as packed cell volume (PCV), white blood cell (WBC), mean corpuscular haemoglobin concentration (MCHC), mean corpuscular haemoglobin (MCH), and mean corpuscular volume (MCV) using standard procedures (Jain 1986; Campbel 1995; Subhashree et al. 2012).
Statistical analysis
Data obtained from the in vitro and in vivo experimental trials were expressed as mean ± SEM and analysed using one-way analysis of variance (ANOVA), followed by Tukey post hoc test to determine significant difference between groups. Similarly, data obtained from the scoring of caecal lesions from the chicks in different groups were analysed using the Kruskal–Wallis non-parametric test. Graphpad Prism version 5.0 for Windows was used for the analyses (Graphpad software, San Diego, CA, USA). Values of P < 0.05 were considered significant.
Result
Phytochemical Screening of the Seeds of Garcinia kola
Phytochemical screening of the crude methanol extract (CME) and butanol fraction (BTF) revealed the presence of cardiac glycosides, saponins, carbohydrates, steroids/triterpenes, tannins, flavonoids, and alkaloids. The ethyl acetate fraction (EAF) contains all the metabolites except saponins whereas the hexane fraction (HXF) contains only cardiac glycosides, tannins, and steroids/triterpenes (Table 1).
Acute toxicity studies
There was neither mortality nor any observable toxic effects during the acute toxicity trial. The CME of Garcinia kola was therefore considered acutely safe in broiler chickens and the LD50 was assumed to be > 5000 mg/kg.
Effect of CME of Garcinia Kola H. in broiler chickens experimentally infected with E. tenella (Houghton Strain)
Clinical observations
The observable clinical manifestations of caecal coccidiosis observed in chickens experimentally infected with the Houghton strain of E. tenella in this study were anorexia, ruffled feathers, somnolence, weight loss/emaciation, wet bedding materials, chocolate-brown to bloody faecal droppings (pasty to watery inconsistency; Fig. 1). However, these signs were milder in the treatment groups included mild weight loss, slightly brown faecal droppings, and mild anorexia when compared with the untreated groups of birds.
Mortality rate
There was no mortality observed in all the experimental groups throughout the course of the study.
Effect on weight gain
Days 0–3 (pretreatment), Days 3–6 (3 days in treatment), and Days 6–8 (3-5 days in treatment) were used to evaluate the mean body weight gain. The mean body weight gain of the birds in all the experimental groups in the pretreatment period (Days 0–3: 17 days of age) was observed to be slightly uniform (Table 2).
The mean body weight values of UC (untreated control) group increased from pretreatment of 372.00 ± 13.44 g to 469.00 ± 18.27 g a day post-treatment (8 days post-infection) with a mean body weight gain of 97 ± 5.86 g. The mean body weight values of AMP (Amprolium) group also increased from 383.2 ± 20.46 g to 569 ± 18.21 g with a mean weight gain of 177 ± 12.99 g. Similarly, the mean body weight values of groups CME200, CME400, and CME600 (Crude Methanolic Extract in graded dosages) also increased from 388.4 ± 10.02 g to 545.6 ± 35.24 g, 382 ± 18.27 g to 540.2 ± 41.22 g, and 413.4 ± 15.18 g to 573.3 ± 16.65 g at day respective days post infection. The mean weight gains for the CME groups at 200, 400 and 600 mg/kg were 157.2 ± 31.24 g, 158.2 ± 29.7 g, and 160.4 ± 16.64 g respectively (Table 2).
The lowest mean body weight gain in this study was recorded in the UC (untreated control) group with 97 ± 5.86 g whereas the highest mean body weight recorded was in the AMP (Amprolium) group with 177 ± 12.99 g. However, the mean body weight gains recorded across the three different periods in all the groups were statistically not significant (P > 0.05).
There was a significant difference (P < 0.05) in the overall mean body weight gains obtained in the UC (untreated control) group, the AMP (Amprolium) group, and the CME groups. The overall values of mean body weight obtained in the UC (untreated control) group when computed with the overall values of mean body weight gains obtained in the CME (200, 400, and 600 mg/kg) groups were observed to be statistically not significant (P > 0.05).
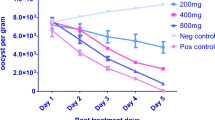
Effect on oocysts shedding
The oocyst count per grams of faeces recorded on day 6 post infections for all the groups were 7.7500 ± 0.1216 × 104(UC), 0.8540 ± 0.1104 × 104(AMP; 30 mg/kg), 6.7587 ± 0.5955 × 104(CME; 200 mg/kg), 5.1113 ± 0.1174 × 104(CME; 400 mg/kg) and 3.0947 ± 0.3898 × 104(CME; 600 mg/kg). At the 7th day post infection, the untreated birds treated and those treated with Amprolium (30 mg/kg), and CME (200, 400 and 600 mg/kg) had oocyst count of 10.7267 ± 0.5193 × 104, 0.5193 ± 0.0540 × 104, 4.0993 ± 0.4426 × 10, 3.0600 ± 0.0968 × 104 and 2.6380 ± 0.1961 respectively. Similarly, the oocyst count of birds on the 8th day post infection i.e. a day after treatment were 9.8893 ± 0.0165 × 104, 0.4380 ± 0.0 511 × 104, 2.2413 ± 0.0416 × 104, 2.0673 ± 0.1630 × 104, and 1.9513 ± 0.1681 × 104 in the untreated control group, and birds treated with Amprolium (30 mg/kg), 200, 400 and 600 mg/kg of the CME respectively (Table 3).
The untreated (UC) group of birds recorded the highest mean oocyst per gram of faeces on both day 6 and 8 post-infection with mean OPG values of 7.7500 ± 0.1216 × 104and 9.8893 ± 0.0165 × 104 respectively. Whereas the least mean oocyst per gram of faeces count on both day 6 and 8 post-infection were recorded in the AMP (Amprolium 30 mg/kg) with mean OPG count of 0.8540 ± 0.1104 × 104 and 0.4380 ± 0.0 511 × 104 respectively (Table 3).
Values of oocyst per gram (OPG) of faeces obtained in the UC group throughout the three days sampling period were statistically significant (p < 0.05) with values obtained in the rest of the groups on days 6, 7, and 8 except for the values obtained on day 6 in the group of birds treated with CME 200 mg/kg. OPG values obtained in the UC (untreated control) throughout the three days sampling period were statistically significant (p < 0.05) with values obtained in the rest of the groups on days 6, 7 and 8. OPG values obtained from the group of birds treated with CME 200 mg/kg also differ significantly with values obtained from the UC (untreated control) and AMP 30 mg/kg group of birds on days 6, 7 and 8 but only differ significantly with values obtained from birds treated with 200 and 600 mg/kg of the CME on day 7. Similarly, OPG values recorded from birds treated with CME 400 mg/kg were statistically significant (p < 0.05) when compared with the rest of the experimental groups only on day 6 and only differ significantly (p < 0.05) with values obtained from the NC and AMP 30 mg/kg groups on day 7 and 8 (Table 3).
Postmortem findings
The observable postmortem findings of caecal coccidiosis observed in chickens experimentally infected with the Houghton strain of E. tenella in this study include: ruffled feathers, emaciation, slightly pale breast muscles, ballooned and distended caecum caeceous materials, chocolate-brown to bloody caecal content (pasty to watery in consistency), and thickened blood-tinged caecal wall. Birds given 0.8% tween 80 (untreated) control group had ballooned and distended caecum with caseous content and thickened walls. The pathologies observed in birds treated with CME (200, 400, and 600 mg/kg) and Amprolium (30 mg/kg) were mild when compared to the birds in the untreated group.
Effect on caecal lesion scoring
The mean lesion score of 1.87 ± 0.34 in the untreated group of birds was recorded. The birds treated with AMP 30 mg/kg and CME (200, 400, and 600 mg/kg) were of mild pathological lesions compared to those obtained in the UC group of birds. The mean lesion scores recorded in the respective groups were 0.63 ± 0.20, 1.67 ± 0.24, 1.13 ± 0.07 and 1.03 ± 0.12 (Table 4).
The mean caecal lesion scores of birds obtained in the untreated control group was not statistically significant (p > 0.05) to those of birds in groups treated with CME at 200, 400, and 600 mg/kg. However, the mean lesion score of birds treated with amprolium differs significantly (p < 0.05) when compared with birds in the other groups (Table 4).
Effect on oocysts per gram (OPG) of caecal content and oocyst decrease ratio (ODR)
UC group recorded the highest mean oocyst per gram of caecal content with mean OPG values of 2.14413 ± 0.3714 × 105 followed by the CME groups with OPG values of 0.43440 ± 0.0097 × 105, 0.60340 ± 0.0333 × 105, and 0.96093 ± 0.0549 × 105 in the 600, 400, and 200 mg/kg doses respectively. The AMP group had the least mean oocyst per gram (OPG) of caecal content with mean OPG values of 0.37140 ± 0.0317 × 105 (Table 5). The percentage oocyst decrease of the AMP (30 mg/kg) and CME (200, 400, and 600 mg/kg) groups were 82%, 55.18%, 71.86% and 76.74% respectively (Table 4).
There was a significant difference (P < 0.05) between OPG values of UC group and the rest of the experimental groups. Similarly OPG values recorded in the CME 200 mg/kg group was significant (P < 0.05) statistically when compared with the rest of the experimental groups. OPG values of AMP group were only highly-very significant and very significant with OPG values obtained from the UC and CME 200 mg/kg groups respectively (Table 4). Similarly, OPG values recorded in the CME 400 mg/kg did not differ significantly (P > 0.05) when compared with the AMP (30 mg/kg) and the CME (600 mg/kg) groups.
Anticoccidial index (ACI)
The calculated anti-coccidial index of AMP (30 mg/kg) and the CME at 200, 400, and 600 mg/kg were 178, 150, 156, and 160 respectively The highest ACI was recorded in the AMP (Amprolium 30 mg/kg) group with 178 while the lowest ACI was recorded in the CME 200 mg/kg group with 149 (Table 5).
Haematology
Effect of CME of G. kola on packed cell volume (PCV) of birds infected with E. tenella oocysts
The mean PCV values recorded in this studies decreased from pre-infection values of 33.67 ± 3.18%, 34.67 ± 2.60%, 35.67 ± 1.86%, 33.33 ± 2.91% and 38.00 ± 0.58% to 17.67 ± 0.88%, 34.00 ± 2.08%, 27.00 ± 2.08%, 29.67 ± 1.20% and 34.33 ± 0.67% on the 8 th day post-infection (Table 6). The lowest mean PCV value in this experiment trial was recorded in the UC group (17.67 ± 0.88%) whereas the highest mean PCV value was recorded in the CME 600 group (34.33 ± 0.67%) (Table 6). There was a significant difference (P < 0.05) between the values of PCV counts in the UC group and the rest of the groups of the experimental birds at the end of the experimental trial.
Effect of CME of G. kola on white blood cells (WBC) of birds infected with E. tenella oocysts
The recorded white blood cell counts prior to experimental infection with E. tenella (Houghton strain) were within the range of 11.47 ± 0.81 × 109 /L to 14.53 ± 1.07 × 109 /L. Shortly after experimental infections, there was a continuous increase in values of WBC in all the experimental groups of birds with a significant increase recorded on day 6 post-infection (Table 6). The UC group recorded the highest mean WBC count of 22.47 ± 2.78 × 109 /L compared to the lower values of WBCs (in a range of 12.57 ± 1.75 to 12.90 ± 0.72) obtained in the AMP (30 mg/kg) and the CME (200, 400 and 600 mg/kg) groups on the 8th day post-infection (a day post-treatment). For the differential WBC (Leucocytes) count, there was lymphocytosis, Heterophilia as well as Eosinophilia. The mean Lymphocyte, mean Heterophil and mean Eosinophil counts obtained from pre-infection to the termination of the experiment are as shown in Table 6.
There was a significant difference (P < 0.05) between the values of WBC and lymphocyte counts in UC group and the rest of the groups of the experimental birds at the end of the experimental trial. While values obtained from the Heterophil as well as the Eosinophil counts were statistically the same (P0.05).
Effect of CME of G. kola on mean corpuscular haemoglobin concentration (MCHC) of birds infected with E. tenella oocysts
The mean Mean Corpuscular Haemoglobin Concentration (MCHC) values for all the experimental groups of birds recorded a slight increase from pre-infection values of 33.26 ± 0.04 g/dL, 33.26 ± 0.04 g/dL, 32.67 ± 0.57 g/dL, 33.20 ± 0.02 g/dL and 33.24 ± 0.05 g/dL to 33.21 ± 0.0604 g/dL, 33.24 ± 0.0604 g/dL, 33.21 ± 0.0704 g/dL, 33.15 ± 0.0304 g/dL and 33.19 ± 0.0704 g/dL on the 8th day post-infection( a day post treatment) (Table 6).
There was no statistically significant difference (P > 0.05) recorded between all the groups of the experimental birds.
Effect of CME of G. kola on mean corpuscular haemoglobin (MCH) in birds infected with E. tenella oocysts
The Mean Corpuscular Haemoglobin (MCH) values for the AMP (30 mg/kg) and the CME (200, 400 and 600 mg/kg) groups recorded a slight increase from pre-infection values of 19.36 ± 0.64 pg, 20.10 ± 0.38 pg, 20.12 ± 0.15 pg and 19.91 ± 0.71 pg to 22.33 ± 2.50 pg, 20.26 ± 0.36 pg, 20.48 ± 0.17 pg, and 20.44 ± 0.62 pg respectively on the 8th day post-infection (a day post-treatment; Table 6). Whereas the UC group (Table 6) had a decrease in MCH value from pre-infection value of 20.24 ± 0.04 pg to 18.93 ± 0.43 pg on the 8th day post infection (Table 6). The lowest mean MCH value in this experiment trial was recorded in the UC group (18.93 ± 0.43 pg) whereas the highest mean MCH value was recorded in the AMP group (22.33 ± 2.50 pg) (Table 6).
There was no significant difference (P > 0.05) in the values of MCH recorded between all the groups of the experimental birds.
Effect of CME of G. kola on mean corpuscular volume (MCV) of birds infected with E. tenella oocysts
The mean Mean Corpuscular Volume (MCV) values for the AMP and CME (200, 400 and 600 mg/kg) groups recorded a slight increase from pre-infection values of 59.23 ± 0.95 fg, 60.60 ± 1.17 fg, 60.60 ± 0.49 fg and 59.90 ± 2.23 fg to 67.18 ± 7.52 fg, 61.01 ± 1.21 fg, 61.77 ± 0.54 fg, and 62.41 ± 5.40 fg respectively on the 8th day post -infection (a day post treatment) (Table 6). Whereas the UCgroup (Table 6) had a decrease in MCV value from pre-infection value of 60.84 ± 0.07 fg to 57.02 ± 1.40 fg on the 8 th day post infection (Table 6).
The lowest mean MCV value recorded was in the UC group (57.02 ± 1.40 fg) whereas the highest mean MCV value was recorded in the AMP group(67.18 ± 7.52 fg) (Table 6).
There was no significant difference (P > 0.05) in the values of MCV recorded between all the groups of the experimental birds.
Histopathology
Sections of the caeca of the broiler chicks in the untreated control group had multiple invasion of the caecal epithelium by different developmental stages (schizonts, merozoites, microgametes, and macrogametes) of E. tenella. The epithelial surfaces had haemorrhages as well as submucosal oedema. Additionally, there were various necrotic lesions with surrounding diffuse infiltration of inflammatory cells (lymphocytes, plasma cells, eosinophils, and heterophils) (Fig. 2a).
Photomicrograph of the cecum of a broiler chick experimentally infected with Eiemeria tenella and treated with a 0.8% tween 80, b Amprolium (30 mg/kg), c Crude methanol extract (200 mg/kg), d Crude methanolic extract (400 mg/kg), e Crude methanol extract (600 mg/kg). Note the developmental stages of Eimeria tenella (arrows), necrotic tissue (N), and diffused mononuclear cells infiltration (C). H and E × 200
In the group treated with amprolium (30 mg/kg), there was mild infiltration of inflammatory cells (lymphocytes, heterophils, and eosinophils). More so, few numbers of developmental stages of E. tenella when compared with findings from the untreated control group were observed (Fig. 2b).
Caeca sections of the broiler chicks treated with CME (200 mg/kg) also had multiple invasion of the caecal epithelium by different developmental stages of E. tenella and diffuse infiltration of inflammatory cells (lymphocytes, plasma cells, eosinophils, and heterophils) (Fig. 2c).
The caecal sections of the chicks in the group treated with CME (400 mg/kg) had a moderate invasion of the epithelial cells by different developmental stages of E. tenella (Houghton strain) and moderate inflammatory cells infiltration (Fig. 2d).
In the group treated with CME (600 mg/kg), there were few numbers of developmental stages of E. tenella with mild infiltration of inflammatory cells (lymphocytes, heterophils, and eosinophils) when compared with the untreated control group (Fig. 2e).
Summarily, in all cases, the intestinal changes observed were associated with the intralesional presence of various developmental stages of E. tenalla with all the described elements fluctuating greatly between experimental groups (Fig. 2a–e). There was a significant reduction in the number of parasites seen as the concentration of the G. kola extract increases.
Discussion
Garcinia kola is well known for its antiprotozoal, antioxidant, antidiabetic antigenotoxic, marked inhibition of gastrointestinal motility, protection against castor oil-induced diarrhoea and hepatoprotective activities (Kerboeuf et al. 2008; Ferreira et al. 2010; Farombi and Owoeye 2011) but less is known about its activity against Eimeria spp.
All birds infected with the Houghton strain of E. tenella showed classical signs of caecal coccidiosis such as droopiness, loss of appetite/weight, and chocolate-brown to bloody faecal droppings. This indicates that the Houghton strain of E. tenella was pathogenic to the experimental broiler chicks. There was however no mortality recorded in the course of the experiment which could be due to the low challenge dose of the parasite used for this experiment. It could as well be due to the low production of chymotrypsin in the younger birds used in the present work, a proteolytic enzyme that contributes immensely to the excystation of sporozoites for epithelial invasion and hence the mild infection observed. Similar observations were made by other workers on E. tenella (Duffy et al. 2005; Song et al. 2009; Gharekhani et al. 2014).
All the birds infected with E. tenella had some levels of weight loss with the least and highest being observed in the group treated with amprolium (standard drug) and the untreated control group respectively. The observed decrease in weight could be associated with the changes in the intestinal villi as a result of damage caused by the invasion and the multiplication of the parasite, thus impairing normal absorption of nutrients (Shaban 2012). The weight gain observed in all the birds treated with the CME of G. kola was similar to that observed in the group treated with amprolium. This could be attributed to the fact that both amprolium and the plant extract might have altered the microenvironment of the host digestive tract that is necessary to stimulate the excystation of the oocyst resulting in the release of sporozoites that invade and destroy cells in the intestinal mucosa (Dalloul and Lillehoj 2005; Jatau et al. 2014).
It was observed that birds treated with CME and amprolium had significantly (p < 0.05) lower oocysts/gram of faeces and shedding of the Eimeria oocyst when compared to birds in the group administered with 0.8% tween 80 (untreated control) at 6 days post-infection. Artemisinin, a sesquiterpene lactone derivative of Artemesia annua, and the extracts of the fruits/leaves of Azadirachta indica and Vernonia amygdalina were shown to have a similar effect on oocysts of E. tenella. This was attributed to the protective effects of these plant materials on the intestinal mucosa against the Eimeria organism (Abbas et al. 2006; Oyegbemi et al., 2012; Pop et al. 2015). Similarly, findings from this study on oocyst decrease ratio (ODR) correlates with that of faecal oocysts shedding in the treated birds.
The postmortem findings observed in the present study were relatively mild and correlates with the observed gross lesion scores as well as the histopathological findings. The findings of the present study were in agreement with the study of Jatau et al. (2014) and this was possibly attributed to the mild nature of the infection. The lesions and the histopathological lesions with the number of parasites observed at histological examination correlate also with the macroscopic lesion score and the oocysts counts for all birds (Pop et al. 2015). The lesion observed in all the birds could be associated with the damages caused by the replicating effect of developmental stages of the Eimeria parasites which might have possibly resulted in inflammatory reactions as well as secondary bacterial infection (Jatau et al. 2014). Perhaps this is due to the observed cellular infiltration.
The PCV values obtained in this study significantly (p < 0.05) decreased in the untreated group on the 8th day postinfection with only a slight decrease in PVC in the groups treated with the extract of G. kola and amprolium. These findings also correlate with the slightly pale breast muscles, chocolate-brown to bloody caecal content (pasty to watery in consistency), thickened blood-tinged caecal wall observed at postmortem. All these alterations observed could be a result of the damages to the gastrointestinal tract caused by the offending parasites leading to loss of blood and fluids in faeces (Irizaary-Rovira 2004; Ogbe et al. 2010).
Three days post-infection, leucocytosis was seen in all the birds. This is observed for up to 6 days post-infection. However, birds in the treated groups showed a decrease in the levels of white blood cells when compared to birds in the untreated control group. This is indicative that peripheral blood leucocytes were likely mobilized in response to the invasive activities of the offending organism (E. tenella) in the gastrointestinal tract (Rose et al. 1979; Ricklefs and Sheldon 2007).
The differential WBC counts in this study, showed mild lymphocytosis, heterophilia as well as eosinophilia in all the birds infected with E.tenalla. The mild lymphocytosis observed was consistent in birds treated with CME but it was more pronounced on the 6th day of infection. Values of lymphocytes count increased continuously in the untreated group up till day 8 of the experiment (end of the experiment). This is contrary to the observable decrease in the WBC counts obtained in the group treated with amprolium and CME. The increase in lymphocyte count may be attributed to their response to the inflammatory reactions caused by E. tenella in the caecum (Irizaary-Rovira 2004). Although the mechanism of action of the anti-inflammatory activity of Garcinia plants as reported by Panthong et al. (2007) is not quite clear, the reduction in lymphocyte counts seen in the group of birds treated with G. kola extract may be related to its possible inhibitory effect on inflammation.
Heterophil count in birds normally increases with acute or chronic inflammatory disease conditions (Irizaary-Rovira 2004) as is the case in this study. They contain basophilic granules that contribute to the first line of defense against bacterial infections. This might be the possible reason relating to the high heterophil count recorded in the infected/untreated group on the 8th day post-infection.
Eosinophilia in birds rarely occurs but may be associated with parasitism as is the case in this study. This was noticed on the 3rd day of experimental infection and the eosinophil values decreased upon treatment with the experimental plant extract and amprolium but remain consistently high in the untreated control group.
The Mean Corpuscular Haemoglobin Concentration (MCHC), Mean Corpuscular Haemoglobin (MCH), and Mean Corpuscular Volume (MCV) values obtained in this study showed slight alterations from the pre-infection values. The MCHC values recorded across all the experimental groups showed a slight observable decrease from the values obtained a day prior to experimental infection. This could possibly be due to the low-grade infection with E.tenella as well as the Packed Cell Volume values recorded in this study.
The MCH values, on the other hand, increased slightly on the 8th day post-infection from pre-infection values in the groups of birds treated with amprolium and G. kola extract. On the contrary, the values of MCH obtained in the untreated group decreased from those obtained before the experimental infection. Values of MCV in the untreated group decreased considerably compared to the slight increase recorded in the group of birds treated with amprolium and CME.
Flavonoids such kolaviron (a biflavonoid of G. kola) are well known for their antioxidant effect due to their redox properties. Some flavonoids act on host-parasite interactions, and others disturb the development or metabolism of protozoan parasites (Fotie 2008; Kerboeuf et al. 2008; Akefe et al. 2020). This could likely be the explanation regarding the increased weight gain, reduced rate of oocysts shedding, high oocyst decrease ratio, and the reduction in both gross and histopathological lesions, which also correlates with the haematological findings of this study.
The Anticoccidial Index (ACI), which is a measure for assessing the protective effect of a substance. It was used here to demonstrate the overall anticoccidial activity of CME of G. kola. In this study, birds treated with CME (600 mg/kg) had an ACI of 160 which compares very well with the group treated with amprolium (ACI = 178). It is possible from the study that higher doses of the extract could have an improved aniticoccidial effect by increasing the ACI values.
Conclusions
The present study demonstrated the anticoccidial potential of the crude methanol extract (CME) of Garcinia kola in broilers experimentally infected with E. tenella. The administration of graded doses of Garcinia kola in broiler chicks infected with E. tenella improved the body weight gain, haematological parameters, anticoccidial index, and in addition, decreased the rate of oocysts shedding, and histological lesions. This study therefore highlights the beneficial effects of administering G. kola in the treatment and control of E. tenella-associated coccidiosis infection in chickens. This is the first report on the anticoccidial activities of G. kola, however, further investigation to identify the active principle responsible for the anticoccidial activities of G. kola is required.
References
Abbas RZ, Colwell DD, Gilleard J, J. (2012) Botanicals: an alternative approach for the control of avian coccidiosis. World’s Poult Sci J 68(2):203–215
Abbas RZ, Iqbal Z, Akhtar MS, Khan MN, Jabbar A, Sandhu Z (2006) Anticoccidial screening of Azadirachta Indica (neem) in broilers. Pharmacologyonline 3:356–371
Abu AH, Amuta PO, Buba E, Inusa TR (2013) Evaluation of antispermatogenic effect of Garcinia kola seed extracts in Albino rats. Asian Pac J Reprod 2(1):15–18
Adedara IA, Vaithinathan S, Jubendradass R, Mathur PP, Farombi EO (2013) Kolaviron prevents Carbendazim—induced steroidogenic dysfunction and apoptosis in testes of rats. Environ Toxicol Pharmacol 35:444–453
Adesuyi AO, Eumm IK, Adaramola FB, Nwokocha AGM (2012) Nutritional and Phytochemical screening of Garcinia Kola. Adv J Food Sci Technol 4(1):9–14
Akefe IO, Ayo JO, Sinkalu VO (2020) Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS ONE 15(7):e0236251. https://doi.org/10.1371/journal.pone.0236251
Akefe IO, Yusuf IL, Adegoke VA (2019) C-glycosyl flavonoid orientin alleviates learning and memory impairment by radiofrequency electromagnetic radiation in mice via improving antioxidant defence mechanism. Asian Pac J Trop Biomed 9:518–523
Allen PC, Fetterer RH (2002) Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev 15:58–65
Ameh MP, Mohammed M, Ofemile YP, Mohammed MG, Gabriel A, Isaac AO (2020) Detoxifying action of aqueous extracts of Mucuna pruriens seed and Mimosa pudica root against venoms of Naja nigricollis and Bitis arietans. Recent Pat Biotechnol 14(2):134–144. https://doi.org/10.2174/1872208313666191025110019
Ayepola OR, Cerf ME, Brooks NL, Oguntibeju OO (2014) Kolaviron, a biflavonoid complex of Garcinia Kola seeds modulates apoptosis by suppressing oxidative stress and inflammation in diabetes – induced nephrotoxic rats. Phytomedicine 12:1785–1793
Bindari YR, Kheravii SK, Morton CL, Wu SB, Walkden-Brown SW, Gerber PF (2021) Molecular detection of Eimeria species and Clostridium perfringens in poultry dust and pooled excreta of commercial broiler chicken flocks differing in productive performance. Vet Parasitol. https://doi.org/10.1016/j.vetpar.2021.109361
Campbel TW (1995) Avian Haematology and Cytology, 2nd edn. Iowa State University Press, Ames, IA, USA, pp 179–180
Chabra A, Rahimi-Esboei B, Habibi E, Monadi T, Azadbakht M, Elmi T, Valian HK, Akhtari J, Fakhar M, Naghshvar F (2019) Effects of some natural products from fungal and herbal sources on Giardia lamblia in vivo. Parasitology 146(9):1188–1198. https://doi.org/10.1017/S0031182019000325
Chapman H (1998) Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int J Parasitol 28:1141–1144
Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis 49(1):1–8
Daryani A, Ebrahimzadeh MA, Sharif M, Ahmadpour E, Edalatian S, Esboei BR, Sarvi S (2015) Anti-toxoplasma activities of methanolic extract of Sambucus nigra (Caprifoliaceae) fruits and leaves. Rev Biol Trop 63(1):07–12
Dryden MW, Payne PA, Ridley R, Smith V (2005) Comparison of common faecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther 6(1):15–28
Duffy CF, Mathis GF, Power RF (2005) Effects of NatustatTM supplementation on performance, feed efficiency and intestinal lesion scores in broiler chickens challenged with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Vet Parasitol 130(3–4):185–190
Eckert J, Carrisosa M, Hauck R (2021) Network meta-analysis comparing the effectiveness of anticoccidial drugs and anticoccidial vaccination in broiler chickens. Veterinary Parasitol. https://doi.org/10.1016/j.vetpar.2021.109387
Farombi EO, Owoeye O (2011) Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health 8:2533–2555
Ferreira JFS, Luthria DL, Sasaki T, Heyerick A (2010) Flavonoids from Artemisia Annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 15:3135–3170
Fotie J (2008) The antiprotozoan potential of flavonoids. Pharmacol Rev 2:6–19
Gaboriaud P, Sadrin G, Guitton E, Fort G, Niepceron A, Lallier N, Rossignol C, Larcher T, Sausset A, Guabiraba R, Silvestre A, Lacroix-Lamandé S, Schouler C, Laurent F, Bussière FI (2021) The absence of gut microbiota alters the development of the apicomplexan parasite Eimeria tenella. Front Cell Infect Microbiol 10:632556. https://doi.org/10.3389/fcimb.2020.632556
Gabriel A, Mohammed M, Magaji MG, Ofemile YP, Matthew AP, Akefe IO (2020) In vitro and in vivo neutralizing activity of uvaria chamae leaves fractions on the venom of Naja nigricollis in Albino Rat and Bovine Blood. Recent Pat Biotechnol 14(4):295–311. https://doi.org/10.2174/1872208314666200903152129
Geng T, Ye C, Lei Z, Shen B, Fang R, Hu M, Zhao J, Zhou Y (2021) Prevalence of Eimeria parasites in the Hubei and Henan provinces of China. Parasitol Res 120(2):655–663. https://doi.org/10.1007/s00436-020-07010-w
Gharekhani J, Sadeghi-Dehkordi Z, Bahrami M (2014) Prevalence of coccidiosis in broiler chicken farms in Western Iran. J. Vet. Med. 2014:4
Habibi H, Firouzi S, Nili H, Razavi M, Asadi SL, Daneshi S (2016) Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J Parasit Dis 40(2):401–407. https://doi.org/10.1007/s12639-014-0517-4
Hansen VL, Kahl S, Proszkowiec-Weglarz M, Jiménez SC, Vaessen S, Schreier LL, Jenkins MC, Russell B, Miska KB (2021) The effects of tributyrin supplementation on weight gain and intestinal gene expression in broiler chickens during Eimeria maxima-induced coccidiosis. Poultry Sci. https://doi.org/10.1016/j.psj.2021.01.007
Ikpesu TO (2014) Therapeutic potential of Garcinia Kolawith reference to the restoration of inhibited acetylcholinestrase activities in induced Clarias gariepinus. Beni – Suef University. J Basic Appl Sci 3:203–300
Irizaary-Rovira AR (2004) Avian and reptilian clinical pathology (Avian hematology and biochemical analysis). Vet Clin Path Secrets 1:282–313
Isaac A, Ibrahim Y, Andrew A, Edward D, Solomon A (2017a) The cortisol steroid levels as a determinant of health status in animals. J Proteomics Bioinform 10:277–283. https://doi.org/10.4172/jpb.1000452
Isaac OA, Joseph OA, Victor OS (2017b) Mitigative Effects of Anitoxidants in Noise Stress. J Clin Nutr Diet 3:21. https://doi.org/10.4172/2472-1921.100056
Jain NC (1986) Haematologic techniques. Schalm’s veterinary hematology, 4th ed. Lea & Febiger, Philadelphia, PA, USA, pp 20–86
Jaksch W (1981) Euthanasia of day-old male chicks in the poultry industry. Int J Study Anim Probl 2(4):203–213
Jang SI, Jun MH, Lillehoj HS, Dalloul RA, Kong IK, Min WG (2007) Antococcidial effect of green tea based diets against Eimeria maxima. J Vet Parasitol 144:172–175
Jatau ID, Odika AN, Thlama M, Talba AM, Bisalla M, Musa IW (2014) Response of 2 breeds of broiler chicks to experimental infection with low dose of Eimeria tenella sporulated oocysts. Turk J Vet Ani Sci 38:398–404
Jeffers T (1974) Eimeria acervulina and E. maxima: incidence and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis 1:331–342
Johnson J, Reid WM (1970) Anti coccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28(1):30–36
Kerboeuf D, Riou M, Guégnard F (2008) Flavonoids and related compounds in parasitic disease control. Mini Rev Med Chem 8:116–128
Kubata BK, Nagamune K, Murakami N, Merkel P, Kabututu Z, Martin SK, Kalulu TM, Mustakuk H, Yoshida M, Ohnishi-Kameyama M, Kinoshita T, Duszenko M, Urade Y (2005) Kola acuminate proanthocyanidins: a class of antitrypanosomal compounds effective against Trypanosome brucei. Int. J. Parasitol. 35(91):103
Kumar S, Garga R, Moftahb A, Clark EL, Macdonaldc SE, Chaudhry AS, Sparagano O, Banerjeea PS, Kundua K, Tomleyc FM, Blake DP (2014) An optimised protocol for molecular identification of Eimeria from chickens. Vet Parasitol 199:24–31
Lamidi IY, Hudu MG, Akefe IO, Adamu S, Salihu SI (2020) Sub-chronic administration of lavonoid fraction Dalon improve lead-induced alterations in delta-aminolevulinic acid dehydratase activity, erythrocytic parameters, and erythrocyte osmotic fragility in Wistar rats. Comp Clin Pathol . https://doi.org/10.1007/s00580-020-03144-6
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54:275–287
Luna LG (1968) Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd edn. McGraw-Hill Book Company, New York
Mackeen MM, Ali AM, Lajis NH, Kawazu K, Hassan Z, Amran M, Habsah M, Mooi LY, Mohamed SM (2000) Antimicrobial, antioxidant, antitumour – promoting and cytotoxic activities of different plant part extract of Garcina atroviridis Griff. Ex T Anders J Ethnopharmacol 72:395–402
Magadula JJ, Mwanbo ZH (2010). Garcinia plant Series of African Origin. Ethnobotanical, pharmacology and phytochemical studies. Open Science publishers, New York, p 62
Malik TA, Kamili AN, Chishti MZ, Tanveers S, Ahad S, Johri RK (2016) Synergistic approach for treatment of chicken coccidiosis using berberine – a plant natural product. Microb Pathog 93:56–62
Mansoori M, Modirsanei M (2012) Effects of dietary Tannic acid and vaccination on the course of coccidiosis in experimentally challenged broiler chicken. J Vet Parasitol 187:119–122
McDougald LR, Fitz-Coy HS (2008) Coccidiosis. In: Saif YM (ed) Diseases of poultry, 12th edn. Blackwell Publishing, Ames, pp 1068–1085
Mohiti-Asli M, Ghanaatparast-Rashti M (2015) Dietary Oregano essential oil alleviates experimentally induced coccidiosis in broilers. J Prev Vet Med 120:195–202
Naidoo V, Mcgaw LJ, Bisschop SPR, Duncan N, Eloff JN (2008) The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. J Vet Parasitol 153:214–219
Ogbadoyi EO, Kabiru AY, Omotosho RF (2011) Preliminary studies of the antitrypanosomal activity of Garcina Kola nut extracts in mice infected with Trypanosoma brucei. J Med Med Sci 2(1):628–631
Ogbe AO, Atawodi SE, Abdu PA, Oguntayo BO, Dus N (2010) Oral treatment of Eimeria tenella infected broilers using aqueous extract of wild mushroom (Ganoderma Sp): Effect on haematological parameters and histopathology lesions. Afr J Biotechnol 9:8923–8927
Okoko T (2009) In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. J Food Chem Toxicol 47:2620–2623
Okunji C, Komarnytsky S, Fear G, Poulev A, Ribnicky DM, Awachie PI, Ito Y, Raskin I (2007) preparative isolation and identification of Tyrosinase inhibitors from the seed of Garcina Kola by high speed counter—current chromatography. J Chromatogr A 1151:45–50
Oyegbemi T, Adejinmi JO (2012) Supplementation of broiler feed with leaves of Vernonia amygdalina and azadirachta indica protected birds naturally infected with Eimeria species. Afr J Biotechnol 11(33):8407–8413
Panthong A, Norkaew P, Kanjanapothi D, Taesotikul T, Anantachoke N, Reutrakul V (2007) Anti-inflammatory, analgesic and antipyretic activities of the extract of gamboge from Garcinia hanburyi Hoof f. J Ethopharmacol 111:335–340
Pop L, Gyorke A, Tabaran AF, Dumitrache MO, Kalmar Z, Mircean V, Zagon D, Balea A, Cozma V (2015) Effects of artemisinin in broiler chickens challenged with Eimeria acervulina, E. maxima and tenella in battery trials. J Vet Parasitol 214:264–271
Qaid MM, Al-Mufarrej SI, Azzam MM, Al-Garadi MA, Albaadani HH, Alhidary IA, Aljumaah RS (2021) Anti-Coccidial Effect of Rumex Nervosus Leaf Powder on Broiler Chickens Infected with Eimeria Tenella Oocyst. Anim: Open Access J MDPI 11(1):167. https://doi.org/10.3390/ani11010167
Rates SMK (2001) Plants as source of drugs. Toxicon 39:603–613
Redfern J, Kinninmonth M, Burdass D, Verran J (2014) Using soxhlet ethanol extraction to produce and test plant material (essential oils) for their antimicrobial properties. J Microbiol Biol Educ 1:45–46
Ricklefs RE, Sheldon KS (2007) Malaria prevalence and White Blood Cell response to infection in a tropical and in a temperate thrush: The American Ornithologists’ Union. Auk 124:1254–1266
Rose ME, Mockett AP (1983) Antibodies to coccidia: detection by the enzyme-linked immunosorbent assay (ELISA). Parasite Immunol 5:479–489
Rose ME, Hesketh P, Ogilvie BM (1979) Peripheral blood leukocyte response to coccidial infection: a comparison of the response in rats and chickens and its correlation with resistance to reinfection. Immunology 36:71–79
Sathyanarayanan L, Ortega Y (2006) Effects of temperature and different food matrices on cyclospora cayetanensis oocyst sporulation. J Parasitol 92(2):218–222
Schneiders GH, Foutz JC, Fuller AL, Nelson J, Rekaya R, Aggrey SE (2020) The effect of increased temperatures on viability, morphology, infectivity, and development of Eimeria tenella. J Parasitol 106(3):428–437. https://doi.org/10.1645/19-17
Senguttuvan J, Paulsamy S, Karthika K (2014) Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata L for in vitro antioxidant activities. Asian Pac J Trop Biomed 4(1):359–367
Shaban KS (2012) Exacerbating effect of Newcastle disease virus (NDV) infection on subclinical caecal coccidiosis in broilers vaccinated against Newcastle disease virus (NDV). Res 4:55–59
Shetshak MA, Jatau ID, Suleiman MM, Ameh MP, Ada G, Akefe I (2021) In Vitro Anticoccidial Activities of the Extract and Fractions of Garcinia kola (Heckel h) against Eimeria tenella Oocyst. Recent Patents Biotechnol. https://doi.org/10.2174/1872208315666210129095213
Snyder RP, Guerin MT, Hargis BM, Page G, Barta JR (2021) Monitoring coccidia in commercial broiler chicken flocks in Ontario: comparing oocyst cycling patterns in flocks using anticoccidial medications or live vaccination. Poult Sci 100(1):110–118. https://doi.org/10.1016/j.psj.2020.09.072
Song X, Xu L, Yan R, Huang X, Shah MAA, Li X (2009) The optimal immunization procedure of DNA vaccine pcDNATA4- IL-2 of Eimeria tenella, its cross immunity to Eimeria necatrix and Eimeria acervulina. Vet Parasitol 159:30–36
Subhashree AR, Parameaswari PJ, Shanthi B, Revathy C, Parijatham BO (2012) The reference intervals for the haematological parameters in healthy adult population of Chennai Southern India. J Clin Diagn Res 6(10):1675–1680
Talebi A, Mulcahy G (2005) Partial protection against Eimeria acervulina and Eimeria tenella induced by synthetic peptide vaccine. Exp Parasitol 110:342–348
Terashima K, Takaya Y, Niwa M (2002) Powerful antioxidative agents based on Garcinoic acid from Gracinia Kola. J Bioorg Med Chem 10:1619–1625
Wallace RJ, Oleszek W, Franz C, Hahn I, Baser KHC, Mathe A, Teichmann K (2010) Dietary plant bioactives for poultry health and productivity. Br Poult Sci 51:461–487
Wang ML, Suo X, Gu JH, Zhang WW, Fang Q, Wang X (2008) Influence of grape seed Proanthocyanidin extract I broiler chickens: effect on chicken coccidiosis and antioxidant status. J Poult Sci 87:2273–2280
Yan X, Han W, Liu X, Suo X (2021) Exogenous nitric oxide stimulates early egress of Eimeria tenella sporozoites from primary chicken kidney cells in vitro. L’oxyde nitrique exogène stimule in vitro la sortie précoce des sporozoïtes d’Eimeria tenella des cellules primaires de rein de poulet. Parasite (Paris, France) 28:11. https://doi.org/10.1051/parasite/2021007.
Yarahmadi M, Fakhar M, Ebrahimzadeh MA, Chabra A, Rahimi-Esboei B (2016) The anti-giardial effectiveness of fungal and commercial chitosan against Giardia intestinalis cysts in vitro. J Parasit Dis 40(1):75–80. https://doi.org/10.1007/s12639-014-0449-z
Yu Y, Dong H, Zhao Q, Zhu S, Liang S, Wang Q, Wang H, Yu S, Huang B, Han H (2021) Molecular characterization and analysis of the ATPase ASNA1 homolog gene of Eimeria tenella in a drug sensitive strain and drug resistant strains. Int J Parasitol Drugs Drug Resist 15:115–125. https://doi.org/10.1016/j.ijpddr.2021.02.005
Zajac AZ, Conboy GA (2012) Veterinary clinical parasitology, 8th Ed. Wiley, pp 8–11.
Zaman MA, Iqbal Z, Abbas RZ, Khan MN (2012) Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitol 139:237–243
Zhang Z, Huang J, Li M, Sui Y, Wang S, Liu L, Xu L, Yan R, Song X, Li X (2014) Identification and Molecular Characterization of Microneme 5 of Eimeria acervulina. PLoS ONE 9(12):115411
Zhou BH, Jia LS, Wei SS, Ding HY, Yang JY, Wang HW (2020) Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult Sci 99(3):1297–1305. https://doi.org/10.1016/j.psj.2019.10.073
Acknowledgements
Authors are grateful to Mr. Denis Otie and Abdulwahab Hashimu of Veterinary Pharmacology and Toxicology Laboratory, Ahmadu Bello University Zaria for their technical inputs in the accomplishment of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shetshak, M.A., Suleiman, M.M., Jatau, I.D. et al. Anticoccidial efficacy of Garcinia kola (Heckel H.) against experimental Eimeria tenella infection in chicks. J Parasit Dis 45, 1034–1048 (2021). https://doi.org/10.1007/s12639-021-01389-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-021-01389-8