Abstract
Nineteen (91%) of 21 rufous-and-white wrens (Thryothorus rufalbus) and five (71%) of seven plain wrens (Cantorchilus modestus) sampled from Costa Rica were positive for a new species of Isospora. Oocysts have a thin, smooth, double, colorless wall and measure 20.1 ± 1.4 × 23.4 ± 1.5 μm (18–24 × 20–26 μm) with an average length–width ratio of 1.2 μm. Sporocysts are ovoid, measure 9.5 ± 0.9 × 15.5 ± 1.1 μm (7–12 × 12–18 μm) with an average length–width ratio of 1.6 μm. A nipple-like steida body continuous with the sporocyst wall and a prominent oval-shaped substeida body are present. In addition to the four sporozoites, a single compact sporocyst residuum was present in each sporocyst. This is the first description of an Isospora species from the family Troglodytidae and the first report of Isospora from the rufous-and-white wren and plain wren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wrens (Aves: Passeriformes: Troglodytidae) include over 60 species and are found throughout the New World with only a single species reported in Eurasia and Northern Africa (Stiles and Skutch 1989). The rufous-and-white wren (Thryothorus rufalbus) is found throughout Central America and portions of North and South America from southwestern Mexico to Northern Columbia and northwestern Venezuela. In Costa Rica, the rufous-and-white wren is common in the northwestern Pacific lowlands at elevations up to 1,100 m. The plain wren (Cantorchilus modestus) is found throughout Central America and Mexico. In Costa Rica, the plain wren is found along the Pacific slope extending into the Central Valley at elevations up to 2,000 m and in lowland areas along the Caribbean slope. At one time, these two geographically isolated populations were considered to be separate species, but the populations are now considered to be one species or potentially two subspecies (Stiles and Skutch 1989).
Many species of coccidia have been reported and described in Passeriformes including one species of Isospora in the white-throated magpie jay (Calocitta formosa) from Costa Rica (Svobodova 1994; McQuistion 2000; Upton et al. 1995). However, to date, few coccidia have been reported from Troglodytidae with only two unnamed Isospora sp. reported from a winter wren (Troglodytes troglodytes) (Rysavy 1954; Svobodova 1994) and a northern house wren (Troglodytes aedon) (Boughton et al. 1938). In this report, we describe a new species of coccidia from two species of wren in Costa Rica.
Material and methods
From July 2005–September 2007, birds were captured via mist nets in the San Luis Valley, approximately 7 km southwest of the Monteverde region in Northwestern Costa Rica (10°16′57.117″ N, 84°47′53.747″ W). This valley is on the Pacific, leeward side of the volcanic Cordillera de Tilarán mountain range, and lies at 1,100 m elevation. Birds were captured primarily from two habitats, shade-grown coffee plantations and secondary tropical, pre-montane forest fragments. Captured birds were held in individual paper bags for a maximum of 30 min, and if a fecal sample was expelled, the feces were placed in 2.5% potassium dichromate and held at room temperature. Feces were examined using an Olympus CH30 microscope at ×100–1,000, oocysts and sporocysts were measured using an ocular micrometer, and measurements are given in micrometers followed by standard deviation. Photos were taken using a Zeiss Axioplan 2 microscope at ×400 using Nomarski direct inference contrast.
Results
Nineteen (91%) of the 21 rufous-and-white wrens were positive for Isospora. Positive birds were found in both of the sampling habitats whereby 11 (85%) of the 13 birds from the forest and 8 (100%) of the birds from the coffee plantation were positive respectively. Five (71%) of the seven plain wrens were positive for Isospora. Positive birds were only found in the coffee plantation, where five (83%) of six birds were positive; however, only one bird was examined from the forested sites.
Description
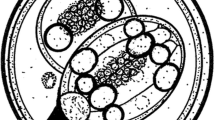
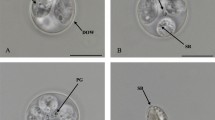
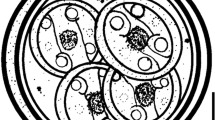
Isospora troglodytes n. sp. (Figs. 1 and 2)
Sporulated oocysts characteristic of Isospora with each oocyst containing two sporocysts that contain four sporozoites. Oocysts (n = 53) are ovoid, 20.1 + 1.4 × 23.4 + 1.5 (18–24 × 20–26) with an average length–width ratio of 1.2. Oocyst wall is double-layered, smooth, and colorless. Oocyst residuum and micropyle absent but single circular to ovoid polar body (2–3 μm) present. Sporocysts (n = 53) ovoid, 9.5 + 0.9 × 15.5 + 1.1 (7–12 × 12–18) with an average length–width ratio of 1.6. A nipple-like steida body continuous with the sporocyst wall and a prominent oval-shaped substeida body present. The sporocysts posses a single compact sporocyst residuum with pitted surface. Sporozoites are smooth with single terminal nuclei typically observed surrounding the compact sporocyst residuum. Centered refractile body was present.
Taxonomic summary
Type host: Thryophilus rufalbus (Lafresnaye, 1845), rufous-and-white wren
Other host: Cantorchilus modestus (Cabanis, 1861), plain wren
Type locality: San Luis, Costa Rica (9°51′0″ N, 84°27′0″ W)
Location in host: Unknown
Sporulation: Exogenous. Before 2 weeks at 25°C.
Type specimens: Photosyntype and a composite drawing of sporulated oocysts submitted to the US National Parasite Collection (USNPC), Beltsville, Maryland, USNPC no. 104839.
Etymology: The name is derived from the species in which the parasite was detected; T. rufalbus and C. modestus which are both species in the family Troglodytidae.
Discussion
This is the first description of an Isospora species from the family Troglodytidae and the first report of Isospora from the rufous-and-white and plain wrens. Although only partially described, the Isospora sp. type 3 reported from the winter wren (Svobodova 1994) can be differentiated from I. troglodytes n. sp. by multiple features. The oocysts of Isospora sp. type 3 are slightly larger (21.5 × 24.0) and subspherical with an average length–width ratio of 1.1 compared to the oocysts of I. troglodytes n. sp. which measure 20.1 × 23.4 and are ovoid with an average length–width ratio of 1.2 (Svobodova 1994). The sporocyst residuum of Isospora sp. type 3 is described as diffuse, compared to the single compact sporocyst residuum observed in I. troglodytes n. sp. (Svobodova 1994). Additionally, the Isospora sp. type 3 has also only been reported in the winter wren, which has a near cosmopolitan distribution throughout Europe, Asia, and North America and is the only species of Troglodytidae outside of the New World, while the rufous-and-white wren and plain wren are restricted to Latin America (Rysavy 1954; Svobodova 1994).
Prevalence rates have not been reported in the three previous reports of Isospora from the Troglodytidae (Boughton et al. 1938; Rysavy 1954; Svobodova 1994), but this information is available for other studies of passerines in Costa Rica and South America (McQuistion 2000; Upton et al. 1995). McQuistion (2000) compared prevalences of coccidian parasites between passerine host species with different behaviors and diets in South America. Ground-feeding and forest-dwelling birds had higher coccidian prevalences (23%) compared to forest border (13%) and canopy (9%)-feeding species, and insectivorous species (23–29%) had higher prevalences than frugivores (1%) (McQuistion 2000). Higher prevalences in ground-feeding passerine species have also been reported in Europe (Zinke et al. 2004). Species in the family Troglodytidae have diverse diets and behaviors, but the diets of both the rufous-and-white wren and plain wren consist of insects, spiders, and invertebrates, and both species forage along the ground or on lower branches (Stiles and Skutch 1989). The prevalence rates we observed in both species of wren were higher than that reported for passerines with similar diets (23–29%) in South America and white-throated magpie jay in Costa Rica (50%) but consistent with passerines sampled in autumn in Europe (McQuistion 2000; Upton et al. 1995; Zinke et al. 2004).
References
Boughton DC, Boughton RB, Volk J (1938) Avian hosts of the genus Isospora (Coccidiida). Oh J Sci 38:149–163
McQuistion TE (2000) The prevalence of coccidian parasites in passerine birds from South America. Trans Ill Acad Sci 93:221–227
Rysavy B (1954) Prispevek k poznani kokcidii nasich i dovezenych obratlovcu. Ceskoslovenska Parasitol 1:131–174
Stiles FG, Skutch AF (1989) A guide to the birds of Costa Rica. Cornell University Press, New York
Svobodova M (1994) Isospora, Caryospora and Eimeria (Apicomplexa: Eimeriidae) in passeriform birds from Czech Republic. Acta Protozool 33:101–108
Upton SJ, Langen TA, Wright TF (1995) A new species of Isospora Schneider, 1881 (Apicomplexa: Eimeriidae) from the white-throated magpie jay Calocitta formosa (Passeriformes: Corvidae) from Costa Rica. Syst Parasitol 31:195–199
Zinke A, Schnebel B, Dierschke V, Ryll M (2004) Prevalence and intensity of excretion of coccidial oocysts in migrating passerines on Helgoland. J Ornithol 145:74–78
Acknowledgments
We would like to acknowledge the property owners in the San Luis community who allowed sampling and Adan Fuentes, our field assistant and community liaison. The authors would like to thank the UGA San Luis Research Station for providing lodging and logistical support. A portion of this project was funded by a National Science Foundation Graduate Student Fellowship to SMH. We also thank S. Dougan (UGA) for providing the microscope used to obtain photomicrographs of the oocysts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keeler, S.P., Yabsley, M.J., Fox, J.M. et al. Isospora troglodytes n. sp. (Apicomplexa: Eimeriidae), a new coccidian species from wrens of Costa Rica. Parasitol Res 110, 1723–1725 (2012). https://doi.org/10.1007/s00436-011-2691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2691-y