Abstract
A new species of Acroeimeria Paperna & Landsberg, 1989 is described from the spotted house gecko, Gekko monarchus (Schlegel) from Peninsular Malaysia. Oöcysts of Acroeimeria grismeri n. sp. are spheroidal to subspheroidal with a smooth bi-layered wall, measure on average 18.4 × 17.3 µm, and have a length/width (L/W) ratio of 1.1; a micropyle and an oöcyst residuum are absent but variable polar granule(s) are present, commonly in Brownian movement. Sporocysts are ellipsoidal and measure on average 8.6 × 6.7 µm, L/W 1.3; Stieda, sub-Stieda and para-Stieda bodies are absent. The sporocyst residuum is composed of numerous spheroidal granules in the center of the sporocyst. This is the initial species of coccidian reported from G. monarchus and one of the few reported from any reptile from Peninsular Malaysia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spotted house gecko, Gekko monarchus (Schlegel) is a medium-sized saurian that occurs in disturbed habitats and human habitations of southern Thailand, Peninsular Malaysia, Taiwan, the Philippines, Singapore, the Kei Islands, Indonesia, and Papua New Guinea (Grismer & Quah, 2019); it has also been accidentally imported into Africa (Bauer, 2004). This gecko is insectivorous, feeding on various types of arthropods (spiders, crickets, flies, roaches, termites, and wasps).
A great deal of information is available on eimeriid coccidians from lizards of the family Gekkonidae (Gray) (see most recent summation by El-Toukhy et al., 2013); however, none have been reported from G. monarchus and only three species have been reported from lizards from Malaysia (Else & Colley, 1975; Maupin et al., 1998). Here, we provide a description of a new species of Acroeimeria Paperna & Landsberg, 1989, from G. monarchus from Peninsular Malaysia.
Materials and methods
During August 2019, 3 (2 male, 1 female) adult (60–95 mm snout-vent length, SVL) G. monarchus were collected by hand on buildings at and around an abandoned country club at Frasers Hill, Peninsular Malaysia. Fresh faecal specimens were preserved in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7). Samples were examined for coccidia using an Olympus BX43 microscope with Nomarski DIC after flotation in Sheather’s sugar solution (specific gravity = 1.30). Measurements were taken on 20 sporulated oöcysts using a calibrated ocular micrometer and Lumenera Infinity Analyze software and reported in micrometres (µm) with the ranges followed by the means in parentheses; photographs were taken using brightfield optics. Oöcysts were c.120 days-old when measured and photographed. Descriptions of oöcysts and sporocysts follow the standard guidelines of Wilber et al. (1998) including: oöcyst length (L) and width (W), their ranges and ratios (L/W), micropyle (M), oöcyst residuum (OR), polar granule(s) (PG), sporocyst length (L) and width (W), their ranges and ratio (L/W), sporocyst (SP), Stieda body (SB), sub-Stieda body (SSB), para-Stieda body (PSB), sporocyst residuum (SR), sporozoites (SZ), anterior (ARB) and posterior (PRB) refractile bodies, and nucleus (N). Voucher specimens of hosts are deposited in the La Sierra University Herpetology Collection (LSUHC), Riverside, California, USA. Photosyntypes of sporulated oöcysts were accessioned into the Harold W. Manter Laboratory of Parasitology (HWML), Lincoln, Nebraska, USA.
Results
A single G. monarchus was found to be passing an unknown species of Acroeimeria Paperna & Landsberg, 1989, that we describe herein as new.
Family Eimeriidae Minchin, 1903
Genus Acroeimeria Paperna & Landsberg, 1989
Acroeimeria grismeri n. sp.
Type-host: Gekko monarchus (Schlegel, 1836) (Reptilia: Sauria: Gekkonidae), adult male (93 mm SVL), LSUHC 14514, collected August, 2019.
Type-locality: Fraser’s Hill (Bukit Fraser in Malay) (3.7237871°N, 101.714207°W), state of Pahang, Peninsular Malaysia.
Type-material: Photosyntypes of sporulated oöcysts are deposited as HWML 216259.
Prevalence: In 1 of 3 (33%).
Sporulation time: Unknown; oöcysts were collected in the field and when examined 120 days later, they were completely sporulated.
Site of infection: Unknown.
Endogenous stages: Unknown.
ZooBank Registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Acroeimeria grismeri n. sp. is urn:lsid:zoobank.org:act:5EA19D2A-ACE3-45A8-B95D-B5CAB6190EF3.
Etymology: The specific epithet is given in honor of Larry Lee Grismer, Professor of Biology, La Sierra University, Riverside, California, for his many contributions to the study of gekkonid lizards in Southeast Asia. He has also educated students for many years with field studies in Peninsular Malaysia.
Description
Sporulated oöcyst
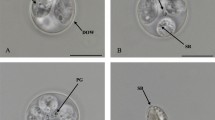
[Based on 20 oöcysts; Figs. 1, 2A–D.] Oöcyst spheroidal to subspheroidal; 15–20 × 13–19 (18.4 × 17.3), length/width (L/W) ratio 1.0–1.2 (1.1). Wall smooth, thick, bi-layered, c.1.0–1.5 (1.2), outer layer 2/3 total thickness and light yellow, inner layer darker. Micropyle and oöcyst residuum absent but polar granule (PG) present and variable; most, 15 of 20 (75%) fragmented into many (15+) fine granules in Brownian movement; 1 of 20 (5%) fragmented into loose mass of fine granules plus scattered fine granules; 2 of 20 (10%) of 1 to 2 PG in compact mass of granules; 2 of 20 (10%) PG (1 or 2) compact mass of granules plus few (5 to 6) scattered fine granules.
A–D, Nomarski-interference contrast photomicrographs of oöcysts of Acroeimeria grismeri n. sp. ex Gekko monarchus. Scale-bars: 10 µm. Abbreviations: ARB, anterior refractile body; OW, outer wall; PG, polar granule; PRB, posterior refractile body; SP, sporocyst; SR, sporocyst residuum; SZ, sporozoite
Sporocyst and sporozoite
[Based on 20 sporocysts.] Sporocysts 4, ellipsoidal; 7–10 × 5–8 (8.6 × 6.7), L/W ratio 1.3–1.5 (1.3). Wall smooth, thin and uni-layered, light brown, c.0.5 thick. Stieda, sub-Stieda and para-Stieda bodies absent; sporocyst residuum typically various sized granules scattered between and across sporozoites; sometimes compact sphere of granules or dense irregular mass located between and across sporozoites. Sporozoites (2), elongate (not measured), posterior end rounded with spheroidal posterior refractile body, anterior end tapered with small spheroidal anterior refractile body.
Remarks
The genus Acroeimeria was erected by Paperna & Landsberg (1989a) to contain coccidians with spheroidal to ellipsoidal oöcysts, which initially develop immediately beneath the brush-border of intestinal columnar intestinal epithelium and are passed unsporulated. When sporulated, they are morphologically similar to those of Eimeria species to which they are closely related. They possess endogenous development within a parasitophorous vacuole that starts to bulge above the surface of the intestinal mucosal cells as meronts and gamonts continue to grow; then, the host cell cytoplasm expands as the parasite matures, giving rise to a short, stalk-like structure forming a layer on the surface of the gut mucosa; this endogenous development occurs above the host cell nucleus and below the brush border in the enterocytes of the ileum. Although we did not obtain tissues for histological sections showing endogenous stages from this host, we still feel confident in placing the new species in the genus Acroeimeria Paperna & Landsberg, 1989.
Oöcysts of the new species are most similar in size and shape to Eimeria tokayae Ball & Daszak, 1995 collected from captive Tokay geckos, Gekko gecko Linnaeus from a pet dealer in the UK, but hosts were reported to originate from South China (Ball & Daszak, 1995). However, in E. tokayae, their oöcysts lack a polar granule (vs quite evident and variable in A. grismeri n. sp.) and their sporocyst shape index (L/W) is considerably larger (1.6 vs 1.3 in the new species). In addition, another similar species, Eimeria simonkingi Ball & Daszak, 1995 from Smith’s green-eyed gecko, Gekko smithii Gray, also from a pet dealer in the UK (originally from Indonesia), has slightly larger oöcysts (20.5 × 19.4 vs 18.4 × 17.3 µm) without polar granules (vs those of the new species containing polar granules) and a greater sporocyst L/W ratio than the new species (1.7 vs 1.3) (Ball & Daszak, 1995).
Discussion
The lizard family Gekkonidae includes about 1,331 species, including those within 58 genera (Uetz et al., 2020). The genus Gekko Laurenti contains only 5% (72) of the species in the family (Uetz et al., 2020) and many have never been examined and/or reported to harbor coccidians. The only species reported to harbor Eimeria spp. (see Table 1) include: G. gecko, Schlegelʼs Japanese Gecko, Gekko japonicus (Schlegel) from Japan, G. smithii, and lined gecko, Gekko vittatus Houttuyn from the Solomon Islands (Tanabe, 1928a, b; Bovee, 1971; Ball & Daszak, 1995; El-Toukhy et al., 2013). There are three additional Eimeria spp. reported from Gekko spp., including Eimeria koidzumii Matsubayasi, 1941 from G. japonicus from Japan (Matsubayasi, 1941), Eimeria japonicus Bovee, 1971 from G. japonicus from Japan (Bovee, 1971) and Eimeria vittati Ball & Daszak, 1995 from G. vittatus from a pet dealer in the UK (Ball & Daszak, 1995); however, all possess elongate to cylindroidal oöcysts possessing L/W ratios of > 2.0, devoid of a Stieda body, and with probable endogenous development in the distal part of the biliary epithelial cells and gall-bladder epithelium. We suggest these be proposed to represent species within the genus Choleoeimeria Paperna & Landsberg, 1989 (see Paperna & Landsberg, 1989a, b; Megía-Palma et al., 2015). Many gekkonid species remain to be surveyed and the prospect of finding new host species and geographical records for coccidians are likely, including the discovery of novel species.
References
Ball, G. H., & Daszak, P. (1995). Description of the oocysts of three new species of Eimeria (Apicomplexa: Eimeriidae) from geckos (Sauria: Gekkonidae). Systematic Parasitology, 32, 101–106.
Bauer, A. M. (2004). An accidental importation of Gekko monarchus into Africa. Hamadryad, 28, 122–123.
Bovee, E. C. (1971). New species of Eimeria from lizards of Japan. Transactions of the American Microscopical Society, 90, 336–343.
Else, J. G., & Colley, F. C. (1975). Eimeria cicaki sp. n. and Isospora thavari sp. n. from the house lizard Gehyra mutilata Boulenger in Malaysia. Journal of Protozoology, 22, 455–457.
El-Toukhy, A. A., Abdel-Aziz, A., Abo-Senna, F. M., & Abou El-Nour, M. F. (2013). Three coccidian parasites from Moorish gecko, Tarentola mauritanica (Gekkonidae) 2-Eimeria alexandriensis n. sp. (Apicomplexa: Eimeriidae). International Journal of Advanced Research, 1, 526–534.
Grismer, L. L., & Quah, E. S. H. (2019). An updated and annotated checklist of the lizards of Peninsular Malaysia, Singapore, and their adjacent archipelagos. Zootaxa, 4545, 230–248.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Matubayasi, H. (1941). On a new species of coccidia parasitic in the gecko, Gekko japonicus. Zoological Magazine (Tokyo), 53, 312–314.
Maupin, R. S., Diong, C. H., & McQuistion, T. E. (1998). Two new coccidian parasites from the grand anglehead lizard, Gonocephalus grandis from Peninsular Malaysia. Journal of Parasitology, 84, 1210–1212.
Megía-Palma, R., Martínez, J., Acevedo, I., Martín, J., García-Roa, R., Ortega, J., et al. (2015). Phylogeny of the reptilian Eimeria: Are Choleoeimeria and Acroeimeria valid generic names. Zoologica Scripta, 44, 1–9.
Paperna, I., & Landsberg, J. H. (1989a). Description and taxonomic discussion of eimerian coccidia from African and Levantine geckoes. South African Journal of Zoology, 24, 345–354.
Paperna, I., & Landsberg, J. H. (1989b). Fine structure and endogenous stages of Eimeria turcicus developing in gall bladder epithelium of the gecko Hemidactylus turcicus. South African Journal of Zoology, 24, 251–259.
Reese, D. J., Kinsella, J. M., Zdziarski, J. M., Zeng, Q.-Y., & Greiner, E. C. (2004). Parasites in 30 captive tokay geckos, Gekko gecko. Journal of Herpetological Medicine and Surgery, 14, 21–25.
Sinha, C. K., & Sinha, S. (1978). Eimeria bongaonensis n. sp. from a gecko, Gekko gecko (Linn.) in West Bengal. India. Indian Journal of Zootomy, 19, 61–62.
Tanabe M. (1928a). Eimeria gekkonis n. sp., a new coccidium from the gecko, (Gecko japonicus Duméril et Bibron). Journal of the Chosen Medical Association, 87 (Abstract of Original Articles), 65–69.
Tanabe, M. (1928b). Eimeria gekkonis n. sp., a new coccidium from the gecko Gekko japonicus (Duméril et Bibron.). Acta Medicinalia Keijo, 11, 207–220.
Uetz, P., Freed, P., & Hošek, J. (eds). The Reptile Database. http://www.reptile-database.org. Accessed on 28 May 2020.
Wilber, P. G., Duszynski, D. W., Upton, S. J., Seville, R. S., & Corliss, J. O. (1998). A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae). Systematic Parasitology, 39, 113–135.
Acknowledgements
We thank Drs Scott L. Gardner and Gábor R. Rácz (HWML) for expert curatorial assistance. We also thank Professor Shahrul Anuar and Dr L. Lee Grismer (La Sierra University), as well as the support of members of the Grismer lab, for assistance with collecting, and Dr Thomas J. Fayton (Cornell University, Ithaca, NY) for locating some literature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. The Department of Wildlife and National Parks, Peninsular Malaysia, issued a research permit (P-00074-15-18) allowing collection of specimens.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub:F70CB6CC-03B0-45F5-BE54-4E17E0DB8E1C. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
Rights and permissions
About this article
Cite this article
McAllister, C.T., Hnida, J.A., Fisher, S.R. et al. A new acroeimerian (Apicomplexa: Eimeriidae) in spotted house gecko, Gekko monarchus (Schlegel) (Sauria: Gekkonidae) from Peninsular Malaysia. Syst Parasitol 97, 529–534 (2020). https://doi.org/10.1007/s11230-020-09929-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-020-09929-1