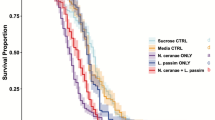
Abstract
There has been increased focus on the role of microbial attack as a potential cause of recent declines in the health of the western honey bee, Apis mellifera. The Nosema species, N. apis and N. ceranae, are microsporidian parasites that are pathogenic to honey bees, and infection by these species has been implicated as a key factor in honey bee losses. Honey bees infected with both Nosema spp. display significant changes in their biology at the cellular, tissue, and organismal levels impacting host metabolism, immune function, physiology, and behavior. Infected individuals lead to colony dysfunction and can contribute to colony disease in some circumstances. The means through which parasite growth and tissue pathology in the midgut lead to the dramatic physiological and behavioral changes at the organismal level are only partially understood. In addition, we possess only a limited appreciation of the elements of the host environment that impact pathogen growth and development. Critical for answering these questions is a mechanistic understanding of the host and pathogen machinery responsible for host-pathogen interactions. A number of approaches are already being used to elucidate these mechanisms, and promising new tools may allow for gain- and loss-of-function experiments to accelerate future progress.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
The western honey bee, Apis mellifera, is crucial to key agricultural and ecological systems, and the pollination of many important crops is dependent on this species (Potts et al. 2016). If this pollination crisis remains unsolved, it will contribute to serious nutritional deficiencies for humans (Smith et al. 2015) and significant economic losses (Gallai et al. 2009) worldwide. Infection by pathogenic microbes represents one critical factor influencing honey bee declines in recent years (Goulson et al. 2015; Steinhauer et al. 2018). The microsporidian species Nosema apis and N. ceranae can cause individual pathology and mortality in honey bees that has been well documented (Fries 2014; Goblirsch 2017; Martín-Hernández et al. 2018). Such infections can also contribute to colony-level disease and collapse in conjunction with other elements, which appear to differ by region. Such factors include genetic variation on the part of both parasite and host and the presence of other stressors (e.g., climate variation or pesticide exposure) (Fries 2014; Goblirsch 2017; Martín-Hernández et al. 2018).
Honey bees infected with either Nosema spp. display significant changes in their biology at the cellular, tissue, and organismal levels with impacts on host metabolism, immune function, physiology, and behavior. Infected individuals lead to colony dysfunction and can contribute to colony disease in some circumstances (Fig. 7.1). Starting with the digestive tract, this review will describe some of the cellular, tissue, and organismal changes associated with infection by Nosema spp., with special focus on N. ceranae, which originated in the eastern honey bee, Apis cerana, and seems to have displaced N. apis in the western honey bee in many regions (Klee et al. 2007; Emsen et al. 2016; Gisder et al. 2017). The impacts of individual bee disease and mortality on the colony and on honey bee health more broadly have been comprehensively reviewed recently and will not be covered here (Fries 2014; Goblirsch 2017; Martín-Hernández et al. 2018).
Remarkable progress has been made in our understanding of Nosema disease in the years since the first report of N. ceranae in western honey bees. Yet many key questions remain unanswered. While there is still some debate, both Nosema spp. appear to display very narrow tissue tropism, being restricted to the digestive tract (Huang and Solter 2013; Higes et al. 2020). Thus, proximal changes to midgut function and the presence of a rapidly developing infectious agent must necessarily be responsible for dysfunction at the organismal level. However, the relative contribution of the host and parasite cells to these larger order changes and the molecular mechanisms through which they are achieved are incompletely understood. Conversely, the factors that influence the growth and development of cells of both Nosema spp. within the host environment remain unclear. Finally, our understanding of the positive and negative consequences of the observed organismal changes on both host and parasite is limited. Understanding the host and pathogen machinery responsible for these negative changes is critical for answering these questions. A number of approaches are already being used to better understand these mechanisms, and promising new tools may allow for the gain- and loss-of-function and biochemical experiments necessary to accelerate future progress.
7.2 Changes in Digestive Tract Structure and Function After Nosema Infection
In worker honey bees, both N. apis and N. ceranae are restricted to infecting cells of the midgut. Typical of microsporidia, environmental spores first inject sporoplasms into host cells where they develop into a meronts that begin to rapidly proliferate. Meronts then mature into sporoblasts, which produce large numbers of primary spores and ultimately new infective environmental spores. These spores are released from the infected cell to begin the cycle anew (Solter et al. 2012). Digestive tract cells infected with Nosema spp. show evidence of important restructuring of subcellular structures, including aggregated ribosomes (Liu 1984) and association of microsporidia with the mitochondria in both N. ceranae (Higes et al. 2007) and N. apis (Graaf et al. 1994) infection. These cells also display extensive lysis, tissue disorganization, and cell sloughing into the lumen of the midgut (Liu 1984; Higes et al. 2007; García-Palencia et al. 2010; Dussaubat et al. 2012). In adults of insect species studied to date, epithelial cells are sloughed off into the lumen at some rate before being replaced by stem and progenitor cell proliferation and differentiation (Apidianakis and Rahme 2011). In the fruit fly, homeostatic self-renewal of the digestive tract has been shown to be critical for maintaining organ function after insult from damage or infection (Jiang et al. 2016; Guo et al. 2016b).
As an intracellular parasite with a long and complex lifecycle, Nosema-mediated regulation of cell turnover is likely paramount for maximum production of mature spores prior to enterocyte shedding and removal. Such regulation could occur through control of a variety of cellular processes including apoptosis and proliferation. In 2013, it was reported that midgut cells of N. ceranae-infected honey bees have reduced rates of apoptosis. The authors hypothesized that this reduced cell death was due to active manipulation of host cell apoptosis to allow for maximal pathogen reproduction (Higes et al. 2013). Further studies have confirmed this finding and also revealed that apoptosis suppression was most pronounced in the posterior regions of the midgut (Kurze et al. 2018). Studies have indicated that N. apis infection is initially restricted to the posterior section of the midgut (Fries 1988; Graaf et al. 1994), although this has not been observed in N. ceranae infection (Snow 2016). The mechanisms through which N. ceranae suppresses apoptosis are incompletely understood. One study implicated augmented expression of the inhibitor of apoptosis protein-2 (Iap2) gene in cell survival (Kurze et al. 2015). Interestingly, both the suppressed apoptosis and the increased Iap2 gene expression were lost in a strain of bees selected for Nosema tolerance. Another study found that a number of other survival factors are aberrantly upregulated in infected midguts, including the Bcl2-like Buffy gene and the Iap family member BIRC5 gene (Martín-Hernández et al. 2017). Alterations in the expression of apoptotic machinery have also been observed in infected midgut tissue using transcriptomic (Dussaubat et al. 2012; Holt et al. 2013; Doublet et al. 2017) and proteomic (Kurze et al. 2016a) approaches. Other microsporidia species infecting vertebrates and invertebrates are known to inhibit host cell apoptosis (Scanlon et al. 1999; Aguila et al. 2006). For example, Nosema bombycis reduces apoptosis in silkworm cells exposed to actinomycin D, an RNA polymerase inhibitor commonly used to induce apoptosis. Here, upregulation of Buffy was also implicated (He et al. 2015).
N. ceranae infection also impacts midgut proliferation. Panek et al. found that proliferation, as assessed by BrdU+ “crypts,” was reduced in N. ceranae-infected bees (Panek et al. 2018). This decrease is correlated with altered transcription of components of a number of pathways known to regulate midgut regeneration in the fruit fly (Jiang et al. 2016; Guo et al. 2016b), such as Hippo (Panek et al. 2018), and Wnt (Dussaubat et al. 2012), as well as cell cycle genes themselves (Martín-Hernández et al. 2017). Proliferation of midgut cells in the honey bee has been shown to be influenced by age, social function, and diet (Ward et al. 2008; Willard et al. 2011). However, a more complete understanding of the pathways responsible for controlling proliferation in the honey bee midgut is vital to understand how these pathways are altered by Nosema infection. For example, due to their sequence divergence, the honey bee JAK/STAT pathway ligands that regulate tissue regeneration in the fruit fly midgut have been elusive and were only recently discovered as being induced by thermal stress in the honey bee midgut (Bach et al. 2021). As new players in honey bee midgut biology are characterized, their role in Nosema infection can be explored.
A number of other interesting alterations in midgut biology have been observed after N. ceranae infection. Relating to cell proliferation, Panek et al. observed that N. ceranae appeared to be excluded from the proliferative stem cell population (Panek et al. 2018) although a previous study did not observe similar findings (Higes et al. 2007). Such exclusion likely has a significant impact on the ability of the digestive tract to retain some functionality during the long course of a typical microsporidia infection by preventing exhaustion of regeneration potential. The peritrophic membrane (PM), a key protective feature of insect digestive tracts (Hegedus et al. 2009), also appears disorganized after N. ceranae infection (Dussaubat et al. 2012), though full characterization of this phenomenon is lacking. Disruptions in PM function may be important for spore production and shedding but may also modify the nature of the interactions between the midgut cells and the cells of the microbiome or even other pathogens. Several studies have also suggested that N. ceranae infection impairs the integrity of the epithelial layer in the midgut (Higes et al. 2007; Dussaubat et al. 2012). While formal demonstration of increased intestinal barrier permeability has not yet been provided, it would be expected to enable diverse entities from the digestive tract to enter the hemocoel and future studies should clarify its role in Nosema disease.
Transcriptomic and proteomic data also suggests dramatic alterations in the expression of genes involved in all major functions of the digestive tract in insects, including nutrient acquisition and processing, host defense, microbiome maintenance, chemical detoxification, and barrier function (Antúnez et al. 2009; Dussaubat et al. 2012; Chaimanee et al. 2012; Holt et al. 2013; Aufauvre et al. 2014; Doublet et al. 2017). Additionally, expressions of a number of host miRNA are altered by infection (Huang et al. 2015), although the targeted mRNA are largely unknown. Our current understanding of how digestive tract function is altered will be discussed in more details in the sections below.
Infection by Nosema spp. Impacts Nutrient Acquisition and Processing
Infection by Nosema spp. causes proximal changes to midgut nutrient acquisition and processing that impact organismal metabolism. The digestive function of the midgut epithelium is compromised at early time points after infection by N. apis, as both trypsin and chymotrypsin levels are decreased (Malone and Gatehouse 1998). Changes to metabolism in the gut have been inferred from gene expression studies that show changes in the genes involved in transport and processing of carbohydrate molecules. In particular, genes encoding trehalose transporters and alpha-glucosidase have been found to be upregulated during infection (Dussaubat et al. 2012), while trehalase has been shown to be downregulated (Aufauvre et al. 2014). Alpha-glucosidase protein levels have also been shown to be impacted (Vidau et al. 2014). It is not currently clear which of the above changes are specific to microsporidia infection versus generic consequences that would be found with diverse stressors. For example, trehalose transporter gene expression (Bach et al. 2021) and alpha-glucosidase protein levels (Huang et al. 2013) have both been shown to be altered by stressors not related to infection state, suggesting that these changes might not be a specific to infection. Regardless, these changes are particularly interesting because trehalose is currently thought to be the most plausible source of glucose for microsporidia (Timofeev et al. 2020). As one of the so-called “Terresporidia,” neither N. apis nor N. ceranae appear to possess the alternative oxidase of more basal microsporidia (Timofeev et al. 2020). Thus, it is unclear how regeneration of reduction equivalents such as NAD+ is achieved. It is possible that novel transport proteins able to move NAD+ (Dean et al. 2018) could provide one solution and the species of the Nosema genus indeed possess a rich transportome (Chetia et al. 2017). A recent study also found that the levels of eight proteins of the oxidative phosphorylation pathway were altered by N. ceranae infection (Houdelet et al. 2020), which is particularly striking in the context of the association between microsporidia and mitochondria previously discussed. RNAi studies indicate that N. ceranae is in fact dependent on its ATP/ADP transporters (Paldi et al. 2010), likely for commandeering host ATP as has been shown for other microsporidia (Jarkass and Reinke 2020). N. bombycis also possesses a putative ATP-binding cassette transporter, which is critical for parasite cell growth (He et al. 2019). Multiple metabolic enzymes of microsporidia are secreted into host cells (Senderskiy et al. 2014), presumably to modify host metabolism (Timofeev et al. 2020). Hexokinase, which catalyzes the first step in glycolysis, was first found to be secreted by Nematocida spp. (Cuomo et al. 2012) and then by diverse microsporidia species (Timofeev et al. 2020). The enzymatic activity of hexokinase has been confirmed for both N. ceranae and N. bombycis (Dolgikh et al. 2019). RNAi studies demonstrated a critical role for this enzyme in N. bombycis growth (Huang et al. 2018b).
Infection leads to dramatic changes in metabolism in a number of tissues that are distal from the site of infection. Reduced trehalose levels are observed in the hemolymph of honey bee foragers, although glucose levels stay the same (Mayack and Naug 2010). Other metabolites including amino acids, lipid biosynthesis components, and the polyamine compound spermidine are also altered in the hemolymph of N. ceranae-infected bees (Aliferis et al. 2012; Jousse et al. 2020). Whole bee lipid loss is observed (Li et al. 2018), which likely represents changes in fat body lipid storage and provides one indicator of reduced energy reserves. Bees infected with N. ceranae are energetically stressed and have higher hunger levels (Alaux et al. 2009; Naug and Gibbs 2009; Mayack and Naug 2009; Martín-Hernández et al. 2011), which have important impacts on their physiology and behavior and overall colony health as discussed below. N. apis infection also impacts bee energetics, but the effect is less pronounced compared to N. ceranae infection (Martín-Hernández et al. 2011). Interestingly, decreased survival of bees infected with N. ceranae can be ameliorated by ad libitum feeding, suggesting that energetic stress may be a critical driver of mortality in infected bees (Mayack and Naug 2009). Energetic stress, as measured by hemolymph trehalose levels, is not observed in the Nosema-tolerant strain after N. ceranae infection (Kurze et al. 2016b), perhaps suggesting that mechanisms increasing tolerance to infection are as critical as those for resistance to infection (Kurze et al. 2016c)
Immune Responses Are Altered by Nosema Disease
The immune response to microsporidiosis in invertebrates is currently thought to be composed of multiple arms. First, microsporidia infection leads to transcriptional induction of immune recognition and effector proteins, including antimicrobial peptides (AMPs). This arm has been most extensively studied in honey bees. At early time points, experimental (Schwarz and Evans 2013; Huang et al. 2016b) and natural (Li et al. 2017b) infection by N. ceranae induces expression of genes encoding AMPs and pattern recognition receptors (PRRs) and alters expression of components of the Imd and Toll pathways, both known to respond to pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) stimuli. After this initial phase, the expression of immune effector (e.g., AMPs) genes and other immune-related transcripts was reduced below the levels of infected controls. There appears to be significant study-related variability in immune gene expression (Antúnez et al. 2009; Dussaubat et al. 2012; Chaimanee et al. 2012; Holt et al. 2013; Aufauvre et al. 2014; Doublet et al. 2017), which may be due to differences in experimental procedures (dose, tissue sampled, or time of sampling) or strain variation on the part of either the host or the parasite. N. bombycis infection induces robust transcriptional activation of immune genes in Bombyx mori, including genes encoding AMPs and serine proteases and serpins (Ma et al. 2013), suggesting that microsporidia infection induces some similar responses in different insect species (Pan et al. 2018). Thus far, there are only a few pieces of evidence that these responses have a protective effect on the host. Naked cuticle (nkd) is a Wnt pathway antagonist that is induced by N. ceranae infection and is thought to regulate immune responses. Knockdown of this gene results in reduced parasite load and induction of immune genes, such as the AMPs abecin, apidecin, and defensin-1 and the Pattern Recognition Receptors (PRR) PGRP-S2 (Li et al. 2016). Exposure to Pathogen-associated molecular patterns (PAMPs) increased survivorship in N. ceranae-infected bees, potentially by inducing a more robust immune response, although no measurement of such responses was made (Valizadeh et al. 2020). Drones from the Nosema-tolerant strain above also appear to have some evidence of more active immune pathways, which may influence their survival (Huang et al. 2012).
Systemic immune responses, including cellular and humoral, have not been emphasized in honey bee studies of microsporidia infection to date, presumably because of the highly restricted tissue tropism of the microsporidia infection in bees. Cellular immunity, as measured by hemocyte numbers (Alaux et al. 2010), does appear impacted by microsporidia infection, although hemocytes have been shown to phagocytose N. apis spores in vitro (Gilliam and Shimanuki 1967). The phenoloxidase cascade, involved in immune-mediated melanization, is similarly unaffected by N. ceranae infection (Alaux et al. 2010; Pasquale et al. 2013; Roberts and Hughes 2014), although serine proteases involved in melanization are upregulated by infection (Aufauvre et al. 2012; Badaoui et al. 2017). However, in silkworms, there is evidence that systemic melanization is an important mechanism of immune defense against microsporidia infection (Ma et al. 2013; Ni et al. 2020) and N. bombycis parasite actively suppresses melanization (Bao et al. 2019).
An intracellular immune mechanism that targets microsporidia was recently discovered in C. elegans. This mechanism, called the “intracellular pathogen response” (IPR), uses ubiquitin to target and clear parasite cells via autophagy (Bakowski et al. 2014; Balla et al. 2019; Reddy et al. 2019). While this specific pathway has not been found in insects, autophagy has been shown to be a critical component of immunity in the fruit fly (reviewed in (Buchon et al. 2014)), and that was recently shown to confer some protection against N. bombycis infection in silkworms (Hua et al. 2021). Characterization of intracellular immune mechanisms in bees that might target microsporidia represents a crucial direction for future research.
Complex Interactions Between Nosema spp. and the Honey bee Microbiome
The microbiomes of insects play key roles in their biology (Engel and Moran 2013), and it is likely that some of the impacts of honey bee disease are caused by interactions between the honey bee microbiome and Nosema spp. Recent studies have shown that the gut microbiota of honey bees is more complex than that found in solitary insects (Kwong and Moran 2016) and that its composition can have a significant impact on honey bee health (Raymann and Moran 2018). The microbiome community provides benefits to the honey bee host that include metabolic contributions (Zheng et al. 2017) and immune modulation (Kwong et al. 2017). In addition, the microbiome can regulate hormonal signaling found to be involved in some of the organismal-level processes perturbed by Nosema infection (described below) (Zheng et al. 2017). Thus, teasing out the relative contributions of host cell pathology and host dysbiosis in inciting Nosema disease will be challenging. Perturbation of the honey bee microbiota by diverse mechanisms, such as antibiotic exposure or dietary alterations, can negatively impact honey bee health. Changes in the microbiome can impact the severity and outcomes of infections by pathogenic microbes, including Nosema spp. Microbiome composition is correlated with infection intensity of N. ceranae infection in A. mellifera (Maes et al. 2016; Rubanov et al. 2019; Paris et al. 2020). The interaction appears to be bidirectional. Penicillin-streptomycin exposure can make bees more susceptible to N. ceranae infection (Li et al. 2017a). Conversely, reducing N. ceranae infection through RNAi increased the abundance of certain bacterial species, suggesting that infection directly or indirectly impacts the digestive tract microbiome (Huang and Evans 2020). Interestingly, recent data from Apis cerana also shows that the microbiota can suppress N. ceranae growth (Wu et al. 2020), suggesting an antagonistic relationship between N. ceranae and the microbiome is a more widespread phenomenon in different bee species.
7.3 Nosema Infection Induces Changes in Organismal Physiology and Behavior
Microsporidia infection in bees is associated with alterations in diverse tissues and organs that impact both physiology and behavior of infected individuals. Hypopharyngeal glands (HPG) are involved in producing secretions for feeding larvae in nurse bees and in foraging bees (Ahmad et al. 2021). HPG are decreased in size (Wang and Moeller 1969) and functionally deficient (Liu 1990) in adult bees infected with N. apis and show alterations in gene expression and enzyme activity in bees infected with N. ceranae (Li et al. 2019). The insect fat body is an important tissue for managing energy homeostasis (Arrese and Soulages 2010). Despite its importance as a metabolic hub for the organism, relatively little is known about the impacts of infection by Nosema spp. on this organ. One study found that fat body genes involved in metabolism and hormonal signaling were impacted by infection (Holt et al. 2013). N. ceranae infection also alters gene expression in the brain (Holt et al. 2013; McDonnell et al. 2013; Mayack et al. 2015; Doublet et al. 2016) and impacts learning and memory (Gage et al. 2017). Collectively, these tissue-/organ-level shifts act in concert to alter organismal physiology and behavior.
Taken together, organismal changes mimic an acceleration of those observed during normal honey bee development. Individuals of the nonreproductive worker caste of honey bees exhibit a phenomenon known as age polyethism, defined as the age-related division of labor for nonreproductive tasks (Seeley 1982). The most pronounced transition with this process is from nurse bees, which perform tasks inside the colony, to forager bees, involved in gathering resources from the environment (Johnson 2010). This shift is associated with significant behavioral and physiological changes and is highly regulated to optimize colony function. Infection by either N. apis (Hassanein 1953; Wang and Moeller 1970; Woyciechowski and Moroń 2009) or N. ceranae (Goblirsch et al. 2013; Dussaubat et al. 2013; Natsopoulou et al. 2015; Lecocq et al. 2016) causes worker bees to accelerate this aging process as manifested by both physiological and behavioral changes. One 2013 study found that infected bees are twice as likely to engage in precocious foraging compared to controls (Goblirsch et al. 2013). Foragers infected by N. ceranae (Naug 2014; Alaux et al. 2014) and N. apis (Lach et al. 2015; Dosselli et al. 2016) perform less efficiently, and N. ceranae-infected foragers are less likely to return to the colony due to energetic stress (Kralj and Fuchs 2010) and reduced homing ability (Wolf et al. 2014). Infected workers also spend more time outside the nest engaged in risky behaviors such as robbing (Kuszewska and Woyciechowski 2014).
Multiple hormonal systems known to control worker division of labor are impacted in bees infected by Nosema spp. The vitellogenin (Vg)/juvenile hormone (JH) axis is critical for controlling age polyethism (Johnson 2010). JH levels are increased in bees infected with either Nosema spp. (Ares et al. 2012), while Vg expression is reduced (Antúnez et al. 2013; Goblirsch et al. 2013; Zheng et al. 2014; Garrido et al. 2016). Bees infected as larva have increased Vg levels as young adults, suggesting different impacts based on the age of infection (BenVau and Nieh 2017). Infected bees also show alterations in the octopamine pathway (Mayack et al. 2015), which is also known to participate in the transition to foraging (Johnson 2010), and insulin signaling (Holt et al. 2013), involved in regulating caste (Ament et al. 2008, 2010).
Interestingly, a number of other stressors are known to induce a similar precociousness in the physiological and behavioral shifts associated with the development from nurse to forager as well as the hormonal systems controlling these transitions. For example, infection by diverse pathogens and parasitization by an arthropod pest induce comparable changes (McDonnell et al. 2013; Doublet et al. 2016). One theory to explain the related outcomes from these distinct stressors is that they all impinge on nutritional status which is known to heavily influence this transition (Ament et al. 2010). Here again, additional foundational knowledge of honey bee biology will likely facilitate a better understanding of Nosema disease. Based on the complexity of the interorgan communication systems used in fruit flies to regulate organismal physiology and behavior (Droujinine and Perrimon 2016; Liu and Jin 2017), it is likely that other molecules yet to be characterized in honey bees integrate signals of nutrition status (and injury) at the physiological levels and couple these inputs with control of life stage transitions in bees. The unpaired family of JAK/STAT pathway ligands described above is also known to regulate both physiology and behavior in flies (Droujinine and Perrimon 2016; Liu and Jin 2017). Characterizing the role of the recently described honey bee homologs of these ligands (Bach et al. 2021) in interorgan communication may represent one potential link between the infected digestive tract and organismal pathology.
The behavioral changes observed in parasitized bees may be seen to have adaptive benefits to both the parasite and host. For example, by engaging in risky behavior, infected bees spend less time in the colony potentially limiting transmission to nestmates and benefitting the colony (Rueppell et al. 2010). This potentially altruistic self-removal by infected bees may represent one of the social immunity strategies found in eusocial insect species (Cremer et al. 2018). However, such behavior could result in more efficient spread to individuals of uninfected colonies, thereby benefiting the pathogen. Similarly, N. ceranae-infected bees appear to seek out the warmer areas in the colony, which could help energetically stressed bees manage thermoregulation or provide the higher temperatures preferred by this microsporidia species and a better opportunity to spread the pathogen (Campbell et al. 2010). Thus, the adaptive benefit, if any, and the beneficiary of such behavioral changes in infected honey bees are unclear (Wagoner et al. 2013).
Many groups have further reported shortened worker lifespans after infection by Nosema spp. (Higes et al. 2006; Alaux et al. 2009; Vidau et al. 2011; Goblirsch et al. 2013; Williams et al. 2014; Doublet et al. 2015). For example, Goblirsch et al. observed a 9-day reduction in worker lifespan (Goblirsch et al. 2013). However, it is important to note that other studies have not observed differences in mortality in infected bees and these inconsistencies may be due to similar factors involved in modulating the colony impacts of Nosema disease, such as genetic variation on the part of both parasite and host and the presence of other stressors (Goblirsch 2017; Martín-Hernández et al. 2018).
7.4 Organismal Pathogenesis Caused by Nosema Disease Disrupts Colony Organization and Function
Honey bee colonies are eusocial (Holldobler and Wilson 2008), and colony physiology is regulated by the collective activities of individual colony members (Friedman et al. 2020). Individual infection by Nosema spp. appears to cause significant disruptions in colony organization and function which likely contribute to colony-level disease. For example, N. ceranae infection alters pheromone production in workers (Dussaubat et al. 2010, 2013) and queens (Alaux et al. 2011). Nosema spp. infection has been observed to alter production of ethyl oleate, which is involved in the regulation of division of labor among workers (Dussaubat et al. 2010). Another recent study found increased levels of alarm pheromone in colonies infected with N. ceranae (Mayack et al. 2021). Other studies have demonstrated differences in cuticular hydrocarbon profiles (McDonnell et al. 2013; Murray et al. 2016), although it is not clear how these changes impact interactions between workers (Murray et al. 2016; Biganski et al. 2018).
In the honey bee colony, the health and function of the reproductive individuals in the colony are critical for colony success. While less studied, both drones and queens can be infected by Nosema spp. Thus far, individuals from the reproductive castes display similar perturbations to worker bees in addition to important changes in biology unique to their colony function (reviewed in (Goblirsch 2017; Martín-Hernández et al. 2018)). The many impacts of microsporidia infection on the physiology and behavior of workers in addition to these effects on reproductive individuals likely contribute to colony death, especially in the presence of other stressors. The negative consequences of Nosema infection on bees are thought to be influenced by the presence of other stressors, including nutritional stress due to loss of appropriate forage, chemical poisoning from pesticides, changes to natural living conditions brought about through large-scale beekeeping practices, myriad environmental changes due to climate change, and infection by arthropod parasites and other pathogenic microbes. For example, N. ceranae infection can interact with chemical stressors, such as pesticides, on honey bees leading to synergistic effects on health and mortality (Alaux et al. 2010; Vidau et al. 2011; Wu et al. 2012; Pettis et al. 2012, 2013; Aufauvre et al. 2012, 2014; Retschnig et al. 2014; Doublet et al. 2015). In addition, chemical stressors are known to increase the prevalence or intensity of N. ceranae infection likely through impacts on host well-being (Wu et al. 2012; Pettis et al. 2013). Bees are also often coinfected by multiple pathogens (Runckel et al. 2011; Cornman et al. 2012), and the impacts on both pathogens and the host are likely to be complex. This topic has been recently covered in great detail (Goblirsch 2017; Martín-Hernández et al. 2018) and will not be explored further here.
7.5 Impact of Host Factors on Microsporidia Growth and Development
The parameters that influence the growth and development of Nosema cells within the host environment are still largely unknown. Microsporidia as a group have significantly reduced genomes relative to free-living fungi, with much of the lost coding content being found in metabolic pathways (Nakjang et al. 2013). To make up for reduced biosynthetic complexity, microsporidia are known to acquire diverse array of metabolites from host cells, often through the use of expanded families of transporters (Chetia et al. 2017), which are sometimes acquired through horizontal gene transfer (Dean et al. 2018). Recent studies of Tubulinosema ratisbonensis which infects fruit flies (Niehus et al. 2012) have shown that specific metabolites, namely, phosphatidic acid and related lipids, are limiting for the proliferation of the microsporidium in host cells (Franchet et al. 2019). Most studies of N. ceranae suggest that increased protein consumption leads to increased infection intensities as measured by spore counts (Porrini et al. 2011; Basualdo et al. 2014; Zheng et al. 2014; Fleming et al. 2015; Jack et al. 2016; Tritschler et al. 2017), suggesting that for this species, amino acids may be limiting. There is also evidence that many other host-derived nutrients are important for N. ceranae growth. For example, iron, which is often a limiting nutrient for diverse pathogenic microbes (Cassat and Skaar 2013), was recently observed to be reduced in forager honey bees (but not nurses) after N. ceranae infection (Rodríguez-García et al. 2021). RNAi-mediated knockdown of transferrin resulted in decreased available iron and led to reduced N. ceranae infection intensity. Limiting iron availability may be useful as a therapeutic strategy (Rodríguez-García et al. 2021).
The life cycle of Nosema spp. is complex and is likely influenced by the host environment and involves multiple developmental transitions characterized by distinct morphological forms and evolving patterns of expression of both mRNA (Huang et al. 2016b) and miRNA (Huang and Evans 2016; Shao et al. 2021) transcripts. For example, many microsporidia species appear to use the “early sporulation” strategy to produce primary spores for spread within host tissues before switching to the production of the secondary or environmental spore. The presence of intracellular empty spore coats indicates that intracellular germination of spores is occurring during N. ceranae infection (Higes et al. 2007; García-Palencia et al. 2010) as has been shown for N. apis (Fries et al. 1992). Another strategy for rapid spread within the tissues without the need to progress to the environmental spore is found in microsporidia infection in nematodes, which involves the removal of lateral membranes to form syncytia (Balla et al. 2016). Here, developmental timing, in particular the switch to sporulation, is triggered by parasite density (Balla et al. 2016). This may be true for N. ceranae as well, as inoculation dose has been shown to play a role in infection intensity and spore production (McGowan et al. 2016). Another possible signal could be related to available host resources, which may not only impact growth directly (see above) but may influence developmental decisions. Other possible cues include cell stress, which alters the developmental program of other fungi (Boyce and Andrianopoulos 2015). One stress that is known to impact N. ceranae infection is temperature (Martín-Hernández et al. 2009; Higes et al. 2010; McNamara-Bordewick et al. 2019), and some evidence suggests this effect is due to changes in the development of the pathogen (Higes et al. 2010). However, detailed analysis of the stage and impact has not heretofore been possible due to the difficulty in isolating the distinct developmental forms from host cells. Similarly, the current frontline drug for treating N. ceranae infection, fumagillin, may also impact development. At lower doses, this drug causes hyperproduction of spores compared to spore production in untreated bees (Huang et al. 2013).
While the typical focus is on the impacts on the honey bee host, the effect of various stressors on the growth and development of Nosema spp. in bees is also important to understand. Many environmental factors are carefully controlled by the healthy host cell, thereby reducing variability. Adaptation to this host-controlled environment would be predicted to have a significant impact on the cell stress machinery in microsporidia. In fact, comparative genomics indicates that microsporidia have lost many of the quality control and cell stress pathways found in free-living eukaryotes (McNamara-Bordewick et al. 2019; Snow 2020), perhaps leading to a loss of the redundancy and flexibility that often allow organisms to withstand cellular stresses. However, data suggests high levels of stress resistance in Nosema spp. For example, although the majority of fungal species prefer the 12°–30 °C range and relatively few species tolerate temperatures higher than 35 °C (Robert and Casadevall 2009), N. ceranae exhibits a striking ability to grow at the high temperatures (34–35 °C) maintained in honey bee colonies (Martín-Hernández et al. 2009; Higes et al. 2010), despite the loss of the Heat Shock Factor (Hsf) gene, encoding the canonical regulator of the heat shock response (McNamara-Bordewick et al. 2019). N. apis, while still missing the Hsf gene, is similar to typical fungi in its sensitivity to high temperatures (Burnside and Revell 1948; Woyciechowski and Czekońska 1999), and honey bees can recover from infection when kept at the slightly elevated temperature of 37 °C (Lotmar 1943). While the canonical cell stress response systems appear reduced, there may be adaptation of the cellular machinery to allow for novel responses to cell stress. This may be achieved through mechanisms that increase tolerance to stress. Microsporidia encode aminoacyl-tRNA synthetases without editing domains, and the wrong amino acid is added in up to 6% of cases for some codons. This diversity of protein variants may allow these parasites to adapt under different stress conditions (Melnikov et al. 2018). Another mechanism may involve manipulation of the host cell. For example, N. ceranae infection induces decreases in both the amount of ROS and oxidative damage (Paris et al. 2017), possibly mediated through pathogen manipulation of host cell machinery. Microsporidia genomes possess a large number of genes encoding proteins with predicted signal peptides. Many of these are likely secreted into the host cell and are thus candidates for interceding in the function of host cell processes (Cuomo et al. 2012; Campbell et al. 2013). In fact, Reinke et al. were able to use exogenous machinery expressed in host cells to label a number of host-exposed proteins from Nematocida species. Based on analysis of other microsporidia genomes, the authors predicted that other microsporidia species might use 6–32% of their proteome to interface with the host (Reinke et al. 2017). While such an experiment is not currently possible in honey bees, there is a need for methods to identify Nosema proteins responsible for host manipulation.
7.6 Conclusion and Future Perspectives
Since the discovery of N. ceranae infecting western honey bees, there have been impressive gains in our understanding of the effects these microsporidia have on honey bee health (Goblirsch 2017; Martín-Hernández et al. 2018). A number of questions and areas for further research are evident. As parasite growth is restricted to the digestive tract, the routes through which parasite growth and tissue dysfunction in the midgut leads to the dramatic physiological and behavioral changes at the organismal level are incompletely understood. If caused by pathogen-mediated manipulation, how are these changes effected at the molecular level? If due to disruptions in normal midgut function and host responses to this pathology, are changes specific to microsporidia infection or are they observed with diverse stressors? The critical host parameters that influence the growth and development of Nosema cells within the host environment also remain obscure. Relatedly, elucidating the positive and negative consequences of observed changes on both host and parasite is also critical.
A number of approaches are already being used to perform gain- and loss-of-function experiments to answer these questions. RNAi machinery exists in N. ceranae (Paldi et al. 2010), and RNAi has been shown to be a powerful tool in elucidating the machinery of both the parasite (Paldi et al. 2010; Huang et al. 2016a, 2018a; Rodríguez-García et al. 2018; Huang and Evans 2020) and the host (Li et al. 2016; Rodríguez-García et al. 2021). New genetic tools are being developed that could greatly facilitate investigations into the mechanisms involved in host-pathogen interactions. Recently, CRISPR/Cas9 protocols have been developed for the honey bee (Kohno et al. 2016; Kohno and Kubo 2018; Roth et al. 2019; Hu et al. 2019; Nie et al. 2021). Such tools could allow confirmation of host pathways and processes suspected to be involved in Nosema-induced pathogenesis. For example, loss-of-function experiments could be used to probe host factors involved in the reduced apoptosis, such as the candidate Iap (Kurze et al. 2015; Martín-Hernández et al. 2017).
Methods to generate genetically modified microsporidia would similarly represent an indispensable resource for making future advances (Reinke and Troemel 2015). Some critical progress has been made in culturing sporoplasm in N. bombycis for this purpose (He et al. 2019 2020) and in delivering genetic material (Guo et al. 2016a). A heterologous cell culture system has been established (Gisder et al. 2011; Gisder and Genersch 2015) that could facilitate biochemical studies although additional more robust cell culture models are necessary.
Such future studies could also provide much needed potential target molecules for treatment strategies for Nosema disease in honey bees. N. apis infection has long been controlled by treatment with the drug fumagillin, a methionine aminopeptidase 2 inhibitor (Heever et al. 2014). Yet, the effectiveness of fumagillin treatment for treating N. ceranae at the colony level appears transient. High doses of this drug impair host cell function, and evidence suggests that N. ceranae can escape suppression in some circumstances, although the mechanisms remain unknown (Huang et al. 2013). Most critically, the long-term availability of fumagillin is uncertain, making efforts to find alternative treatment strategies critical to protect honey bees from this parasite (Heever et al. 2014; Holt and Grozinger 2016). New treatment strategies to reduce the impact of Nosema disease on this pollinator species are currently being explored (see (Huntsman et al. 2021) and references therein), which could have significant positive impacts on the health of both agricultural and ecological systems.
Taxonomic Note
Nosema apis and N. ceranae have recently been redefined as Vairimorpha apis and V. ceranae based on a recent molecular phylogenetics analysis of the Nosema and Vairimorpha clades (Tokarev et al. 2020). For the purposes of this review the Nosema Genus will still be used.
References
Aguila C del, Izquierdo F, Granja AG, et al (2006) Encephalitozoon microsporidia modulates p 53-mediated apoptosis in infected cells. Int J Parasitol 36:869–876. https://doi.org/10.1016/j.ijpara.2006.04.002
Ahmad S, Khan SA, Khan KA, Li J (2021) Novel insight into the development and function of Hypopharyngeal glands in honey bees. Front Physiol 11:615830. https://doi.org/10.3389/fphys.2020.615830
Alaux C, Sinha S, Hasadsri L et al (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc Natl Acad Sci 106:15400
Alaux C, Brunet J-L, Dussaubat C et al (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12:774–782. https://doi.org/10.1111/j.1462-2920.2009.02123.x
Alaux C, Folschweiller M, McDonnell C et al (2011) Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J Invertebr Pathol 106:380–385. https://doi.org/10.1016/j.jip.2010.12.005
Alaux C, Crauser D, Pioz M et al (2014) Parasitic and immune modulation of flight activity in honey bees tracked with optical counters. J Exp Biol 217:3416–3424. https://doi.org/10.1242/jeb.105783
Aliferis KA, Copley T, Jabaji S (2012) Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J Insect Physiol 58:1349–1359. https://doi.org/10.1016/j.jinsphys.2012.07.010
Ament S, Corona M, Pollock H, Robinson G (2008) Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci 105:4226
Ament S, Wang Y, Robinson G (2010) Nutritional regulation of division of labor in honey bees: toward a systems biology perspective. Wiley Interdiscip Rev Syst Biol Med 2:566–576
Antúnez K, Martín-Hernández R, Prieto L et al (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (microsporidia). Environ Microbiol 11:2284–2290
Antúnez K, Mendoza Y, Santos E, Invernizzi C (2013) Differential expression of vitellogenin in honey bees (Apis mellifera) with different degrees of Nosema ceranae infection. J Apicult Res 52:227–234. https://doi.org/10.3896/ibra.1.52.5.09
Apidianakis Y, Rahme L (2011) Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech 4:21
Ares AM, Nozal MJ, Bernal JL et al (2012) Liquid chromatography coupled to ion trap-tandem mass spectrometry to evaluate juvenile hormone III levels in bee hemolymph from Nosema spp. infected colonies. J Chromatogr B 899:146–153. https://doi.org/10.1016/j.jchromb.2012.05.016
Arrese EL, Soulages JL (2010) Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55:207–225. https://doi.org/10.1146/annurev-ento-112408-085356
Aufauvre J, Biron DG, Vidau C et al (2012) Parasite-insecticide interactions: a case study of Nosema ceranae and fipronil synergy on honeybee. Sci Rep 2:326. https://doi.org/10.1038/srep00326
Aufauvre J, Misme-Aucouturier B, Viguès B et al (2014) Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS One 9:e91686. https://doi.org/10.1371/journal.pone.0091686.s005
Bach DM, Holzman MA, Wague F et al (2021) Thermal stress induces tissue damage and a broad shift in regenerative signaling pathways in the honey bee digestive tract. J Exp Biol
Badaoui B, Fougeroux A, Petit F et al (2017) RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS One 12:e0173438. https://doi.org/10.1371/journal.pone.0173438.s013
Bakowski MA, Desjardins CA, Smelkinson MG et al (2014) Ubiquitin-mediated response to microsporidia and virus infection in C. elegans. PLoS Pathog 10:e1004200. https://doi.org/10.1371/journal.ppat.1004200
Balla KM, Luallen RJ, Bakowski MA, Troemel ER (2016) Cell-to-cell spread of microsporidia causes Caenorhabditis elegans organs to form syncytia. Nat Microbiol 1(11):16144. https://doi.org/10.1038/nmicrobiol.2016.144
Balla KM, Lažetić V, Troemel ER (2019) Natural variation in the roles of C. elegans autophagy components during microsporidia infection. PLoS One 14:e0216011. https://doi.org/10.1371/journal.pone.0216011
Bao J, Liu L, An Y et al (2019) Nosema bombycis suppresses host hemolymph melanization through secreted serpin 6 inhibiting the prophenoloxidase activation cascade. J Invertebr Pathol 168:107260. https://doi.org/10.1016/j.jip.2019.107260
Basualdo M, Barragán S, Antúnez K (2014) Bee bread increases honeybee haemolymph protein and promote better survival despite of causing higher Nosema ceranae abundance in honeybees. Environ Microbiol Rep 6:396–400. https://doi.org/10.1111/1758-2229.12169
BenVau LR, Nieh JC (2017) Larval honey bees infected with Nosema ceranae have increased vitellogenin titers as young adults. Sci Rep 7:14144. https://doi.org/10.1038/s41598-017-14702-4
Biganski S, Kurze C, Müller MY, Moritz RFA (2018) Social response of healthy honeybees towards Nosema ceranae-infected workers: care or kill? Apidologie 49:325–334. https://doi.org/10.1007/s13592-017-0557-8
Boyce KJ, Andrianopoulos A (2015) Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev 39:797–811. https://doi.org/10.1093/femsre/fuv035
Buchon N, Silverman N, Cherry S (2014) Immunity in Drosophila melanogaster — from microbial recognition to whole-organism physiology. Nat Rev Immunol 14:796–810. https://doi.org/10.1038/nri3763
Burnside CF, Revell IL (1948) Observations on Nosema disease of honey bees. J Econ Entomol 41(4):603–607
Campbell J, Kessler B, Mayack C, Naug D (2010) Behavioural fever in infected honeybees: parasitic manipulation or coincidental benefit? Parasitology 137:1487–1491. https://doi.org/10.1017/s0031182010000235
Campbell SE, Williams TA, Yousuf A et al (2013) The genome of Spraguea lophii and the basis of host-microsporidian interactions. PLoS Genet 9:e1003676–e1003615. https://doi.org/10.1371/journal.pgen.1003676
Cassat JE, Skaar EP (2013) Iron in infection and immunity. Cell Host Microbe 13:509–519. https://doi.org/10.1016/j.chom.2013.04.010
Chaimanee V, Chantawannakul P, Chen Y et al (2012) Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J Insect Physiol 58:1090–1095. https://doi.org/10.1016/j.jinsphys.2012.04.016
Chetia H, Kabiraj D, Sharma S, Bora U (2017) Comparative insights to the transportome of Nosema: a genus of parasitic microsporidians. bioRxiv. https://doi.org/10.1101/110809
Cornman RS, Tarpy DR, Chen Y et al (2012) Pathogen webs in collapsing honey bee colonies. PLoS One 7:e43562. https://doi.org/10.1371/journal.pone.0043562
Cremer S, Pull CD, Fuerst MA (2018) Social immunity: emergence and evolution of Colony-level disease protection. Annu Rev Entomol 63:105–123. https://doi.org/10.1146/annurev-ento-020117-043110
Cuomo CA, Desjardins CA, Bakowski MA et al (2012) Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res 22:2478–2488. https://doi.org/10.1101/gr.142802.112
Dean P, Sendra KM, Williams TA et al (2018) Transporter gene acquisition and innovation in the evolution of microsporidia intracellular parasites. Nat Commun 9:1709. https://doi.org/10.1038/s41467-018-03923-4
Dolgikh VV, Tsarev AA, Timofeev SA, Zhuravlyov VS (2019) Heterologous overexpression of active hexokinases from microsporidia Nosema bombycis and Nosema ceranae confirms their ability to phosphorylate host glucose. Parasitol Res 118:1511–1518. https://doi.org/10.1007/s00436-019-06279-w
Dosselli R, Grassl J, Carson A et al (2016) Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite Nosema apis. Sci Rep 6:36649. https://doi.org/10.1038/srep36649
Doublet V, Labarussias M, de Miranda JR et al (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol 17:969–983. https://doi.org/10.1111/1462-2920.12426
Doublet V, Paxton RJ, McDonnell CM et al (2016) Brain transcriptomes of honey bees (Apis mellifera) experimentally infected by two pathogens: black queen cell virus and Nosema ceranae. GDATA 10:79–82. https://doi.org/10.1016/j.gdata.2016.09.010
Doublet V, Poeschl Y, Gogol-Döring A et al (2017) Unity in defence: honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genomics 18(1):207. https://doi.org/10.1186/s12864-017-3597-6
Droujinine IA, Perrimon N (2016) Interorgan communication pathways in physiology: focus on drosophila. Annu Rev Genet 50:539–570. https://doi.org/10.1146/annurev-genet-121415-122024
Dussaubat C, Maisonnasse A, Alaux C et al (2010) Nosema spp. infection alters pheromone production in honey bees (Apis mellifera). J Chem Ecol 36:522–525. https://doi.org/10.1007/s10886-010-9786-2
Dussaubat C, Brunet J-L, Higes M et al (2012) Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS One 7:e37017. https://doi.org/10.1371/journal.pone.0037017.t001
Dussaubat C, Maisonnasse A, Crauser D et al (2013) Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J Invertebr Pathol 113:42–51. https://doi.org/10.1016/j.jip.2013.01.002
Emsen B, Guzman-Novoa E, Hamiduzzaman MM et al (2016) Higher prevalence and levels of Nosema ceranae than Nosema apis infections in Canadian honey bee colonies. Parasitol Res 115:175–181. https://doi.org/10.1007/s00436-015-4733-3
Engel P, Moran NA (2013) The gut microbiota of insects – diversity in structure and function. FEMS Microbiol Rev 37:699–735. https://doi.org/10.1111/1574-6976.12025
Fleming JC, Schmehl DR, Ellis JD (2015) Characterizing the impact of commercial pollen substitute diets on the level of Nosema spp. in honey bees (Apis mellifera L.). PLoS ONE. https://doi.org/10.1371/journal.pone.0132014.s001
Franchet A, Niehus S, Caravello G, Ferrandon D (2019) Phosphatidic acid as a limiting host metabolite for the proliferation of the microsporidium Tubulinosema ratisbonensis in drosophila flies. Nat Publ Group 4:645–655. https://doi.org/10.1038/s41564-018-0344-y
Friedman DA, Johnson BR, Linksvayer TA (2020) Distributed physiology and the molecular basis of social life in eusocial insects. Horm Behav 122:104757. https://doi.org/10.1016/j.yhbeh.2020.104757
Fries I (1988) Infectivity and multiplication of Nosema-Apis Z in the Ventriculus of the honey bee. Ann Abeille 19:319–328
Fries I (2014) Microsporidia: pathogens of opportunity. First Edition Microsporidia: Pathogens of Opportunity, 571–577. https://doi.org/10.1002/9781118395264.ch22
Fries I, Granados RR, Morse RA (1992) Intracellular germination of spores of Nosema-Apis Z. Ann Abeille 23:61–70
Gage SL, Kramer C, Calle S et al (2017) Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees(Apis mellifera). J Exp Biol 21(Pt 4):jeb161489. https://doi.org/10.1242/jeb.161489
Gallai N, Salles J-M, Settele J, Vaissière BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821. https://doi.org/10.1016/j.ecolecon.2008.06.014
García-Palencia P, Martín-Hernández R, González-Porto A-V et al (2010) Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J Apicult Res 49:278–283. https://doi.org/10.3896/ibra.1.49.3.08
Garrido PM, Porrini MP, Antúnez K et al (2016) Sublethal effects of acaricides and Nosema ceranae infection on immune related gene expression in honeybees. Vet Res 47:51. https://doi.org/10.1186/s13567-016-0335-z
Gilliam M, Shimanuki H (1967) In vitro phagocytosis of Nosema apis spores by honey-bee hemocytes* 1,* 2. J Invertebr Pathol 9:387–389
Gisder S, Genersch E (2015) Identification of candidate agents active against N. ceranae infection in honey bees: establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS One 10:e0117200. https://doi.org/10.1371/journal.pone.0117200.t002
Gisder S, Moeckel N, Linde A, Genersch E (2011) A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ Microbiol 13:404–413. https://doi.org/10.1111/j.1462-2920.2010.02346.x
Gisder S, Schüler V, Horchler LL et al (2017) Long-term temporal trends of Nosema spp. infection prevalence in Northeast Germany: continuous spread of Nosema ceranae, an emerging pathogen of honey bees (Apis mellifera), but no general replacement of Nosema apis. Front Cell Infect Microbiol 7:1–14. https://doi.org/10.3389/fcimb.2017.00301
Goblirsch M (2017) Nosema ceranae disease of the honey bee (Apis mellifera). Ann Abeille 49:131–150. https://doi.org/10.1007/s13592-017-0535-1
Goblirsch M, Huang ZY, Spivak M (2013) Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS One 8:e58165. https://doi.org/10.1371/journal.pone.0058165.t002
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science (New York, NY) 347:1255957. https://doi.org/10.1126/science.1255957
Graaf DCD, Raes H, Sabbe G et al (1994) Early development of Nosema-Apis (Microspora, Nosematidae) in the midgut epithelium of the honeybee (Apis-Mellifera). J Invertebr Pathol 63:74–81
Guo R, Cao G, Lu Y et al (2016a) Exogenous gene can be integrated into Nosema bombycis genome by mediating with a non-transposon vector. Parasitol Res 115(8):3093–3098. https://doi.org/10.1007/s00436-016-5064-8
Guo Z, Lucchetta E, Rafel N, Ohlstein B (2016b) Maintenance of the adult drosophila intestine: all roads lead to homeostasis. Curr Opin Genet Dev 40:81–86. https://doi.org/10.1016/j.gde.2016.06.009
Hassanein MH (1953) The influence of infection with Nosema apis on the activities and longevity of the worker honeybee. Ann Appl Biol 40:418–423. https://doi.org/10.1111/j.1744-7348.1953.tb01093.x
He X, Fu Z, Li M et al (2015) Nosema bombycis(microsporidia) suppresses apoptosis in BmN cells (Bombyx mori). Acta Biochim Biophys Sin 47:696–702. https://doi.org/10.1093/abbs/gmv062
He Q, Luo J, Xu J-Z et al (2019) In-vitro cultivation of Nosema bombycis sporoplasms: a method for potential genetic engineering of microsporidia. J Invertebr Pathol 174:107420. https://doi.org/10.1016/j.jip.2020.107420
He Q, Luo J, Xu J-Z et al (2020) Morphology and transcriptome analysis of Nosema bombycis sporoplasm and insights into the initial infection of microsporidia. mSphere 5:e00958-19. https://doi.org/10.1128/msphere.00958-19
Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54:285–302. https://doi.org/10.1146/annurev.ento.54.110807.090559
Higes M, Martín R, Meana A (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol 92:93–95. https://doi.org/10.1016/j.jip.2006.02.005
Higes M, García-Palencia P, Martín-Hernández R, Meana A (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (microsporidia). J Invertebr Pathol 94:211–217. https://doi.org/10.1016/j.jip.2006.11.001
Higes M, García-Palencia P, Botías C et al (2010) The differential development of microsporidia infecting worker honey bee (Apis mellifera) at increasing incubation temperature. Environ Microbiol Rep 2:745–748. https://doi.org/10.1111/j.1758-2229.2010.00170.x
Higes M, Juarranz Á, Dias-Almeida J et al (2013) Apoptosis in the pathogenesis of Nosema ceranae (microsporidia: Nosematidae) in honey bees (Apis mellifera). Environ Microbiol Rep 5:530–536. https://doi.org/10.1111/1758-2229.12059
Higes M, García-Palencia P, Urbieta A et al (2020) Nosema apis and Nosema ceranae tissue tropism in worker honey bees (Apis mellifera). Vet Pathol 57:132–138. https://doi.org/10.1177/0300985819864302
Holldobler B, Wilson EO (2008) The superorganism: the beauty, elegance, and strangeness of insect societies. W. W. Norton, New York
Holt HL, Grozinger CM (2016) Approaches and challenges to managing Nosema (Microspora: Nosematidae) parasites in honey bee (hymenoptera: Apidae) colonies. J Econ Entomol 109:1487–1503. https://doi.org/10.1093/jee/tow103
Holt HL, Aronstein KA, Grozinger CM (2013) Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genomics 14:799. https://doi.org/10.1186/1471-2164-14-799
Houdelet C, Sinpoo C, Chantaphanwattana T et al (2020) Proteomics of anatomical sections of the gut of Nosema -infected Western honeybee (Apis mellifera) reveals different early responses to Nosema spp. isolates. J Proteome Res 20:804–817. https://doi.org/10.1021/acs.jproteome.0c00658
Hu XF, Zhang B, Liao CH, Zeng ZJ (2019) High-efficiency CRISPR/Cas9-mediated gene editing in honeybee (Apis mellifera) embryos. G3 (Bethesda, md) 9(5):1759–1766. https://doi.org/10.1534/g3.119.400130
Hua X, Xu W, Ma S, Xia Q (2021) STING-dependent autophagy suppresses Nosema bombycis infection in silkworms. Bombyx Mori Dev Comp Immunol 115:103862. https://doi.org/10.1016/j.dci.2020.103862
Huang Q, Evans JD (2016) Identification of microRNA-like small RNAs from fungal parasite Nosema ceranae. J Invertebr Pathol 133:107–109. https://doi.org/10.1016/j.jip.2015.12.005
Huang Q, Evans JD (2020) Targeting the honey bee gut parasite Nosema ceranae with siRNA positively affects gut bacteria. BMC Microbiol 20:258. https://doi.org/10.1186/s12866-020-01939-9
Huang W-F, Solter LF (2013) Comparative development and tissue tropism of Nosema apis and Nosema ceranae. J Invertebr Pathol 113:35–41. https://doi.org/10.1016/j.jip.2013.01.001
Huang Q, Kryger P, Conte YL, Moritz RFA (2012) Survival and immune response of drones of a Nosemosis tolerant honey bee strain towards N. ceranae infections. J Invertebr Pathol 109:297–302. https://doi.org/10.1016/j.jip.2012.01.004
Huang W-F, Solter LF, Yau PM, Imai BS (2013) Nosema ceranae Escapes Fumagillin Control in Honey Bees. PLoS Pathog 9:e1003185. https://doi.org/10.1371/journal.ppat.1003185
Huang Q, Chen Y, Wang RW et al (2015) Honey bee microRNAs respond to infection by the microsporidian parasite Nosema ceranae. Sci Rep 5:17494. https://doi.org/10.1038/srep17494
Huang Q, Chen Y, Neumann P, Evans JD (2016a) Effective silencing of dicer decreases spore load of the honey bee parasite Nosema ceranae. Fungal Genom Biol 6:1000144. https://doi.org/10.4172/2165-8056.1000144
Huang Q, Chen YP, Wang RW et al (2016b) Host-parasite interactions and purifying selection in a microsporidian parasite of honey bees. PLoS One 11:e0147549. https://doi.org/10.1371/journal.pone.0147549
Huang Q, Li W, Chen Y et al (2018a) Dicer regulates Nosema ceranae proliferation in honey bees. Insect Mol Biol 28(1):74–85. https://doi.org/10.1111/imb.12534
Huang Y, Zheng S, Mei X et al (2018b) A secretory hexokinase plays an active role in the proliferation of Nosema bombycis. Peerj 6:e5658. https://doi.org/10.7717/peerj.5658
Huntsman EM, Cho RM, Kogan HV et al (2021) Proteasome inhibition is an effective treatment strategy for microsporidia infection in honey bees. Biomol Ther 11:1600. https://doi.org/10.3390/biom11111600
Jack CJ, Uppala SS, Lucas HM, Sagili RR (2016) Effects of pollen dilution on infection of Nosema ceranae in honey bees. J Insect Physiol 87:12–19. https://doi.org/10.1016/j.jinsphys.2016.01.004
Jarkass HTE, Reinke AW (2020) The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell Microbiol 14:e0216011–e0216012. https://doi.org/10.1111/cmi.13247
Jiang H, Tian A, Jiang J (2016) Intestinal stem cell response to injury: lessons from drosophila. Cell Mol Life Sci: CMLS 73:3337–3349. https://doi.org/10.1007/s00018-016-2235-9
Johnson BR (2010) Division of labor in honeybees: form, function, and proximate mechanisms. Behav Ecol Sociobiol 64:305–316. https://doi.org/10.1007/s00265-009-0874-7
Jousse C, Dalle C, Abila A et al (2020) A combined LC-MS and NMR approach to reveal metabolic changes in the hemolymph of honeybees infected by the gut parasite Nosema ceranae. J Invertebr Pathol 176:107478. https://doi.org/10.1016/j.jip.2020.107478
Klee J, Besana AM, Genersch E et al (2007) Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J Invertebr Pathol 96:1–10. https://doi.org/10.1016/j.jip.2007.02.014
Kohno H, Kubo T (2018) mKast is dispensable for normal development and sexual maturation of the male European honeybee. Sci Rep-uk 8:11877. https://doi.org/10.1038/s41598-018-30380-2
Kohno H, Suenami S, Takeuchi H et al (2016) Production of knockout mutants by CRISPR/Cas9 in the European honeybee, Apis mellifera L. Zool Sci 33:505–512. https://doi.org/10.2108/zs160043
Kralj J, Fuchs S (2010) Nosema sp influences flight behavior of infected honey bee (Apis mellifera) foragers. Ann Abeille 41:21–28. https://doi.org/10.1051/apido/2009046
Kurze C, Conte YL, Dussaubat C et al (2015) Nosema tolerant honeybees (Apis mellifera) escape parasitic manipulation of apoptosis. PLoS One 10:e0140174–e0140178. https://doi.org/10.1371/journal.pone.0140174
Kurze C, Dosselli R, Grassl J et al (2016a) Differential proteomics reveals novel insights into Nosema–honey bee interactions. Insect Biochem Mol Biol 79:42–49. https://doi.org/10.1016/j.ibmb.2016.10.005
Kurze C, Mayack C, Hirche F et al (2016b) Nosema spp. infections cause no energetic stress in tolerant honeybees. Parasitol Res 115:2381–2388. https://doi.org/10.1007/s00436-016-4988-3
Kurze C, Routtu J, Moritz RFA (2016c) Parasite resistance and tolerance in honeybees at the individual and social level. Zoology 119(4):290–297. https://doi.org/10.1016/j.zool.2016.03.007
Kurze C, Conte YL, Kryger P et al (2018) Infection dynamics of Nosema ceranae in honey bee midgut and host cell apoptosis. J Invertebr Pathol 154:1–4. https://doi.org/10.1016/j.jip.2018.03.008
Kuszewska K, Woyciechowski M (2014) Risky robbing is a job for short-lived and infected worker honeybees. Ann Abeille 45:537–544. https://doi.org/10.1007/s13592-014-0267-4
Kwong WK, Moran NA (2016) Gut microbial communities of social bees. Nat Rev Microbiol 14:374–384. https://doi.org/10.1038/nrmicro.2016.43
Kwong WK, Mancenido AL, Moran NA (2017) Immune system stimulation by the native gut microbiota of honey bees. R Soc Open Sci 4:170003. https://doi.org/10.1098/rsos.170003
Lach L, Kratz M, Baer B (2015) Parasitized honey bees are less likely to forage and carry less pollen. J Invertebr Pathol 130:64–71. https://doi.org/10.1016/j.jip.2015.06.003
Lecocq A, Jensen AB, Kryger P, Nieh JC (2016) Parasite infection accelerates age polyethism in young honey bees. Sci Rep 6:22042. https://doi.org/10.1038/srep22042
Li W, Evans JD, Huang Q et al (2016) Silencing the honey bee (Apis mellifera) Naked Cuticle Gene (NKD) improves host immune function and reduces Nosema ceranae infections. Appl Environ Microbiol 82:6779–6787. https://doi.org/10.1128/aem.02105-16
Li JH, Evans JD, Li WF et al (2017a) New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PLoS One 12:e0187505. https://doi.org/10.1371/journal.pone.0187505.s004
Li W, Evans JD, Li J et al (2017b) Spore load and immune response of honey bees naturally infected by Nosema ceranae. Parasitol Res 116:3265–3274. https://doi.org/10.1007/s00436-017-5630-8
Li W, Chen Y, Cook SC (2018) Chronic Nosema ceranae infection inflicts comprehensive and persistent immunosuppression and accelerated lipid loss in host Apis mellifera honey bees. Int J Parasitol 48:433–444. https://doi.org/10.1016/j.ijpara.2017.11.004
Li Z, He J, Yu T et al (2019) Transcriptional and physiological responses of hypopharyngeal glands in honeybees (Apis mellifera L.) infected by Nosema ceranae. Apidologie 50:51–62. https://doi.org/10.1007/s13592-018-0617-8
Liu TP (1984) Ultrastructure of the midgut of the worker honey bee Apis mellifera heavily infected with Nosema apis. J Invertebr Pathol 44:282–291. https://doi.org/10.1016/0022-2011(84)90026-0
Liu TP (1990) Ultrastructural analysis on the gland secretion in the extracellular ducts of the hypopharyngeal glands of the honeybee infected by Nosema apis. Tissue Cell 4:533–540
Liu Q, Jin LH (2017) Organ-to-organ communication: a drosophila gastrointestinal tract perspective. Front Cell Dev Biol 5:321–316. https://doi.org/10.3389/fcell.2017.00029
Lotmar R (1943) Ueber den Einfluss der Temperatur auf den Parasiten Nosema apis. Beiheft Schweizerische Bienenzeitung 1:261–284
Ma Z, Li C, Pan G et al (2013) Genome-wide transcriptional response of silkworm (Bombyx mori) to infection by the microsporidian Nosema bombycis. PLoS One 8:e84137. https://doi.org/10.1371/journal.pone.0084137
Maes PW, Rodrigues PAP, Oliver R et al (2016) Diet related gut bacterial Dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honey bee (Apis mellifera). Mol Ecol 25(21):5439–5450. https://doi.org/10.1111/mec.13862
Malone LA, Gatehouse HS (1998) Effects of Nosema apis infection on honey bee (Apis mellifera) digestive proteolytic enzyme activity. J Invertebr Pathol 71:169–174
Martín-Hernández R, Meana A, García-Palencia P et al (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol 75:2554–2557. https://doi.org/10.1128/aem.02908-08
Martín-Hernández R, Botías C, Desai SD et al (2011) Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res 109:605–612. https://doi.org/10.1007/s00436-011-2292-9
Martín-Hernández R, Higes M, Sagastume S et al (2017) Microsporidia infection impacts the host cell’s cycle and reduces host cell apoptosis. PLoS One 12:e0170183. https://doi.org/10.1371/journal.pone.0170183.s003
Martín-Hernández R, Bartolomé C, Chejanovsky N et al (2018) Nosema ceranae in Apis mellifera: a 12 years postdetection perspective. Environ Microbiol 20:1302–1329. https://doi.org/10.1111/1462-2920.14103
Mayack C, Naug D (2009) Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J Invertebr Pathol 100:185–188. https://doi.org/10.1016/j.jip.2008.12.001
Mayack C, Naug D (2010) Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J Insect Physiol 56:1572–1575. https://doi.org/10.1016/j.jinsphys.2010.05.016
Mayack C, Natsopoulou ME, McMahon DP (2015) Nosema ceranae alters a highly conserved hormonal stress pathway in honeybees. Insect Mol Biol. https://doi.org/10.1111/imb.12190
Mayack C, Broadrup RL, Schick SJ et al (2021) Increased alarm pheromone component is associated with Nosema ceranae infected honeybee colonies. R Soc Open Sci 8:210194. https://doi.org/10.1098/rsos.210194&domain=pdf&date_stamp=2021-04-28
McDonnell CM, Alaux C, Parrinello H et al (2013) Ecto- and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC Ecol 13:25. https://doi.org/10.1186/1472-6785-13-25
McGowan J, la Mora AD, Goodwin PH et al (2016) Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J Microbiol Methods 131:16–22. https://doi.org/10.1016/j.mimet.2016.09.021
McNamara-Bordewick NK, McKinstry M, Snow JW (2019) Robust transcriptional response to heat shock impacting diverse cellular processes despite lack of heat shock factor in microsporidia. mSphere 4:e00219-19. https://doi.org/10.1128/msphere.00219-19
Melnikov SV, Rivera KD, Ostapenko D et al (2018) Error-prone protein synthesis in parasites with the smallest eukaryotic genome. Proc Natl Acad Sci U S A 115:E6245–E6253. https://doi.org/10.1073/pnas.1803208115
Murray ZL, Keyzers RA, Barbieri RF et al (2016) Two pathogens change cuticular hydrocarbon profiles but neither elicit a social behavioural change in infected honey bees, Apis mellifera (Apidae: hymenoptera). Austral Entomol 55:147–153. https://doi.org/10.1111/aen.12165
Nakjang S, Williams TA, Heinz E et al (2013) Reduction and expansion in microsporidian genome evolution: new insights from comparative genomics. Genome Biol Evol 5:2285–2303. https://doi.org/10.1093/gbe/evt184
Natsopoulou ME, McMahon DP, Paxton RJ (2015) Parasites modulate within-colony activity and accelerate the temporal polyethism schedule of a social insect, the honey bee. Behav Ecol Sociobiol 70:1019–1031. https://doi.org/10.3390/insects4040609
Naug D (2014) Infected honeybee foragers incur a higher loss in efficiency than in the rate of energetic gain. Biol Lett 10:20140731. https://doi.org/10.1098/rsbl.2014.0731
Naug D, Gibbs A (2009) Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 40:595–599. https://doi.org/10.1051/apido/2009039
Ni W, Bao J, Mo B et al (2020) Hemocytin facilitates host immune responses against Nosema bombycis. Dev Comp Immunol 103:103495. https://doi.org/10.1016/j.dci.2019.103495
Nie H-Y, Liang L-Q, Li Q-F et al (2021) CRISPR/Cas9 mediated knockout of Amyellow-y gene results in melanization defect of the cuticle in adult Apis mellifera. J Insect Physiol 132:104264. https://doi.org/10.1016/j.jinsphys.2021.104264
Niehus S, Giammarinaro P, Liégeois S et al (2012) Fly culture collapse disorder: detection, prophylaxis and eradication of the microsporidian parasite Tubulinosema ratisbonensis infecting Drosophila melanogaster. Fly 6:193–204. https://doi.org/10.4161/fly.20896
Paldi N, Glick E, Oliva M et al (2010) Effective gene silencing in a microsporidian parasite associated with honeybee (Apis mellifera) Colony declines. Appl Environ Microbiol 76:5960
Pan G, Bao J, Ma Z et al (2018) Invertebrate host responses to microsporidia infections. Dev Comp Immunol 83:104–113. https://doi.org/10.1016/j.dci.2018.02.004
Panek J, Paris L, Roriz D et al (2018) Impact of the microsporidian Nosema ceranae on the gut epithelium renewal of the honeybee, Apis mellifera. J Invertebr Pathol 159:121–128. https://doi.org/10.1016/j.jip.2018.09.007
Paris L, Roussel M, Pereira B et al (2017) Disruption of oxidative balance in the gut of the western honeybee Apis mellifera-exposed to the intracellular parasite Nosema ceranae and to the insecticide fipronil. Microb Biotechnol 12:774. https://doi.org/10.1002/elps.200405890
Paris L, Peghaire E, Mone A et al (2020) Honeybee gut microbiota dysbiosis in pesticide/parasite co-exposures is mainly induced by Nosema ceranae. J Invertebr Pathol 172:107348. https://doi.org/10.1016/j.jip.2020.107348
Pasquale GD, Salignon M, Conte YL et al (2013) Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One 8:e72016. https://doi.org/10.1371/journal.pone.0072016.s003
Pettis JS, vanEnglesdorp D, Johnson J, Dively G (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99:153–158. https://doi.org/10.1007/s00114-011-0881-1
Pettis JS, Lichtenberg EM, Andree M et al (2013) Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One 8:e70182. https://doi.org/10.1371/journal.pone.0070182
Porrini MP, Sarlo E, Medici SK (2011) Nosema ceranae development in Apis mellifera: influence of diet and infective inoculum. J Apic Res. https://doi.org/10.1016/j.jip.2007.08.005
Potts SG, Imperatriz-Fonseca V, Ngo HT et al (2016) Safeguarding pollinators and their values to human well-being. Nature 540:220–229. https://doi.org/10.1038/nature20588
Raymann K, Moran NA (2018) The role of the gut microbiome in health and disease of adult honey bee workers. Curr Opin Insect Sci 26:97–104. https://doi.org/10.1016/j.cois.2018.02.012
Reddy KC, Dror T, Underwood RS et al (2019) Antagonistic paralogs control a switch between growth and pathogen resistance in C. elegans. PLoS Pathog 15:e1007528. https://doi.org/10.1371/journal.ppat.1007528
Reinke AW, Troemel ER (2015) The development of genetic modification techniques in intracellular parasites and potential applications to microsporidia. PLoS Pathog 11:e1005283. https://doi.org/10.1371/journal.ppat.1005283.g001
Reinke AW, Balla KM, Bennett EJ, Troemel ER (2017) Identification of microsporidia host-exposed proteins reveals a repertoire of rapidly evolving proteins. Nat Commun 8:14023. https://doi.org/10.1038/ncomms14023
Retschnig G, Neumann P, Williams GR (2014) Thiacloprid-Nosema ceranae interactions in honey bees: host survivorship but not parasite reproduction is dependent on pesticide dose. J Invertebr Pathol 118:18–19. https://doi.org/10.1016/j.jip.2014.02.008
Robert VA, Casadevall A (2009) Vertebrate Endothermy restricts Most fungi as potential pathogens. J Infect Dis 200:1623–1626. https://doi.org/10.1086/644642
Roberts KE, Hughes WOH (2014) Immunosenescence and resistance to parasite infection in the honey bee, Apis mellifera. J Invertebr Pathol 121:1–6. https://doi.org/10.1016/j.jip.2014.06.004
Rodríguez-García C, Evans JD, Li W et al (2018) Nosemosis control in European honey bees, Apis mellifera, by silencing the gene encoding Nosema ceranae polar tube protein 3. J Exp Biol 221:jeb184606. https://doi.org/10.1242/jeb.184606
Rodríguez-García C, Heerman MC, Cook SC et al (2021) Transferrin-mediated iron sequestration suggests a novel therapeutic strategy for controlling Nosema disease in the honey bee. Apis mellifera PLoS Pathogens 17:e1009270. https://doi.org/10.1371/journal.ppat.1009270
Roth A, Vleurinck C, Netschitailo O et al (2019) A genetic switch for worker nutrition-mediated traits in honeybees. PLoS Biol 17:e3000171–e3000118. https://doi.org/10.1371/journal.pbio.3000171
Rubanov A, Russell KA, Rothman JA et al (2019) Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci Rep 9:3820–3828. https://doi.org/10.1038/s41598-019-40347-6
Rueppell O, Hayworth MK, Ross N (2010) Altruistic self-removal of health-compromised honey bee workers from their hive. J Evol Biol 23:1538–1546. https://doi.org/10.1111/j.1420-9101.2010.02022.x
Runckel C, Flenniken ML, Engel JC et al (2011) Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 6:e20656. https://doi.org/10.1371/journal.pone.0020656
Scanlon M, Leitch GJ, Shaw AP et al (1999) Susceptibility to apoptosis is reduced in the Microsporidia-infected host cell. J Eukaryot Microbiol 5:34S–35S
Schwarz RS, Evans JD (2013) Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev Comp Immunol 40:300–310. https://doi.org/10.1016/j.dci.2013.03.010
Seeley T (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11:287–293
Senderskiy IV, Timofeev SA, Seliverstova EV et al (2014) Secretion of Antonospora (Paranosema) locustae proteins into infected cells suggests an active role of microsporidia in the control of host programs and metabolic processes. PLoS One 9:e93585–e93512. https://doi.org/10.1371/journal.pone.0093585
Shao SS, Yan WY, Huang Q (2021) Identification of novel miRNAs from the microsporidian parasite Nosema ceranae. Infect Genetics Evol 93:104930. https://doi.org/10.1016/j.meegid.2021.104930
Smith MR, Singh GM, Mozaffarian D, Myers SS (2015) Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. Lancet 386:1964–1972. https://doi.org/10.1016/s0140-6736(15)61085-6
Snow JW (2016) A fluorescent method for visualization of Nosema infection in whole-mount honey bee tissues. J Invertebr Pathol 135:10–14. https://doi.org/10.1016/j.jip.2016.01.007
Snow JW (2020) Prolyl-tRNA synthetase inhibition reduces microsporidia infection intensity in honey bees. Ann Abeille 51:557–569. https://doi.org/10.1007/s13592-020-00742-9
Solter LF, Becnel JJ, Oi DH (2012) Insect pathology (Second edition), pp 221–263. https://doi.org/10.1016/b978-0-12-384984-7.00007-5
Steinhauer N, Kulhanek K, Antúnez K et al (2018) Drivers of colony losses. Curr Opin Insect Sci 26:142–148. https://doi.org/10.1016/j.cois.2018.02.004
Timofeev S, Tokarev Y, Dolgikh V (2020) Energy metabolism and its evolution in microsporidia and allied taxa. Parasitol Res 119(5):1433–1441. https://doi.org/10.1007/s00436-020-06657-9
Tokarev YS, Huang W-F, Solter LF et al (2020) A formal redefinition of the genera Nosema and Vairimorpha (microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J Invertebr Pathol 169:107279. https://doi.org/10.1016/j.jip.2019.107279
Tritschler M, Vollmann JJ, Yañez O et al (2017) Protein nutrition governs within-host race of honey bee pathogens. Sci Rep 7(1):14988. https://doi.org/10.1038/s41598-017-15358-w
Valizadeh P, Guzman-Novoa E, Goodwin PH (2020) Effect of immune inducers on Nosema ceranae multiplication and their impact on honey bee (Apis mellifera L.) survivorship and behaviors. Insects 11:572. https://doi.org/10.3390/insects11090572
van den Heever JP, Thompson TS, Curtis JM et al (2014) Fumagillin: An overview of recent scientific advances and their significance for apiculture. J Agric Food Chem 62:2728–2737. https://doi.org/10.1021/jf4055374
Vidau C, Diogon M, Aufauvre J et al (2011) Exposure to sublethal doses of Fipronil and Thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS One 6:e21550. https://doi.org/10.1371/journal.pone.0021550.g004
Vidau C, Panek J, Texier C et al (2014) Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J Invertebr Pathol 121:89–96. https://doi.org/10.1016/j.jip.2014.07.002
Wagoner KM, Boncristiani HF, Rueppell O (2013) Multifaceted responses to two major parasites in the honey bee (Apis mellifera). BMC Ecol 13:26. https://doi.org/10.1186/1472-6785-13-26
Wang D-I, Moeller FE (1969) Histological comparisons of the development of hypopharyngeal glands in healthy and Nosema-infected worker honey bees. J Invertebr Pathol 14:135–142. https://doi.org/10.1016/0022-2011(69)90098-6
Wang D-I, Moeller FE (1970) The division of labor and queen attendance behavior of Nosema-infected worker honey bees. J Econ Entomol 5:1539–1541
Ward K, Coleman J, Clinnin K, Fahrbach S (2008) Age, caste, and behavior determine the replicative activity of intestinal stem cells in honeybees (Apis mellifera L.). Exp Gerontol 43(6):530–537
Willard LE, Hayes AM, Wallrichs MA, Rueppell O (2011) Food manipulation in honeybees induces physiological responses at the individual and colony level. Ann Abeille 42:508–518. https://doi.org/10.1007/s13592-011-0006-z
Williams GR, Shutler D, Burgher-MacLellan KL, Rogers REL (2014) Infra-population and -community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS One 9:e99465. https://doi.org/10.1371/journal.pone.0099465
Wolf S, McMahon DP, Lim KS et al (2014) So near and yet so far: harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS One 9:e103989. https://doi.org/10.1371/journal.pone.0103989.s003
Woyciechowski M, Czekońska K (1999) The effect of temperature on Nosema apisZander (Microsporida, Nosematidae) infection in honey bees (Apis mellifera). Parasite 6:185–187. https://doi.org/10.1051/parasite/1999062185
Woyciechowski M, Moroń D (2009) Life expectancy and onset of foraging in the honeybee (Apis mellifera). Insect Soc 56:193–201. https://doi.org/10.1007/s00040-009-0012-6
Wu JY, Smart MD, Anelli CM, Sheppard WS (2012) Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (microsporidia) infection. J Invertebr Pathol 109:326–329. https://doi.org/10.1016/j.jip.2012.01.005
Wu Y, Zheng Y, Chen Y et al (2020) Apis cerana gut microbiota contribute to host health though stimulating host immune system and strengthening host resistance to Nosema ceranae. Roy Soc Open Sci 7:192100. https://doi.org/10.1098/rsos.192100
Zheng H-Q, Lin Z-G, Huang SK et al (2014) Spore loads may not be used alone as a direct indicator of the severity of Nosema ceranae infection in honey bees Apis mellifera (hymenoptera:Apidae). J Econ Entomol 107:2037–2044. https://doi.org/10.1603/ec13520
Zheng H, Powell JE, Steele MI et al (2017) Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1701819114
Acknowledgments
The author acknowledges Melissa E. Flores and Helen V. Kogan for their helpful comments and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding
None.
Ethical Approval
The chapter is a review of previously published accounts, as such, no animal or human studies were performed.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Snow, J.W. (2022). Nosema apis and N. ceranae Infection in Honey bees: A Model for Host-Pathogen Interactions in Insects. In: Weiss, L.M., Reinke, A.W. (eds) Microsporidia. Experientia Supplementum, vol 114. Springer, Cham. https://doi.org/10.1007/978-3-030-93306-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-93306-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93305-0
Online ISBN: 978-3-030-93306-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)