Abstract
Heat shock proteins (HSPs) are highly conserved among different organisms. A mycobacterial HSP65 DNA vaccine was previously shown to have prophylactic and immunotherapeutic effects against Mycobacterium tuberculosis infection in mice. Here, BALB/c mice were immunized with mycobacterial DNA-hsp65 or with DNA-hsp65 and trehalose dymicolate (TDM), both carried by biodegradable microspheres (MHSP/TDM), and challenged with Leishmania (Leishmania) major. MHSP/TDM conferred protection against L. major infection, as indicated by a significant reduction of edema and parasite loads in infected tissues. Although high levels of interferon-γ and low levels of interleukin (IL)-4 and IL-10 were detected in mice immunized with DNA-hsp65 or MHSP/TDM, only animals immunized with MHSP/TDM displayed a consistent Th1 immune response, i.e., significantly higher levels of anti-soluble Leishmania antigen (SLA) immunoglobulin G (IgG)2a and low anti-SLA IgG1 antibodies. These findings indicate that encapsulated MHSP/TDM is more immunogenic than naked hsp65 DNA, and has great potential to improve vaccine effectiveness against leishmaniasis and tuberculosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a serious health problem worldwide, with negative effects on the economy of the affected populations (Desjeux 1996). There are two million new cases each year, and 367 million people are estimated to live in risk areas (Grimaldi and Tesh 1993; WHO 2005). In these areas, the affected population is also under the risk of infection by Mycobacterium tuberculosis (Reed and Campos-Neto 2003). No protective and effective anti-Leishmania vaccine is available at the moment in spite of several tested vaccine protocols (Fernandes et al. 1997; Gurunathan et al. 1998; Khalil et al. 2000; Ghosh et al. 2001; Misra et al. 2001; Campbell et al. 2003; Coelho et al. 2003). Live bacterial Bacillus Calmette Guérin (BCG) has been used as adjuvant in anti-Leishmania experimental vaccines (Fernandes et al. 1997; Cabrera et al. 2000; Alimoharrunadian et al. 2002; Santos et al. 2002).
Leishmania share various pathological and immunological features with M. tuberculosis (Russell 1995; Reed and Campos-Neto 2003). Both pathogens multiply inside macrophages and produce granulomatous diseases. In addition, cure and resistance to infection are normally associated with Th1 cell responses. Interferon (IFN)-γ production is essential for resistance and protection against L. major infection (Launois et al. 1997; Gurunathan et al. 1998). On the other hand, susceptibility is linked to a polarized Th2 response characterized by early interleukin (IL)-4 production and by differential expression of the IL-12 receptor (Kaye et al. 1991; Gurunathan et al. 1998; Jones et al. 2002; McMahon-Pratt and Alexander 2004).
DNA vaccines have been largely studied in infection models where cell-mediated immune responses, cytotoxic CD8+ and Th1 CD4+ T cells are required for protection (Seder and Gurunathan 1999; Gurunathan et al. 2000; Ghosh et al. 2001; Ahmed et al. 2004; Nagata et al. 2004). These vaccines are able to provide antigen presentation by class I major histocompatibility complex (MHC) molecules, and the DNA by itself has an intrinsic adjuvant property due to CpG signaling through TLR9 receptor (Krieg 2002). DNA vaccines, expressing Leishmania antigens such as LACK, heat shock protein (HSP), or A2, were also shown to induce protection in experimental models against different species (Lussow et al. 1991; Ghosh et al. 2001; Reed and Campos-Neto 2003; Ahmed et al. 2004; Scott et al. 2004; Coler and Reed 2005). We have previously shown that a formulation containing the DNA-hsp65 vaccine and trehalose dymicolate (TDM) as immune stimulator, both carried into biodegradable microspheres, was able to protect Bagg Albino (BALB)/c mice from challenge infection with virulent strain of M. tuberculosis in a single-dose-based vaccination. The microsphere particles, after intramuscular injection, form a depot, which recruits antigen-presenting cell (APC) to the site of administration facilitating capture of particles and improving APC transfection (Lima et al. 2003a).
Several findings are suggesting that HSP play critical roles in generating specific immune responses against cancers and infectious agents (Kaufmann 1990; Minowada and Welch 1995). These proteins have also been involved in the assembly process of molecular components of the immune system (Pierce et al. 1991; DeNagel and Pierce 1993). In addition, HSP from some pathogens apparently possess modulatory properties when used as carriers in immunization protocols. Immunization with peptides or oligosaccharides conjugated to the M. tuberculosis HSP70 produced high titers of immunoglobulin G (IgG) antibodies in the absence of any adjuvant (Barrios et al. 1992). Furthermore, Suzue and Young (1996) demonstrated that the immunization of a recombinant HIV p24 fused to the M. tuberculosis HSP70 protein elicited both humoral and cellular immune responses against p24 in the absence of adjuvant. Immunostimulatory properties are also present in the Leishmania infantum HSP70 (Rico et al. 1998, 1999). Based on these evidences, as well as in the fact that BCG has been successfully used as adjuvant in Leishmania vaccine protocols, in this work, we evaluated the effectiveness of the DNA-hsp65/TDM entrapped into polyglycolic-co-lactic acid (PLGA) microspheres (MHSP/TDM) to protect mice against L. major infection.
Materials and methods
Parasites
Leishmania major (MHOM/IL/1980/FRIEDLIN) was maintained as promastigotes grown at 23°C in Schneider’s (Sigma, St. Louis, MO, USA) medium supplemented with 20% heat-inactivated fetal bovine serum (FBS; Sigma), 20 mM l-glutamine, 200 U ml−1 of penicillin, 100 μg ml−1 of streptomycin, and 50 μg ml−1 of gentamicin at pH 7.4.
Vaccine preparation
DNA plasmid containing the mycobacterial hsp65 gene (pCDNA3-hsp65) was obtained as previously described (Lima et al. 2003b) and was kindly provided by Nanocore Biotecnologia Ltda (Ribeirão Preto, SP, Brazil). Plasmid pCDNA3, without the hsp65 gene, was used as control. DH5α Escherichia coli transformed with pCDNA3 or pCDNA3-hsp65 plasmids was cultured in Luria–Bertani (LB) liquid medium (Gibco BRL, Gaithersburg, MD, USA) containing ampicillin (100 μg ml−1). Endotoxin-free plasmid DNA was isolated using a Wizard plus SV maxipreps DNA purification kit (Promega, Amersham Biosciences, Sweden). Plasmid concentrations were determined at 260:280 nm using a Gene Quant II apparatus (Pharmacia Biotech, Buckinghamshire, UK).
Microspheres were obtained by the double-emulsion/double-solvent evaporation technique (Lima et al. 2003a). Briefly, 30 ml of a dichloromethane solution containing 400 mg of PLGA polymer 50:50 [Resomer RG 505, molecular weight (MW) 78,000, from Boehringer Ingelheim, Ingelheim, Germany] and TDM (Nanocore) were emulsified with an aqueous inner solution containing pCDNA3 or pCDNA3-hsp65 DNA using a T50 Ultraturrax homogenizer (IKA, Labortechnik, Germany) to produce a primary water-in-oil emulsion. This emulsion was then mixed with 100 ml of an external aqueous phase containing 3% polyvinyl alcohol (Mowiol 40–88, Aldrich Chemicals, Wankee, WI, USA) as surfactant to form a stable water-in-oil-in-water emulsion. The mixture was stirred for 6 h with a Eurostar homogenizer for solvent evaporation. Microspheres were collected and washed three times with sterile water, freeze-dried, and stored at 4°C. Plasmid encapsulation rate was determined by resuspending 10 mg of microspheres in 0.5 ml of methylene chloride and 0.2 ml of TE buffer (Tris 10 mM, EDTA 1 mM, pH 8.0). Samples were incubated at 37°C under rotation end-to-end for 1 h and centrifuged. The DNA was measured in the upper aqueous phase as described before using the Gene Quant II.
Antigen preparation
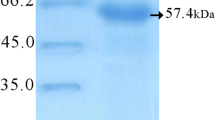
Soluble Leishmania antigen was prepared from stationary-phase promastigotes of L. major after a few passages in Schneider’s medium as described previously (Coelho et al. 2003). Briefly, 2×108 promastigotes were washed five times in cold sterile phosphate-buffered saline (PBS). After five cycles of freezing (at −196°C) and thawing (37°C), suspension was centrifuged at 8,000×g for 30 min at 4°C. The supernatant was then collected, quantified by Bradford method (Bradford 1976), and stored at −80°C. Recombinant hsp 65 kDa (rhsp65) protein was kindly provided by Nanocore.
Immunization
Bagg Albino/c female mice (n=8 per group; 4–6 weeks old) were immunized intramuscularly with 100 μg of pCDNA3-hsp65 or empty pCDNA3 vector. Two vaccine doses were administered at 3 weeks interval. pCDNA3 or hsp65 DNA loaded in microspheres (MpCDNA3 or MHSP/TDM, respectively) was also administered by intramuscular injection following the same immunization regimen. Control group received two doses (50 μl per dose) of sterile PBS.
Challenge infection
Four weeks after the last vaccine dose, mice were challenged with 1×106 stationary-phase promastigotes of L. major in their right hind footpad. The course of the disease was monitored at weekly intervals by measuring footpad thickness with a metric caliper and expressed as the increase in thickness of the infected hind foot compared to the uninfected left foot. All groups of mice were evaluated for lesion development for up to 8 weeks. Then, animals were killed, and the spleen, serum samples, and skin tissue fragments were collected for immunological analysis and parasite burden evaluation.
Parasite burden evaluation
The number of viable parasites at the site of infection was determined by a limiting dilution assay (Afonso and Scott 1993). Briefly, skin fragments were excised and homogenized in Schneider’s medium supplemented with 20% FBS and antibiotics. Each tissue homogenate was serially diluted in a 96-well Maxisorb plate (Nunc, Roskilde, Denmark). Samples, in duplicate, were incubated at 23°C. The wells containing motile promastigotes were identified with a microscope, and the number of viable parasites per milligram of tissue was determined from the highest dilution at which promastigotes have grown after 7 days of incubation.
Cytokine production assays
Splenocyte cultures and cytokine assays were performed as described previously (Coelho et al. 2003). Briefly, single-cell preparations from spleen tissue were plated, in duplicate, in 24-well plates (Nunc) at 2×105 cells per milliliter. Cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM) (background control) or stimulated with either 5 μg concanavalin A, as positive control, SLA L. major (50 μg), or rhsp65 protein (10 μg) at 37°C in 5% CO2 for 48 h. Supernatants were then collected and stored to −80°C. IFN-γ and IL-4 levels were assessed by using Inter Test mouse IFN-γ and IL-4 (Pharmingen, San Diego, CA, USA), respectively, according to technical recommendations. IL-10 levels were measured using a Duo Set enzyme-linked immunosorbent assay (ELISA) development system (R&D System, MN, USA) according to technical recommendations.
ELISA for parasite-specific IgG1 and IgG2a isotypes
Before the serological analysis, a titration curve was performed to determine the best protein concentration. The specific IgG1 and IgG2a isotypes were measured by ELISA according to Coelho et al. (2003). Briefly, 96-well plates (Nunc) were sensitized with rhsp65 protein (250 ng 100 μl−1 well−1) or SLA L. major (1 μg 100 μl−1 well−1) overnight at 4°C. Plates were blocked with PBS/casein 2% at 37°C for 2 h and incubated with 1:100 dilutions of mouse serum samples for 2 h at 37°C. After washing with PBS 1×/0.05% Tween 20, peroxidase-labeled antibodies specific to mouse IgG1 or IgG2a isotypes (Sigma), diluted at 1:5,000, were added for 2 h at 37°C. The plates were washed seven times with PBS 1×/0.05% Tween 20 and incubated with H2O2 and o-phenylenediamine for reaction development. Reactions were stopped by the addition of 20 μl of H2SO4 2 N. Optical densities were determinate at 492 nm in a ELISA reader (BioRad, model 2,550, CA, USA).
Western blot analysis
Recombinant hsp65 protein (10 μg) and SLA L. major (50 μg) were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (0.2-μm pore size, Sigma) by using standard protocols (Towbin et al. 1979). Membranes were blocked with PBS/casein 2% and incubated for 16 h. Afterward, membranes were incubated for 2 h with a pool of sera (1:100 diluted) collected from hsp65 DNA-immunized mice before challenge infection. Peroxidase-labeled antibody specific to mouse IgG (Sigma), diluted 1:10,000, was used as second antibody, and the reactions were incubated for 2 h. After washing, reactions were revealed by the addition of cloronafitol, diaminobenzidine, and H2O2, and stopped with sterile water. Serum from nonimmunized mice (1:100) was used as negative control.
Statistical analysis
All data comparisons were tested for significance by using Student’s t test. P values were considered statistically significant at P<0.05.
Results
The protective effect of immunization with mycobacterial hsp65 in BALB/c mice against L. major infection was evaluated by measuring lesion development (Fig. 1a) and parasite loads in the infected footpad (Fig. 1b). No significant protection or reduction on parasite loads was observed in the control group or in the animals immunized with the naked pCDNA3 vector or entrapped into PLGA/TDM microspheres (MpCDNA3). Lesions developed progressively during all the experiments in these mice, resulting, at the end of experiments, in a high level of swelling and necrosis of the infected footpad. Similarly, no significant protective effect was achieved in mice immunized with TDM or with PLGA alone (data not shown). In contrast, mice immunized with hsp65-DNA load microspheres (MHSP/TDM) showed a delay in the course of L. major infection, resulting in a significant decrease in edema and no signs of necrosis in the infected footpad as compared to control group.
Leishmania major protection assays in BALB/c mice. BALB/c mice (n=8, per group) were immunized intramuscularly with two injections, 3 weeks interval, with 100 μg of naked pCDNA3-hsp65 DNA or empty pCDNA3 vector. pCDNA3-hsp65 DNA and pCDNA3 were entrapped into PLGA/TM microspheres (MHSP/TDM and MpCDNA3, respectively) and were administered in the same immunization regimen. Control mice received only sterile PBS. Each mouse was challenged with 1×106 stationary-phase promastigotes of L. major in their right hind footpad as described in the Materials and methods. a Lesion development was monitored weekly by measuring the footpad swelling with a caliper. Each point represents average plus standard error. b Results of parasite loads in infected footpads of each group 8 weeks after challenge infection. Numbers of viable parasites were determined by a limiting dilution as described in Material and methods. Each bar represents average plus standard deviation. Results are representative of two experiments performed
The reduction in lesion size observed in MHSP-/TDM-immunized animals correlated with a 105-fold decrease in parasite loads in the infected footpad as compared to control group (Fig. 1b). These results indicated that partial protection was achieved in MHSP-/TDM-immunized animals, which was comparable to the level observed in mice immunized with a LACK-DNA vaccine (data not shown). However, the presence of remaining parasites and an inflammatory response may have contributed for the maintenance of a significant level of footpad swelling in MHSP-/TDM-immunized animals (Fig. 1a).
Sera from hsp65 DNA (data not shown) or MHSP-/TDM-immunized mice, collected before infection, recognized the rhsp65 protein and native proteins from L. major SLA in a Western Blot analysis (Fig. 2). A major band of similar size (∼65 kDa) and additional ones, ranging from 60 to approximately 100 kDa, were reactive on the protein extracts.
Western blot analysis of sera from immunized mice in reaction with rhsp65 protein and SLA L. major. Soluble Leishmania antigen (SLA) of L. major (lane 2) was submitted to 10% SDS-PAGE, transferred to nitrocellulose membranes, and incubated with a pool of sera of MHSP-/TDM-immunized mice collected before challenge infection. The rhsp65 protein was used as positive control (lane 1) and sera of nonimmunized and noninfected mice as negative control for reactivity with SLA L. major (lane 3)
Since activation of a Th1 immune response, i.e, increased and sustained IFN-γ production and low levels of IL-4 and IL-10, are important requirements for protection against L. major in BALB/c mice, we analyzed the IFN-γ, IL-4, and IL-10 production by spleen cells of immunized mice (hsp65 DNA or MHSP/TDM) stimulated with rhsp65 protein prior (30 days after the first vaccine dose) and 8 weeks after challenge infection.
Prior to infection, spleen cells taken from hsp65 DNA or MHSP-/TDM-immunized animals produced, in response to rhsp65, high levels of IFN-γ and very low levels of IL-4 or IL-10 (Fig. 3a). Significant differences were observed in the levels of IFN-γ produced by splenocytes of animals immunized with MHSP/TDM, as compared to the levels produced by animals immunized with hsp65-DNA, in response to rhsp65 protein. Low levels of IFN-γ were produced by spleen cells of these animals after stimulation with L. major SLA, possibly because HSP antigens represent proportionally a small fraction among the more than 3,000 proteins found in SLA.
Levels of IFN-γ, IL-4, and IL-10 produced by spleen cells from control and immunized groups before (a) and 8 weeks after (b) L. major challenge infection. Single-cell suspensions (2×105 cells per milliliter) were obtained from spleen and stimulated with rhsp65 protein (10 μg) or with SLA L. major (50 μg) for 48 h at 37°C 5% CO2. IFN-γ, IL-4, and IL-10 levels were measured by ELISA capture in culture supernatants of splenocytes collected before (a) and after (b) infection as described in Materials and methods. Each bar represents average plus standard deviation
After infection, mice immunized with hsp65-DNA or MHSP/TDM showed a sustained IFN-γ production (Fig. 3b). Moreover, spleen cells from MHSP-/TDM-immunized mice produced significantly higher levels of IFN-γ and lower levels of IL-4 and IL-10 in comparison to levels detected in the hsp65 DNA-immunized group in response to both rhsp65 protein and SLA L. major. In contrast, spleen cells from control mice produced increased levels of IL-4 and IL-10 in response to SLA L. major and, in some extent, to rhsp65 protein. Similar results were observed in mice immunized with pCDNA3 or MpCDNA3. Low IFN-γ levels were detected after stimulation of spleen cells of these animals, using both stimuli. This cytokine pattern in response to SLA is consistent with an ongoing infection and a Th2 response.
Immunoglobulin G levels (isotypes 1 and 2a) were measured in sera of control and immunized animals after challenge infection (Fig. 4). This analysis revealed that, while no significant trend toward either increased levels of IgG1 or IgG2a anti-HSP65 or anti-SLA antibodies were detected in sera of animals immunized with hsp65 DNA (IgG2a/IgG1 ∼1), indicating a mixed Th1/Th2 immune response, significantly higher levels of anti-HSP65 and anti-SLA IgG2a antibodies (IgG2a/IgG1>1) were present in sera of animals immunized with MHSP/TDM. This pattern is consistent with the Th1 immune responses observed in these animals. In contrast, anti-SLA IgG1 antibodies levels, which correlate with Th2 immune responses, were significantly higher after infection in control mice or in mice immunized with the empty vector (IgG2a/IgG1<1).
IgG1 and IgG2a isotype levels in sera of immunized mice 8 weeks after L. major challenge infection. Levels of anti-rhsp65 or anti-SLA L. major IgG1 and IgG2a isotypes were assessed in sera of control and immunized groups by ELISA as described in Materials and methods. White and black bars represent average plus standard deviation for IgG2a and IgG1 isotypes, respectively. Gray bars represent the ratio between average of IgG2a/IgG1 isotype levels, respectively
Discussion
Leishmania vaccine preparations have evolved from crude parasite preparations to defined molecules administered as recombinant proteins or DNA vaccines (Mayrink et al. 1979; Gurunathan et al. 1998; Webb et al. 1998; Piedrafita et al. 1999; Streit et al. 2001; Reed and Campos-Neto 2003; Coelho et al. 2003; Scott et al. 2004). BCG has been used as adjuvant for anti-Leishmania vaccine preparations (Satti et al. 2001; Alimoharrunadian et al. 2002; Santos et al. 2002; Srivastava et al. 2003). The role of BCG as a specific modulator of the protective response against Leishmania is not completely understood, but it is known that BCG immunization induces the production of Th1 cytokines such as IL-12 and IFN-γ, and this may contribute to an adequate cytokine environment for leishmanial antigen presentation and development of specific Th1 cell responses. On the other hand, homology between Leishmania and Mycobacterium antigens may contribute for recognition of common protective epitopes (Russell 1995).
Our data show that, after infection, DNA-hsp65-vaccinated mice responded to SLA L. major stimulation, producing high levels of IFN-γ and specific IgG antibodies. Additional data revealed that sera of hsp65 DNA or MHSP-/TDM-immunized mice recognized a group of proteins, with molecular sizes ranging from 60 to 100 kDa in soluble promastigote extracts of L. major. These findings suggest that cross-reactivity between Mycobacterium leprae hsp65 and native proteins of L. major might be involved in the observed protection. HSP proteins are highly conserved among different species and organisms, and the hsp60 of L. major shares 64.4% of similarity with M. leprae hsp65, including identical conservative amino acid substitutions (Rey-Ladino et al. 1997). Significant cross-protection was also observed in BALB/c mice immunized with Leishmania amazonensis hsp70 DNA, in association with the P4 nuclease, against L. major infection (Campbell et al. 2003).
Protection against L. major infection was observed only in encapsulated Mycobacterium hsp65 DNA (MHSP/TDM)-immunized mice, and the immune response was characterized by significantly higher levels of IFN-γ in response to both rhsp65 and SLA as compared to nonencapsulated hsp65 DNA-immunized animals. In addition, comparison of anti-SLA IgG1 and IgG2a antibody levels indicated that only animals immunized with MHSP/TDM displayed a marked shift in the type of antibodies produced following vaccination, showing significantly higher levels of anti-SLA or anti-hsp65 IgG2a antibodies compared to anti-SLA or anti-hsp65 IgG1 (IgG2a/Ig1>1), which indicates a consistent Th1 immune response. On the other hand, naked DNA elicited a Th1 response (IgG2a/IgG1 ∼1) that was not sufficient to protect mice against challenge, although it is possible that after additional vaccine doses, protection would be achieved.
The encapsulated hsp65 DNA, through its intrinsic adjuvant effect, could have provided a microenvironment suitable to drive the response against Leishmania antigens to a Th1 pattern. A nonspecific effect of TDM or PLGA alone can be discharged because mice immunized with MpCDNA3 or with these adjuvants were not protected against L. major challenge infection. Another hypothesis is related to the chaperone effect of heat shock proteins that could improve the presentation of Leishmania antigens after infection contributing to generate a protective immune response (Becker et al. 2002; Robert et al. 2002). This adjuvant effect would be more pronounced in mice vaccinated with microspheres containing hsp65-DNA plus TDM (Lima et al. 2003a).
The higher production of IFN-γ elicited by microsphere formulation could be in part attributed to TDM stimuli. Ryll et al. (2001) showed that TDM could trigger an innate immune response, which was evident by the proliferation of natural killer (NK) cells. It could also induce an early immune response, resulting in the release of IFN-γ, activation of macrophages, and up-regulation of MHC class II. Thus, TDM could provide optimal conditions to initiate an immune response, making it a valuable Th1-promoting component of an associated vaccine against tuberculosis and leishmaniasis.
In conclusion, the vaccine formulation composed by microspheres containing hsp65 DNA and TDM was able to elicit partial protective immune response against L. major. The ability of PLGA microspheres to slowly release the entrapped plasmid indicates that this system is also able to sustain the protein expression without the need of a booster compared to naked DNA administration (Lima et al. 2003a). Association to this system of other Leishmania-specific antigens such as HSP, LACK and A2, which were previously shown to induce protection against different Leishmania species, is also a promising perspective. Thus, this microsphere-based vaccine has great potential for improving the effectiveness of vaccination for tuberculosis and leishmaniasis, especially in the underdeveloped and developing countries, reducing costs and improving quality of life of the population.
References
Afonso LC, Scott P (1993) Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect Immun 61:2952–2959
Ahmed SB, Bahloul C, Robbana C, Askri S, Dellagi K (2004) A comparative evaluation of different DNA vaccine candidates against experimental murine leishmaniasis due to L. major. Vaccine 22:1631–1639
Alimoharrunadian MH, Khamesipour A, Darabi H, Firooz A, Malekzadeh S, Bahonar A, Dowlati Y, Moddaber F (2002) The role of BCG in human immune responses induced by multiple injections of autoclaved Leishmania major as a candidate vaccine against leishmaniasis. Vaccine 21:174–180
Barrios C, Lussow AR, Van Embden J, Van Der Zee R, Rappuoli R, Costantino P, Louis JA, Lambert PH, Del Giudice G (1992) Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming. Eur J Immunol 22:1365–1372
Becker T, Hartl FU, Wieland F (2002) CD40, an extracellular receptor for binding and uptake of hsp70-peptide complexes. J Cell Biol 158:1277–1285
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cabrera M, Blackwell JM, Castes M, Trujillo D, Convit J, Shaw MA (2000) Immunotherapy with live BCG plus heat killed Leishmania induces a T helper 1-like response in American cutaneous leishmaniasis patients. Parasite Immunol 22:73–79
Campbell K, Diao H, Ji J, Soong L (2003) DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous leishmaniasis. Infect Immun 71:6270–6278
Coelho EA, Tavares CA, Carvalho FA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune response induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994
Coler RN, Reed SG (2005) Second-generation vaccines against leishmaniasis. Trends Parasitol 21:244–249
DeNagel DC, Pierce SK (1993) Heat shock proteins in immune responses. Crit Rev Immunol 13:71–81
Desjeux P (1996) Leishmaniasis. Public health aspects and control. Clin Dermatol 14:417–423
Fernandes AP, Herrera EC, Mayrink W, Gazzinelli RT, Liu WY, Costa CA, Tavares CAP, Melo MN, Michalick MSM, Gentz R, Nascimento E (1997) Immune responses induced by a Leishmania (Leishmania) amazonensis recombinant antigen in mice and lymphocytes from vaccinated subjects. Rev Inst Med Trop São Paulo 39:70–78
Ghosh A, Labrecque S, Matlashewski G (2001) Protection against Leishmania donovani infection by DNA vaccination: increased DNA vaccination efficiency through inhibiting the cellular p53 response. Vaccine 19:3169–3178
Grimaldi G Jr, Tesh RB (1993) Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev 6:230–250
Gurunathan S, Prussin C, Sacks DL, Seder RA (1998) Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat Med 4:1409–1415
Gurunathan S, Wu C, Freidag BL, Seder RA (2000) DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol 12:442–447
Jones DE, Ackermann MR, Wille U, Hunter CA, Scott P (2002) Early enhanced Th1 response after Leishmania amazonensis infection of C57BL/6 interleukin-IL-10-deficient mice does not lead to resolution of infection. Infect Immun 70:2151–2158
Kaye PM, Curry AJ, Blackwell JM (1991) Differential production of Th1- and Th2-derived cytokines does not determine genetically controlled vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol 146:2763–2770
Kaufmann SH (1990) Heat shock proteins and the immune response. Immunol Today 11:129–136
Khalil EA, Elhassam AM, Zijlstra EE, Osman OF, Eljack IA, Ibrahim ME, Mukhtar MM, Ghalib HW, Moddaber F (2000) Safety and immunogenicity of an autoclaved Leishmania major vaccine. East Afr Med J 77:468–470
Krieg AM (2002) CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760
Launois P, Maillard I, Pingel S, Swihart KG, Xénarios I, Acha-Orbea H, Diggelmann H, Locksley RM, Mac-Donald HR, Louis JA (1997) IL-4 rapidly produced by Vβ4+ Vα8+ CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541–549
Lima KM, Dos Santos AS, Lima VM, Coelho-Castelo AA, Rodrigues JM Jr, Silva CL (2003a) Single dose of a vaccine based on DNA encoding mycobacterial hsp65 protein plus TDM-loaded PLGA microspheres protects mice against a virulent strain of Mycobacterium tuberculosis. Gene Ther 10:678–685
Lima KM, Dos Santos AS, Santos RR, Brandão IT, Rodrigues JM Jr, Silva CL (2003b) Efficacy of DNA-hsp65 vaccination for tuberculosis varies with method of DNA introduction in vivo. Vaccine 22:49–56
Lussow AR, Barrios C, Van Ebden J, Van Der Zee R, Verdini AS, Pessi A, Louis JA, Lambert PH, Del Giudice G (1991) Mycobacterial heat-shock protein as carrier molecules. Eur J Immunol 21:2297–2302
Mayrink W, Da Costa CA, Magalhães PA, Melo MN, Dias M, Lima AO, Michalick MS, Williams P (1979) A field triad of a vaccine against American dermal leishmaniasis. Trans R Soc Trop Med Hyg 73:385–387
McMahon-Pratt D, Alexander J (2004) Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniasis or the visceral disease? Immunol Rev 201:206–224
Minowada G, Welch WJ (1995) Clinical implications of the stress response. J Clin Invest 95:3–12
Misra A, Dube A, Srivastava B, Sharma P, Srivastava JK, Katiyar JC, Naik S (2001) Successful vaccination against Leishmania donovani infection in Indian langur using alum-precipitated autoclaved Leishmania major with BCG. Vaccine 19:3485–3492
Nagata T, Aoshi T, Uchijima M, Suzuki M, Koide Y (2004) Cytotoxic T-lymphocyte and helper T-lymphocyte-oriented DNA vaccination. DNA Cell Biol 23:93–106
Piedrafita D, Xu D, Hunter D, Harrison RA, Liew FY (1999) Protective immune responses induced by vaccination with an expression genomic library of Leishmania major. J Immunol 163:1467–1472
Pierce SK, DeNagel DC, Vanbuskirk AM (1991) A role for heat shock proteins in antigen processing and presentation. Curr Top Microbiol Immunol 167:83–92
Reed SG, Campos-Neto A (2003) Vaccines for parasitic and bacterial infections. Curr Opin Immunol 15:456–460
Rey-Ladino JA, Joshi PB, Sing B, Gupta R, Reiner RE (1997) Leishmania major: molecular cloning, sequencing, and expression of the heat shock protein 60 gene reveals unique carboxyl terminal peptide sequences. Exp Parasitol 85:249–263
Rico AI, Del Real G, Soto M, Quijada L, Martinez AC, Alonso C, Requena JM (1998) Characterization of the immunostimulatory properties of Leishmania infantum HSP70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice. Infect Immun 66:347–352
Rico AI, Angel SO, Alonso C, Requena JM (1999) Immunostimulatory properties of the Leishmania infantum heat shock protein HSP70 and HSP83. Mol Immunol 36:1131–1139
Robert J, Gantress J, Rau L, Bell A, Cohen N (2002) Minor histocompatibility antigen-specific MHC-restricted CD8 T cell responses elicited by heat shock proteins. J Immunol 168:1697–1703
Russell DG (1995) Mycobacterium and Leishmania: stowaways in the endosomal network. Trends Cell Biol 5:125–128
Ryll R, Watanabe K, Fujiwara N, Takimoto H, Hasunuma R, Kumazawa Y, Okada M, Yano I (2001) Mycobacterial cord factor, but not sulfolipid, causes depletion of NKT cells and upregulation of CD1d1 on murine macrophages. Microbes Infect 3:611–619
Santos WR, De Lima VM, De Souza EP, Bernardo RR, Palatnik M, Palatnik De Souza CB (2002) Saponins, IL-12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine 21:30–43
Satti IN, Osman HY, Daifalla NS, Younis SA, Khalil EAG, Zijstra EE, Hassan E, Ghalib HW (2001) Immunogenicity and safety of autoclaved Leishmania major plus BCG vaccine in healthy Sudanese volunteers. Vaccine 19:2100–2106
Scott P, Artis D, Uzonna J, Zaph C (2004) The development of effector and memory T cells in cutaneous leishmaniasis: the implications for vaccine development. Immunol Rev 201:318–338
Seder RA, Gurunathan S (1999) DNA vaccines—designer vaccines for the 21st century. N Engl J Med 341:277–278
Srivastava JK, Misra A, Sharma P, Srivastava B, Naik S, Dube A (2003) Prophylactic potential of autoclaved Leishmania donovani with BCG against experimental visceral leishmaniasis. Parasitology 127:107–114
Streit JA, Recker TJ, Filho FG, Beverley SM, Wilson ME (2001) Protective immunity against the protozoan Leishmania chagasi is induced by subclinical cutaneous infection with virulent but not avirulent organisms. J Immunol 166:1921–1929
Suzue K, Young RA (1996) Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol 156:873–879
Towbin H, Staehelin T, Bordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, Reed SG (1998) Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun 66:3279–3289
World Health Organization (2005) Program for surveillance and control of leishmaniaisis. http://who.int/emc/diseases/leish/index.html.2005 (generic)
Acknowledgements
This work received financial support from FAPEMIG, FAPESP, CNPq, and Institutes of Millennium REDE-TB (MCT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coelho, E.A.F., Tavares, C.A.P., de Melo Lima, K. et al. Mycobacterium hsp65 DNA entrapped into TDM-loaded PLGA microspheres induces protection in mice against Leishmania (Leishmania) major infection. Parasitol Res 98, 568–575 (2006). https://doi.org/10.1007/s00436-005-0088-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0088-5