Abstract
Although Lake Tanganyika hosts the most diverse endemic cichlid fish assemblage, its monogenean parasite fauna has hardly been documented. The cichlid tribe Tropheini has generated great interest because of its systematic position within the Haplochromini s.l. and its diversity in trophic morphology, reproductive behaviour and population structure. It has the potential to host a diverse Monogenea fauna. Here, we describe the first Cichlidogyrus spp.: Cichlidogyrus steenbergei sp. n., Cichlidogyrus irenae sp. n. and Cichlidogyrus gistelincki sp. n. The three host species, Limnotilapia dardennii, Ctenochromis horei and Gnathochromis pfefferi, are all infected by a single unique Cichlidogyrus sp. The genital and haptoral structure of the new species suggests a close relationship, which might mirror the close affinities between the hosts within the Tropheini. Based on haptoral configuration, the new species belong to a morphological group within the genus containing parasites both of West African cichlids and of Haplochromini, and hence, do not represent a new organisation of the attachment organ (as has recently been described of congeners infecting the ectodine cichlid Ophthalmotilapia).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lake Tanganyika is the deepest and oldest among the African Great Lakes (Cohen et al. 1997). It is home to the morphologically, behaviourally and genetically most diverse cichlid fish fauna (Snoeks 2000). The diversity makes the Cichlidae in general, and the Lake Tanganyika cichlid radiation in particular, a well-established study system, especially for mechanisms underlying speciation (Kornfield and Smith 2000; Koblmüller et al. 2008 and references therein). This requires a good understanding of the phylogenetic relationships within Cichlidae. Parasites are well-established tools in speciation research and to understand the host’s phylogeny (Page and Holmes 1998; Nieberding and Olivieri 2007). Furthermore, there is significant evidence that parasites may influence speciation in cichlids by influencing sexual selection (Blais et al. 2007; Maan et al. 2008). Hence, the study of parasite diversity and evolution seems a useful complementary approach in Tanganyika cichlids. A particularly useful group of parasites in this respect is Monogenea, in view of their species diversity, relatively high host specificity and direct life cycle. Conversely, highly diverse assemblages of closely related and sympatric host species, such as Cichlidae, are fruitful study systems to disentangle factors behind monogenean species richness (Pariselle et al. 2003b).
Cichlid Monogenea belonging to the Ancyrocephalidae have mainly been studied as parasites of West African tilapiine hosts. The gill parasite Cichlidogyrus Paperna, 1960 is the most widespread and speciose genus with 75 recognised species (Pariselle and Euzet 2009; Vanhove et al. 2011b). While its representatives are not known to harm fish stocks in Africa and the Middle East (Paperna 1996), considerable pathogenicity was reported under anthropogenic conditions in Southeast Asia (Kabata 1985). A co-phylogenetic analysis on species from West African Tilapiini taught that species richness seems underestimated due to cryptic species and demonstrated ecological transfers and parallel speciation events (Pouyaud et al. 2006). However, Monogenea of Lake Tanganyika did not receive attention, until a remarkable genetic and phenotypic diversity was shown in Gyrodactylus von Nordmann, 1832 (Vanhove et al. 2011a) and morphologically atypical representatives of Cichlidogyrus were described (Vanhove et al. 2011b).
Lake Tanganyika cichlids were classified into tribes by Poll (1986). One of these tribes, the endemic and monophyletic Tropheini, is a rather species-rich assemblage, mostly of rock-dwelling algae scrapers and invertivores. They are maternal mouthbrooders and display a wide range of trophic morphological adaptations (Sturmbauer et al. 2003). They are a lineage belonging to the widespread Haplochromini s.l. and represent a sister clade to several riverine Cichlidae and the Malawi and Victoria species flocks (Salzburger et al. 2005). Their interesting phylogenetic position, as well as their diverse mating behaviour, variable extent of colour polymorphism and genetic population structuring and trophic diversity makes them very well studied (Koblmüller et al. 2010 and references therein). They potentially harbour a species-rich assemblage of Monogenea (nobis). Considering the advantages of using cichlid hosts in parasitological research (Pariselle et al. 2003b), the closely related and often sympatric Tropheini constitutes a promising subject for a “natural experiment”. This enables the assessment of which effects e.g. host ecology or host population structure have on parasite communities. In this paper, we describe the first Cichlidogyrus spp. recorded from Tropheini hosts. The host fishes under scrutiny are Ctenochromis horei (Günther, 1894), Gnathochromis pfefferi (Boulenger, 1898) and Limnotilapia dardennii (Boulenger, 1899), all Tanganyika endemics with a lake-wide distribution. As in most other Tropheini of rather sediment-rich habitats, their intraspecific phenotypic and genetic differentiation is limited (Koblmüller et al. 2010). While L. dardennii and C. horei are omnivores, G. pfefferi is a predator, mainly of shrimps (Konings 1998; Yuma et al. 1998).
Materials and methods
Host cichlid fish were collected in April 2008 (Zambia and Tanzania) and March and April 2010 (Democratic Republic of the Congo—DRC) using gill nets (cfr. infra for location details). They were identified to species level on site by C. Sturmbauer (Karl-Franzens University of Graz, Austria) and D. Muzumani Risasi (Centre de Recherche en Hydrobiologie, Uvira, DRC), respectively. The fish were kept alive in aerated tanks until they were sacrificed and dissected. The right branchial arches were stored in 96% ethanol for further examination. In the laboratory, the gills were inspected for parasites under an Olympus SZX12 stereomicroscope. Monogenea were removed with a dissection needle. They were mounted on a slide in milli-Q water and fixed under a coverslip using ammonium picrate-glycerine (Malmberg 1957). On some individuals, partial digestion through proteinase K treatment was carried out following Harris and Cable (2000). Some worms collected in the DRC were mounted in the field directly.
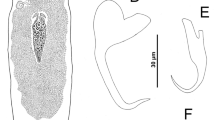
Pictures and measurements of the hard parts of the haptor and the male copulatory organ (MCO) were taken based on Gussev (1962) using an Olympus BX50 microscope at a magnification of ×100 (oil immersion, ×10 ocular) with Olympus DP-Soft 3.2 software. The numbering of haptoral parts was adopted from ICOPA IV (Euzet and Prost 1981); the terminology follows Pariselle and Euzet (1995) (i.e. “uncinuli” for marginal hooks) and the metrics taken are those from Pariselle et al. (2003a) (Fig. 1). Measurements are in micrometers and presented in Table 1. Taxon and author names of fishes follow Eschmeyer and Fricke (2011).
Measurements used to study the three new Cichlidogyrus spp. DB dorsal transverse bar: h length of dorsal bar auricle, w dorsal bar maximum width, x dorsal bar total length, y distance between auricles. A anchor: a anchor total length, b anchor blade length, c anchor shaft length, d anchor guard length, e anchor point length. MA male apparatus: Ap accessory piece length, Pe penis total length, He heel length. U uncinuli length. VB ventral transverse bar: w ventral bar maximum width, x length of one ventral bar branch
Results
Following Paperna (1960) and Pariselle et al. (2003a), the monogenean species described below belong to Cichlidogyrus Paperna, 1960. Generic diagnosis: Ancyrocephalidae. Three pairs of cephalic glands. Two posterior ocellae with crystalline lenses. Two small inconsistent anterior ocellae. Median muscular pharynx. Simple intestinal caeca joined posteriorly. Two pairs of anchors, one dorsal and one ventral. Two transverse bars, one dorsal with two auricles, one ventral curved and articulated. Fourteen uncinuli. Median posterior testis. Vas deferens at right side, not encircling intestinal caecum. Seminal vesicle present. One prostatic reservoir. Male copulatory complex with penis and accessory piece (Vanhove et al. (2011b) mention that the latter is not always present); auxiliary plate sometimes present. Median pre-testicular ovary. Submedian vaginal opening. Sclerotised vagina. Seminal receptacle present. Gill parasites of African Cichlidae, Cyprinodontidae and Nandidae.
Cichlidogyrus steenbergei sp. n. (Fig. 2; Table 1)
Type host: L. dardennii (Boulenger, 1899)
Site of infection: gills
Type locality: Kalambo Lodge, Lake Tanganyika, Zambia (8°37′ S, 31°12′ E)
Additional locality: Mugayo, Lake Tanganyika, DRC (6°47′ S, 29°34′ E).
Material studied: 21 individuals
Type material: the holotype has been deposited at the Natural History Museum, London, UK (NHMUK 2011.6.21.1). Paratypes have been deposited at the Royal Museum for Central Africa, Tervuren, Belgium (MRAC 37683) and at the Iziko South African Museum, Cape Town, Republic of South Africa (SAMCTA 29509).
Etymology: named after mathematician and biologist Maarten Van Steenberge (Belgium), specialist of African freshwater fish diversity, and a good colleague and friend of the authors.
Diagnosis: adults 330 (211–409) long. Dorsal anchor with guard much longer than shaft and regularly curved blade. Large dorsal transverse bar thick and arched, tapering towards the extremities, with auricules relatively far apart. Ventral anchor with guard and shaft more equal in size, as ventral anchor guard is shorter than that of dorsal anchor. Thick ventral transverse bar widest at mid-length of branches; branches straight. First and third to seventh uncinuli short (sensu Pariselle and Euzet 2009, i.e. when considered in proportion to the second uncinuli, which retain their larval size). Very large MCO with broad and thin-walled tubular penis, widening towards the end, and starting in clearly striated bulb with thin, not very prominent heel; accessory piece not reaching end of penis, sharply bent, tapering towards the extremities and slightly curved in the middle. No sclerotised vagina observed.
Remarks: The overall resemblance to Cichlidogyrus halli (Price and Kirk 1967), found on a variety of Tilapiini and Haplochromini, is quite high; however, the smaller diameter of the penis and less pronounced heel of the MCO of C. steenbergei sp. n. represent clear differences. This species also resembles Cichlidogyrus arfii Pariselle and Euzet 1995 (large penis and simple accessory piece) found on Pelmatochromis buettikoferi (Steindachner, 1894), but the latter has large uncinuli I.
Cichlidogyrus irenae sp. n. (Fig. 3; Table 1)
Type host: G. pfefferi (Boulenger, 1898).
Site of infection: gills.
Type locality: Kalambo Lodge, Lake Tanganyika, Zambia (8°37′ S, 31°12′ E)
Additional locality: Luhanga, Lake Tanganyika, DRC (3°31′ S, 29°9′ E)
Material studied: 23 individuals
Type material: the holotype has been deposited at the Natural History Museum, London, UK (NHMUK 2011.6.21.2). Paratypes have been deposited at the Royal Museum for Central Africa, Tervuren, Belgium (MRAC 37684) and at the Iziko South African Museum, Cape Town, Republic of South Africa (SAMCTA 29510).
Etymology: named for “Irene”, the vernacular name for the host species used by the fishermen/snorkelers at Kalambo Lodge who caught the majority of fish specimens.
Diagnosis: adults 364 (193–545) long. Dorsal anchor with guard relatively long as compared to shaft and of irregular shape; rather wide opening between shaft and guard; blade point only curves towards end of the slender blade. Dorsal transverse bar curved and thinner towards the extremities than in the middle; auricles relatively longer than in C. steenbergei sp. n. and planted at mid-width of bar. Ventral anchor also with slender blade curving towards the end, and guard of irregular shape; guard planted at almost right angle of shaft; length difference between both less pronounced than in dorsal anchor. Branches of ventral transverse bar straight; incision where both branches join. First and third to seventh uncinuli short (cfr. supra). Very large MCO. Penis tubular with swollen portion in the middle, starting in pear-shaped bulb with distinct and blunt heel; accessory piece of comparable length as penis, narrowing towards the end. No sclerotised vagina observed.
Remarks: because of the overall haptor morphology and the presence of a swollen portion in the penis, C. irenae sp. n. resembles Cichlidogyrus karibae Douëllou, 1993 and Cichlidogyrus zambezensis Douëllou, 1993 [described from Sargochromis codringtonii (Boulenger, 1908) and Serranochromis macrocephalus (Boulenger, 1899), respectively]. However, C. karibae has an S-shaped MCO accessory piece, more uneven in thickness than the rather straight accessory piece of C. irenae sp. n. The shape of the accessory piece of C. zambezensis, including a finger-like extension ending in a hook, clearly distinguishes this species from C. irenae sp. n. as well, while both C. karibae and C. zambezensis are larger in size. The MCO of C. irenae is reminiscent of C. halli and C. arfii, both in shape and size. However, the penis of these latter species lacks the diffuse swollen portion (sensu Pariselle and Euzet 2009) present in the new species. The haptoral elements of C. halli also generally exceed those of C. irenae sp. n. in size, while the haptor of C. arfii possesses large uncinuli I.
Cichlidogyrus gistelincki sp. n. (Fig. 4; Table 1)
Type host: C. horei (Günther, 1894)
Site of infection: gills
Type locality: Kalambo Lodge, Lake Tanganyika, Zambia (8°37′ S, 31°12′ E)
Additional localities: Mbita Island, Lake Tanganyika, Zambia (8°45′ S, 31°05′ E); Mtosi, Lake Tanganyika, Tanzania (7°35′ S, 30°38′ E); Kalemie, near Lukuga outflow, DRC (5°54′ S, 29°12′ E)
Material studied: 24 individuals
Type material: the holotype has been deposited at the Natural History Museum, London, UK (NHMUK 2011.6.21.3). Paratypes have been deposited at the Royal Museum for Central Africa, Tervuren, Belgium (MRAC 37685) and at the Iziko South African Museum, Cape Town, Republic of South Africa (SAMCTA 29511).
Etymology: named after biochemist and aquariologist Marc Gistelinck (Belgium), a special friend of first author C.G.
Diagnosis: adults 287 (150–392) long. Dorsal anchor with relatively short blade, the base of which is clearly separated from the guard; guard considerably longer than shaft. Dorsal transverse bar with similar width troughout, only tapering towards the extremities; auricles planted at mid-width. Ventral anchor with stubby guard and shaft at right angle to each other; ventral anchor blade relatively longer than that of dorsal anchor. Ventral transverse bar with straight branches widest at mid-length and incised where branches meet. First and third to seventh uncinuli short (cfr. supra). Rather small-sized MCO; tubular penis, beginning in a heart-shaped bulb with trapezoid heel, narrowing and bending backwards towards the opening; slender accessory piece twisted and with distal end covered by a pointed cap. No sclerotised vagina observed.
Remarks: The overall similarities to C. halli or C. arfii are quite high; however, the dimensions of body, haptoral parts, penis and MCO accessory piece are considerably smaller in C. gistelincki sp. n. The more slender penis, more pronounced heel and capped accessory piece allow clear distinction between C. gistelincki sp. n. and C. steenbergei sp. n. on the basis of their MCO.
Discussion
The first representatives of Cichlidogyrus (Monogenea, Ancyrocephalidae) living on Tropheini are described, namely on L. dardennii, G. pfefferi and C. horei hosts. Apart from Gyrodactylus zimbae Vanhove, Snoeks, Volckaert and Huyse, 2011, described from Simochromis diagramma (Günther, 1894) and also recorded on C. horei (Vanhove et al. 2011a), no helminth parasites are known from these hosts, to the best of our knowledge. Throughout the Tropheini, Cichlidogyrus spp. seems to be more prevalent and host-specific compared to monogeneans belonging to Gyrodactylus (nobis).
The current descriptions corroborate the assertion of Pouyaud et al. (2006) that similar species can be distinguished on the basis of their MCO. On a higher level, Cichlidogyrus can be divided in groups based on haptoral configuration (mainly considering relative lengths of the various pairs of uncinuli) (Pariselle and Euzet 2003; Vignon et al. 2011). Molecular information suggests that they reflect genuine evolutionary relationships (Pouyaud et al. 2006). The first pair of uncinuli, as well as pairs three to seven, of all three new species are short. In this sense, the new species do not represent a new haptoral configuration, and thus seem morphologically less unusual, than some of the species described from Ophthalmotilapia Pellegrin, 1904 (Ectodini) in Lake Tanganyika. Indeed, while C. vandekerkhovei Vanhove, Volckaert and Pariselle, 2011 and C. makasai Vanhove, Volckaert and Pariselle, 2011 possess extremely long dorsal bar auricles, C. centesimus Vanhove, Volckaert and Pariselle, 2011 displays several features previously unknown from Cichlidogyrus: a spirally coiled thickening at the end of the penis, the lack of an MCO accessory piece, and a unique haptoral organisation.
Haptor characteristics are useful to define morphological groups within Cichlidogyrus (cfr. supra). The group of Cichlidogyrus spp. in which both the first pair and pairs three to seven of the uncinuli are short, includes, in addition to a variety of West African parasite species, several parasites of Haplochromini s.l., such as C. haplochromii Paperna, 1979, C. karibae and C. zambezensis. On the other hand, C. philander Douëllou, 1993 does not belong to this group (Vignon et al. 2011) and C. bifurcatus Paperna, 1960 seems to have longer UIII – UVII (Paperna 1979). Nevertheless, these species also infect representatives of the Haplochromini s.l., such as Pseudocrenilabrus Fowler, 1934 and Haplochromis Hilgendorf, 1888 (Paperna 1979; Douëllou 1993). In view of the interesting phylogenetic position of the Tropheini with regards to the Haplochromini (cfr. supra), and of the role of Lake Tanganyika as the evolutionary reservoir to all Haplochromini in the region (Salzburger et al. 2005), molecular data and broader taxon sampling are needed to reconstruct the history of Central African Cichlidogyrus. Further molecular work should also elucidate relationships to the already mentioned C. halli, a species with a broad host range, occasionally even parasitizing Haplochromini s.l. (Douëllou 1993: S. macrocephalus), which probably represents a species complex (Pouyaud et al. 2006; Pariselle and Euzet 2009).
In any case, the haptor configuration (similar uncinuli length, hence belonging to the same morphological group within the genus, and similar dorsal bar), the same MCO type (simple accessory piece, broad penis) and the shared absence of a sclerotised vagina suggest a close relationship between the three new species. Interestingly, the three host species are relatively closely related to each other within the Tropheini (Koblmüller et al. 2010). Although it is too early for conclusions, the hypothesis that these species evolved from a shared ancestor on an ancestral Tropheini host should be retained. This is in stark contrast to the only other monogeneans known from Tropheini, namely the Gyrodactylus spp. described from S. diagramma. Indeed, in view of their genetic and morphological differences, ecological transfer needs to be invoked to explain their co-occurrence on one host species (Vanhove et al. 2011a).
Dispersal capacity, leading to gene flow across even unfavourable habitats, is frequently linked to a lack of genetic and morphological substructuring in Tanganyika cichlids (Meyer et al. 1996; Wagner and McCune 2009). For example, L. dardennii, a dweller on preferably vegetated shallow sediment-rich grounds, is known to be a good disperser, also over sandy stretches. It contrasts with other Tropheini cichlids such as Tropheus Boulenger, 1898, which are strongly bound to rocky substrates (Konings 1998). Indeed, the hosts under study hardly display genetic and phenotypic within-species diversity (Koblmüller et al. 2010). It has been suggested that the mobility of the host species positively influences parasite species richness (Gregory 1990; Mwita and Nkwengulila 2008). As the new species are the only Cichlidogyrus spp. retrieved from their respective hosts, with sampling sites several hundreds of kilometres apart, this hypothesis is not supported here. There is strong evidence from other Monogenea that genetic data may contribute to the detection of cryptic speciation and the recognition of underestimated host specificity (Ziętara and Lumme 2003; Huyse and Malmberg 2004; Pouyaud et al. 2006). Hence, the next phase includes the molecular characterization of the parasite fauna. Preliminary molecular data (nuclear rDNA) do not suggest cryptic speciation in parasites collected from the three hosts species (Vanhove et al. unpublished data).
References
Blais J, Rico C, van Oosterhout C, Cable J, Turner GF, Bernatchez L (2007) MHC adaptive divergence between closely related and sympatric African cichlids. PLoS ONE 2:8
Cohen AS, Lezzar KE, Tiercelin JJ, Soreghan M (1997) New palaeogeographic and lake-level reconstructions of Lake Tanganyika: implications for tectonic, climatic and biological evolution in a rift lake. Basin Res 9:107–132
Douëllou L (1993) Monogeneans of the genus Cichlidogyrus Paperna, 1960 (Dactylogyridae: Ancyrocephalinae) from cichlid fishes of Lake Kariba (Zimbabwe) with descriptions of five new species. Syst Parasitol 25:159–186
Eschmeyer WN, Fricke R (eds) (2011). Catalog of Fishes electronic version. http://research.calacademy.org/ichthyology/catalog/fishcatmain.asp. Accessed 10 February 2011
Euzet L, Prost M (1981) Report of the meeting on Monogenea: problems of systematics, biology and ecology. In: Slusarski W (ed) Review of advances in parasitology. P.W.N. Polish Scientific Publishers, Warsaw, pp 1003–1004
Gregory RD (1990) Parasites and host geographic range as illustrated by waterfowl. Funct Ecol 4:645–654
Gussev AV (1962, 1964) Order Dactylogyridea. In: Bychovskaya-Pavlovskaya IE, Gussev AV, Dubinina MN, Izymova NA, Smirnova TS, Sokolovskaya IL, Shtein GA, Shul’man SS, Epsthein VM (eds) Key to the parasites of freshwater fish of the USSR. Israel Program for Scientific Translations, Jerusalem, pp 204–342 [Russian original: Opredelitel’ parazitov presnovohnyh ryb SSSR. Moscow-Leningrad: Izadtel’stovo Akademii Nauk SSSR]
Harris PD, Cable J (2000) Gyrodactylus poeciliae n. sp. and G. milleri n. sp. (Monogenea: Gyrodactylidae) from Poecilia caucana (Steindachner) in Venezuela. Syst Parasitol 47:79–85
Huyse T, Malmberg G (2004) Molecular and morphological comparisons between Gyrodactylus ostendicus n. sp. (Monogenea: Gyrodactylidae) on Pomatoschistus microps (Krøyer) and G. harengi Malmberg, 1957 on Clupea harengus membras L. Syst Parasitol 58:105–113
Kabata Z (1985) Parasites and diseases of fish cultured in the tropics. Taylor & Francis, London and Philadelphia
Koblmüller S, Sefc KM, Sturmbauer C (2008) The Lake Tanganyika cichlid species assemblage: recent advances in molecular phylogenetics. Hydrobiologia 615:5–20
Koblmüller S, Egger B, Sturmbauer C, Sefc KM (2010) Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Mol Phylogenet Evol 55:318–334
Konings A (1998) Tanganyika cichlids in their natural habitat. Cichlid Press, El Paso
Kornfield I, Smith PF (2000) African cichlid fishes: model systems for evolutionary biology. Annu Rev Ecol Syst 31:163–196
Maan M, Van Rooijen AMC, Van Alphen JJM, Seehausen O (2008) Parasite-mediated sexual selection and species divergence in Lake Victoria cichlid fish. Biol J Linn Soc 94:53–60
Malmberg G (1957) On the occurence of Gyrodactylus on Swedish fishes. Skr Söd Sver Fiskför Asskr 1956:19–76 [in Swedish]
Meyer A, Knowles LL, Verheyen E (1996) Widespread geographical distribution of mitochondrial haplotypes in rock-dwelling cichlid fishes from Lake Tanganyika. Mol Ecol 5:341–350
Mwita C, Nkwengulila G (2008) Determinants of the parasite community of clariid fishes from Lake Victoria, Tanzania. J Helminthol 82:7–16
Nieberding CM, Olivieri I (2007) Parasites: proxies for host genealogy and ecology? Trends Ecol Evol 22(3):156–165
Page RDM, Holmes EC (1998) Molecular evolution: a phylogenetic approach. Blackwell, Malden, Oxford and Carlton
Paperna I (1960) Studies on monogenetic trematodes in Israel. 2. Monogenetic trematodes of cichlids. Bamidgeh 12:20–33
Paperna I (1979) Monogenea of inland water fish in Africa. Annalen Koninklijk Museum voor Midden-Afrika Zoologische Wetenschappen 226, Tervuren
Paperna I (1996) Parasites, infections and diseases of fishes in Africa. An update. CIFA Technical Paper 31. Food and Agriculture Organization, Rome
Pariselle A, Euzet L (1995) Gill parasites of the genus Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae) from Tilapia guineensis (Bleeker, 1862), with descriptions of six new species. Syst Parasitol 30:187–198
Pariselle A, Euzet L (2003) Four new species of Cichlidogyrus (Monogenea: Ancyrocephalidae), gill parasites of Tilapia cabrae (Teleostei: Cichlidae), with discussion on relative length of haptoral sclerites. Folia Parasit 50:195–201
Pariselle A, Euzet L (2009) Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema 31(4):849–898
Pariselle A, Bilong Bilong C, Euzet L (2003a) Four new species of Cichlidogyrus Paperna, 1960 (Monogenea, Ancyrocephalidae), all gill parasites from African mouthbreeder tilapias of the genera Sarotherodon and Oreochromis (Pisces, Cichlidae), with a re-description of C. thurstonae Ergens, 1981. Syst Parasitol 56:201–210
Pariselle A, Morand S, Deveney M, Pouyaud L (2003b) Parasite species richness of closely related hosts: historical scenario and “genetic” hypothesis. In: Combes C, Jourdan J (eds) Hommage à Louis Euzet—taxonomie, écologie et évolution des métazoaires parasites. Taxonomy, ecology and evolution of metazoan parasites. Presses Universitaires de Perpignan, Perpignan, pp 147–166
Poll M (1986) Classification des Cichlidae du lac Tanganika. Tribus, genres et espèces. Mémoires de la Classe des Sciences XLV, 2nd edn. Académie Royale de Belgique, Brussels
Pouyaud L, Desmarais E, Deveney M, Pariselle A (2006) Phylogenetic relationships among monogenean gill parasites (Dactylogyridae, Ancyrocephalidae) infesting tilapiine hosts (Cichlidae): systematic and evolutionary implications. Mol Phylogenet Evol 38:241–249
Salzburger W, Mack T, Verheyen E, Meyer A (2005) Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol 5:17
Snoeks J (2000) How well known is the ichthyodiversity of the large East African lakes? Adv Ecol Res 31:17–38
Sturmbauer C, Hainz U, Baric S, Verheyen E, Salzburger W (2003) Evolution of the tribe Tropheini from Lake Tanganyika: synchronized explosive speciation producing multiple evolutionary parallelism. Hydrobiologia 500:51–64
Vanhove MPM, Snoeks J, Volckaert FAM, Huyse T (2011a) First description of monogenean parasites in Lake Tanganyika: the cichlid Simochromis diagramma (Teleostei, Cichlidae) harbours a high diversity of Gyrodactylus species (Platyhelminthes, Monogenea). Parasitology 138(3):364–380
Vanhove MPM, Volckaert FAM, Pariselle A (2011b) Ancyrocephalidae (Monogenea) of Lake Tanganyika: I: Four new species of Cichlidogyrus from Ophthalmotilapia ventralis (Teleostei, Cichlidae), the first record of this family in the basin. Zoologia (Curitiba, Impr) 28(2):253–263
Vignon M, Pariselle A, Vanhove MPM (2011) Modularity in attachment organs of African Cichlidogyrus (Platyhelminthes, Monogenea, Ancyrocephalidae) reflects phylogeny rather than host specificity or geographic distribution. Biol J Linn Soc 102(3):694–706
Wagner CE, McCune AR (2009) Contrasting patterns of spatial genetic structure in sympatric rock-dwelling cichlid fishes. Evolution 63:1312–1326
Yuma M, Narita T, Hori M, Kondo T (1998) Food resources of shrimp-eating cichlid fishes in Lake Tanganyika. Env Biol Fish 52(1–3):371–378
Ziętara MS, Lumme J (2003) The crossroads of molecular, typological and biological species concepts: two new species of Gyrodactylus Nordmann, 1832 (Monogenea: Gyrodactylidae). Syst Parasitol 55:39–52
Acknowledgements
Prof. J. Snoeks (RMCA/K.U.Leuven) is gratefully acknowledged for his input and for commenting on an earlier version of the manuscript; Prof. C. Sturmbauer (Karl-Franzens University of Graz) for organizing the expedition to Zambia and Tanzania, and for host identification; M. Van Steenberge (K.U.Leuven/RMCA), J. Raeymaekers (K.U.Leuven), J.K. Zimba, L. Makasa (Lake Tanganyika Research Station, Mpulungu, Zambia), T. Veall, O.R. Mangwangwa (Rift Valley Tropicals, Zambia/Tanzania), M. Nshombo, N. Mulimbwa, R. Muzumani, B. Muterezi, N. Mbirize, L. Kapepula (Centre de Recherche en Hydrobiologie, Uvira, DRC), C. Katongo (University of Zambia, Lusaka) and their technical staff, for scientific, logistical and administrative contributions to the fieldwork; the Tanzania Commission for Science and Technology (COSTECH) for research permit no. 2007-258-CC-2006-151 to M.P.M.V. and T.H., respectively, are PhD and post-doctoral fellows of the Research Foundation–Flanders (FWO–Vlaanderen). Fieldwork was funded by two travel grants from the Research Foundation–Flanders and one from the King Leopold III Fund for Nature Conservation and Exploration to M.P.M.V.
Author information
Authors and Affiliations
Corresponding author
Additional information
Céline Gillardin and Maarten P.M. Vanhove contributed equally to this paper.
Tine Huyse and Filip A. M. Volckaert are joint senior authors.
Rights and permissions
About this article
Cite this article
Gillardin, C., Vanhove, M.P.M., Pariselle, A. et al. Ancyrocephalidae (Monogenea) of Lake Tanganyika: II: description of the first Cichlidogyrus spp. parasites from Tropheini fish hosts (Teleostei, Cichlidae). Parasitol Res 110, 305–313 (2012). https://doi.org/10.1007/s00436-011-2490-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2490-5