Abstract
In the present study, a loop-mediated isothermal amplification (LAMP) assay was developed and validated for the detection of Paragonimus westermani adults, metacercariae, and eggs in human and animal samples. The LAMP amplification can be finished in 45 min under isothermal condition at 60°C by employing a set of four species-specific primer mixtures and the results can be checked by naked-eye visualization. No amplification products were detected with deoxyribunucleic acid (DNA) of related trematode species including Fasciola hepatica, Fasciola gigantica, Clonorchis sinensis, Opisthorchis viverrini, Schistosoma mansoni, and Schistosoma japonicum. The method was further validated by examining P. westermani DNA in intermediate hosts including freshwater crabs and crayfish, as well as in sputum and pleural fluid samples from patients of paragonimiasis. These results indicated that the LAMP assay was highly specific, sensitive, and rapid, and it was approximately 100 times more sensitive than conventional specific PCR. The LAMP assay established in this study provides a rapid and sensitive tool for the detection of P. westermani DNA in freshwater crabs, crayfish, sputum, and pleural fluid samples, which has important implications for effective control of human paragonimiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human paragonimiasis is caused by trematodes of the genus Paragonimus which parasitize in the lungs of humans. The disease is mainly endemic in China, Korea, Japan, and some other Asian countries, where people have the habit of eating raw or undercooked freshwater crabs or crayfish which may be infected with infective Paragonimus metacercariae (Kim et al. 2009). Now, paragonimiasis is having a wilder distribution due to the movement of the world population and the pursuit of eating exotic and delicate foods (Liu et al. 2008; Lane et al. 2009; Sohn et al. 2009; Ikehara et al. 2010). Paragonimiasis is considered an important food-borne parasitic disease in China (Liu et al. 2008; Zhou et al. 2008).
At present, approximately 28 species of Paragonimus genus are recognized: two species in Africa, five species in the Americas, and 21 species in Asia (Miyazaki 1982). Among them, Paragonimus westermani has long been considered to be the most important causative agent of paragonimiasis in Asia (Miyazaki 1978). P. westermani causes pulmonary, neurologic, and abdominal diseases by infecting lungs, brains, spinal cords, and other organs in humans and animals including dogs, tigers, cats, pigs, cattle, mink, and feral carnivores.
Various techniques have been established for the diagnosis of human paragonimiasis, including parasitological and immunological methods. These methods are conventionally performed by the detection of P. westermani eggs in human sputum or pleural fluid. However, it remains difficult to examine the infection intensity of P. westermani in crabs or crayfishes. It is also difficult to determine metacercariae and eggs of P. westermani to species level, since they are very similar to that of other digeneans morphologically. Furthermore, freshwater crabs or crayfish with only a few metacercariae harbored cannot be reliably recognized as infected using microscopic examination (Devi et al. 2010). Detection of eggs in early or late infection stage or extrapulmonary infection stage is difficult, because eggs rarely exist in these stages. Immunodiagnostic methods have proven sensitive for early diagnosis of human paragonimiasis. Although they are useful for the screening of patients in endemic areas, they are not suitable for the survey of intermediate hosts or sputum and pleural fluid samples (Lee et al. 2006, 2007; Na et al. 2006; Zhao et al. 2007; Kirino et al. 2009).
Recently, polymerase chain reaction (PCR) assays have been described for the detection of P. westermani in infected freshwater crabs or crayfishes and have significantly increased our ability in the detection of P. westermani infection (Sugiyama et al. 2002; Li et al 2005; Tandon et al. 2007; Doanh et al. 2007, 2009; Devi et al. 2010). However, highly precision thermocyclers are needed in PCR techniques, which prevent the widespread use of these approaches in field conditions.
A novel nucleic acid amplification method named loop-mediated isothermal amplification (LAMP) was developed by Notomi et al. (2000). This assay can amplify target deoxyribunucleic acid (DNA) to a quantity as high as 109 copies in less than 1 h under isothermal conditions, and no thermocycler is needed (Notomi et al. 2000; Nagamine et al. 2002). Further, the amplification product can be visually detected with the addition of fluorescent dyes such as SYBR Green I (Poon et al. 2006). Four LAMP primers, which are designed to recognize six distinct regions on the target gene, assure the specific amplification of the target DNA.
LAMP assays have been developed successfully for the detection of many viral, bacterial, and fungal diseases (Maruyama et al. 2003; Endo et al. 2004; Okafuji et al. 2005). Recently, this method has also been developed successfully for the diagnosis of parasitic infections such as malaria, trypanosomiasis, theileriosis, babesiosis, schistosomiasis, and clonorchiasis (Kuboki et al. 2003; Poon et al. 2006; Alhassan et al. 2007; Iseki et al. 2007; Thekisoe et al. 2007; Xu et al. 2009; Cai et al. 2010).
The objective of the present study was to develop a simple and cost-effective LAMP assay for rapid detection of P. westermani DNA in humans and animal samples based on its second internal transcribed spacers (ITS-2) of nuclear ribosomal DNA (rDNA). Data on the sensitivity and specificity of the method were reported, and the applicability of the LAMP assay for the detection of adult, metacercariae, and egg stages of P. westermani was demonstrated.
Materials and methods
Parasite samples
Adult worms of P. westermani were collected from dogs experimentally infected in our laboratory. P. westermani metacercariae were collected from freshwater crabs and crayfish muscles from Fujian and Guangdong provinces, China. P. westermani eggs were collected from sputum and pleural fluid samples from patients who have been diagnosed and identified by morphology (Table 1). Several trematodes infecting humans, namely, Fasciola hepatica, Fasciola gigantica, Clonorchis sinensis, Opisthorchis viverrini, Schistosoma mansoni, and Schistosoma japonicum, were included as ‘heterologous control samples’ for assessing the specificity of the LAMP assay. All parasite materials were preserved in 70% ethanol and kept at −20°C until the extraction of genomic DNA. Thirty-five freshwater crab and crayfish muscle samples with P. westermani metacercariae infected (infection intensity ranged from 1 to 8 metacercariae per gram of muscle), as well as sputum and pleural fluid samples from patients who have been diagnosed and identified by morphology, were used. Furthermore, two crab and two crayfish samples without P. westermani metacercariae, as well as three sputum samples from healthy persons confirmed by microscopic examination, were used as control. All these materials were kept at −86°C until the extraction of genomic DNA.
DNA preparation
Total genomic DNA from P. westermani adults, metacercariaes, eggs, noninfected freshwater crab and crayfish muscles, sputum, and pleural fluid samples from healthy persons, as well as the heterologous control samples, were extracted, respectively, by SDS/proteinase K treatment. Then the DNA samples were column-purified (Wizard® SV Genomic DNA Purification System, Promega; Zhao et al. 2009; Ai et al. 2010) and eluted into 60 μl H2O, respectively, according to the manufacturer’s recommendations. The integrity of all the DNA samples were validated by successful amplification of the mitochondrial cytochrome c oxidase subunit 1 (cox1) using primers and protocols described previously (Bowles et al. 1992; data not shown).
LAMP assay
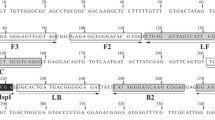
The successful amplification of LAMP assay depends on the specificities of primers designed, and a set of four specific primers (B3, F3, BIP, and FIP) that recognize a total of six distinct sequences (B1, B2c, B3, F1c, F2, and F3) of the target DNA region are required. The ITS-2 sequence of P. westermani rDNA was retrieved from GenBank (AF159604) and was used for the design of species-specific primers with Primer Explorer V4 software (http://primerexplorer.jp/e; Table 2). The primers were designed and selected based on the criteria described by Notomi et al. (2000).
The LAMP assay was carried out in a 25-μl reaction mixture containing 10 × Bst-DNA polymerase buffer (2.5 mM each), betaine (10 μM each), deoxynucleotide triphosphates (2.5 mM each), MgSO4 (10 mM each), FIP and BIP (1.6 mM each), loop-F and loop-B (0.8 μM each), F3 and B3 primers (0.2 mM each), Bst DNA polymerase (8 U, New England BioLabs), ddH2O, and template DNA (1 μl). The template DNA was omitted in the reaction of negative control. The mixtures were incubated at 60°C and then heated at 80°C for 10 min to terminate the reaction. Because of the high sensitivity of LAMP reaction, controls (positive and negative) were included in each run, and all precautions to prevent cross-contamination were considered such as minimizing manipulation of the reaction tubes, performing the steps of LAMP assay in different rooms, and adding sterile paraffin wax to the mix to prevent contamination.
Detection and confirmation of LAMP products
The LAMP amplification results were visually detected by adding 1 μl of 1:10 diluted 10,000 × concentration fluorescent dye SYBR Green I (Invitrogen) to the reaction tube. The solution would turn to green if LAMP reaction was successful; otherwise, it would remain orange. The LAMP products were also monitored in a 2% agarose gel stained with ethidium bromide. The stained gel and the reaction tubes were then photographed using the ultraviolet (UV) image system (Gel documentation system, UVItec, UK).
Conventional PCR with the outer primers B3 and F3 of LAMP was performed. The PCR mix was a 25 μl system with 10 × PCR buffer (2.5 μl), 0.2 mM of each dNTPs, 0.4 μM of each B3 and F3 primers, 1.25 U of ExTaq polymerase (Takara), and 1 μl of DNA sample in a thermocycler (Biometra). The performing conditions were as follows: an initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturation (30 s at 94°C), annealing (1 min at 55°C), and extension (30 s at 72°C), with a final extension for 5 min at 72°C. PCR products (5 μl) were examined in a 2% agarose gel, stained with SYBR green I and photographed.
Results and discussion
To determine the specificity of the LAMP assay for P. westermani, DNA samples from other trematode parasites including F. hepatica, F. gigantica, C. sinensis, O. viverrini, S. mansoni, and S. japonicum were used as control. It was found that target DNA was amplified only from DNA samples of P. westermani adults, metacercariae, and eggs, and no amplification of target DNA was found in the heterologous control samples (Fig. 1, upper). These products displayed typically ladder-like bands on agarose gels (Fig. 1, bottom). The specificity of the primers was also confirmed by BLAST search (http://www.ncbi.nlm.nih.gov/Blast) in the NCBI database (data no shown).
Specificity assessment of the LAMP assay for detection of Paragonimus westermani. Upper: Visual examination of LAMP products. Bottom: Agarose gel electrophoresis of amplified products. Lanes 1–9 represent P. westermani adult, P. westermani metacercariae, P. westermani eggs, Fasciola hepatica, Fasciola gigantica, Clonorchis sinensis, Opisthorchis viverrini, Schistosoma mansoni, and Schistosoma japonicum, respectively. Lane 10 represents no-DNA control. M represents a DNA size marker (ordinate values in bp)
To determine the sensitivity of the LAMP assay for P. westermani, serial concentration of DNA samples were prepared. Briefly, the concentration of DNA samples of P. westermani metacercariae and eggs was measured three times by spectrophotometry to obtain an average concentration. Then they were diluted with 10 mM Tris–HCl (pH 8.8) to a final concentration of 100 ng/μl. Subsequently, tenfold serial dilutions ranging from 1 × 10−3 to 1 × 10−9 ng/μl were prepared and used as templates for LAMP or conventional PCR. The LAMP products were visualized under UV light, and the fluorescent signals of the solutions were observed in the positive reactions without opening the tubes. Results showed that the detection limit of the LAMP assay was 10−8 ng/μl (Fig. 2A and B), while it was only 1 × 10−6 ng/μl for conventional PCR (Fig. 2C). Therefore, the sensitivity of the LAMP assay for the detection of P. westermani DNA was 100 times higher than that of the conventional PCR.
Sensitivity assessment of the LAMP assay and comparison with conventional PCR for the detection of Paragonimus westermani. The conventional PCR was performed with primers F3 and B3. A, B Sensitivity of the LAMP assay. C Sensitivity of a conventional PCR. Lanes 1–6 represent serial concentrations of P. westermani DNA in the range of 10−4–10−9 ng/μl for A and B. Lanes 1–5 represent serial concentrations of P. westermani DNA in the range of 10−3–10−7 ng/μl for C. Lane 7 in A and B and Lane 6 in C represent no-DNA control. M represents a DNA size marker (ordinate values in bp)
The applicability and practicality of the LAMP assay were evaluated for the detection of P. westermani metacercariae in freshwater crabs, crayfishes, or in sputum and pleural fluid samples of human paragonimiasis patients (Fig. 3). Totally, 35 muscle samples from freshwater crabs or crayfishes infected with P. westermani metacercariae, and 17 egg samples from sputum and pleural fluid of human paragonimiasis patients were used for positive evaluation. Two crab samples, two crayfish samples without metacercariae, and three sputum samples without eggs from healthy persons (confirmed by microscopic examination) were used as negative control. As expected, the 35 infected samples and the 17 sputum and pleural fluid samples produced positive results in the LAMP assay, even some samples with infection intensity as low as 1 metacercariae/g. However, no products were detected from the negative control samples of noninfected crabs or sputum samples from healthy persons (Fig. 3).
LAMP amplification of the second internal transcribed spacer of Paragonimus westermani from freshwater crabs and crayfish samples infected with metacercariae, and sputum and pleural fluid samples from patients of paragonimosis. Tube 1 represents P. westermani adult. Tubes 2–4 represent freshwater crab samples infected with metacercariae of P. westermani. Tubes 5–7 represent crayfish samples infected with metacercariae of P. westermani. Tubes 8–13 represent sputum samples from paragonimosis patients. Tubes 14–18 represent pleural fluid samples from paragonimosis patients. Tube 19 represents a crab sample without metacercariae of P. westermani. Tube 20 represents a sputum sample from healthy person. Tube 21 represents no-DNA control
Freshwater crabs and crayfishes are the second intermediate hosts of P. westermani, and humans get infected with P. westermani metacercariae by ingesting raw or undercooked freshwater crabs or crayfishes. Hence, the accurate identification and detection of P. westermani metacercariae of P. westermani in freshwater crabs and crayfishes has important implications for the control and prevention of human paragonimiasis in endemic areas. Microscopic examination has still been the routine method for the detection of metacercariae in freshwater crabs, crayfishes, and sputum and pleural fluid samples from patients of paragonimiasis, but there is limitation of such method in that metacercariae are difficult to be detected and identified due to low intensity of infection. PCR techniques for the detection of P. westermani have been developed in recent years (Sugiyama et al. 2002; Li et al 2005; Tandon et al. 2007; Doanh et al. 2009; Devi et al. 2010). But complicated equipment and expert techniques are required, which makes the techniques not to be readily available in rural endemic regions. Moreover, the Taq DNA polymerase used in PCR assays is easily to be inhibited by biological substances.
The LAMP assay established in the present study is economic and easy to be performed, and it is more sensitive than conventional PCR in the detection of P. westermani infection. The designed loop primers allow the amplification to be finished in 45 min. Only a water bath or a heat block is required to carry out the amplification, which is readily convenient even in poorly equipped laboratories or in large-scale epidemiological studies in poor areas. The Bst DNA polymerase acts at a relatively high temperature, which helps to reduce nonspecific priming. Moreover, this DNA polymerase is also more resistant to inhibitors than Taq DNA polymerase (Poon et al. 2006). A large amount of white magnesium pyrophosphate precipitate will generate in positive samples, which allows the presence of P. westermani DNA to be easily identified by visual inspection, and the positive amplification can be viewed by adding fluorescent dyes such as SYBR Green I.
In conclusion, the results of the present study demonstrated that the established LAMP assay is a rapid and sensitive technique for detection of P. westermani metacercariae in freshwater crabs, crayfishes, or eggs in sputum and pleural fluid samples of human paragonimiasis patients, which provides a useful tool for the prevalence survey of P. westermani in freshwater crabs and crayfishes. Moreover, the technique has the potential to be applied in many clinical and epidemiological investigations of P. westermani infection in humans, which will contribute to the effective control of human paragonimiasis.
References
Ai L, Dong SJ, Zhang WY, Elsheikha HM, Mahmmod YS, Lin RQ, Yuan ZG, Shi YL, Huang WY, Zhu XQ (2010) Specific PCR-based assays for the identification of Fasciola species: their development, evaluation and potential usefulness in prevalence surveys. Ann Trop Med Parasitol 104:65–72
Alhassan A, Govind Y, Tam NT, Thekisoe OM, Yokoyama N, Inoue N, Igarashi I (2007) Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol Res 100:1165–1168
Bowles J, Blair D, McManus DP (1992) Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 54:165–173
Cai XQ, Xu MJ, Wang YH, Qiu DY, Liu GX, Lin A, Tang JD, Zhang RL, Zhu XQ (2010) Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP). Parasitol Res 106:1379–1383
Devi KR, Narain K, Agatsuma T, Blair D, Nagataki M, Wickramasinghe S, Yatawara L, Mahanta J (2010) Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Trop 116:31–38
Doanh PN, Shinohara A, Horii Y, Habe S, Nawa Y, The DT, Le NT (2007) Morphological and molecular identification of two Paragonimus spp., of which metacercariae concurrently found in a land crab, Potamiscus tannanti, collected in Yenbai Province, Vietnam. Parasitol Res 100:1075–1082
Doanh PN, Shinohara A, Horii Y, Habe S, Nawa Y (2009) Discovery of Paragonimus westermani in Vietnam and its molecular phylogenetic status in P. westermani complex. Parasitol Res 104:1149–1155
Endo S, Komori T, Ricci G, Sano A, Yokoyama K, Ohori A, Kamei K, Franco M, Miyaji M, Nishimura K (2004) Detection of gp43 of Paracoccidioides brasiliensis by the loop-mediated isothermal amplification (LAMP) method. FEMS Microbiol Lett 234:93–97
Ikehara M, Komase Y, Morita A, Yamaguchi H, Yamamoto T (2010) Paragonimiasis in a person whose symptoms were shown 22 years after emigrating to Japan from Laos. J Infect Chemother [Epub ahead of print]
Iseki H, Alhassan A, Ohta N, Thekisoe OM, Yokoyama N, Inoue N, Nambota A, Yasuda J, Igarashi I (2007) Development of a multiplex loop-mediated isothermal amplification (mLAMP) method for the simultaneous detection of bovine Babesia parasites. J Microbiol Methods 71:281–287
Kim EM, Kim JL, Choi SI, Lee SH, Hong ST (2009) Infection status of freshwater crabs and crayfish with metacercariae of Paragonimus westermani in Korea. Korean J Parasitol 47:425–426
Kirino Y, Nakano N, Doanh PN, Nawa Y, Horii Y (2009) A seroepidemiological survey for paragonimosis among boar-hunting dogs in central and southern Kyushu, Japan. Vet Parasitol 161:335–338
Kuboki N, Inoue N, Sakurai T, Di Cello F, Grab DJ, Suzuki H, Sugimoto C, Igarashi I (2003) Loop-mediated isothermal amplification for detection of African trypanosomes. J Clin Microbiol 41:5517–5524
Lane MA, Barsanti MC, Santos CA, Yeung M, Lubner SJ, Weil GJ (2009) Human paragonimiasis in North America following ingestion of raw crayfish. Clin Infect Dis 49:e55–e61
Lee JC, Cho GS, Kwon JH, Shin MH, Lim JH, Kim WK (2006) Macrophageal/microglial cell activation and cerebral injury induced by excretory-secretory products secreted by Paragonimus westermani. Neurosci Res 54:133–139
Lee JS, Lee J, Kim SH, Yong TS (2007) Molecular cloning and characterization of a major egg antigen in Paragonimus westermani and its use in ELISA for the immunodiagnosis of paragonimiasis. Parasitol Res 100:677–681
Li AH, Na BK, Kong Y, Cho SH, Zhao QP, Kim TS (2005) Molecular cloning and characterization of copper/zinc-superoxide dismutase of Paragonimus westermani. J Parasitol 91:293–299
Liu Q, Wei F, Liu W, Yang S, Zhang X (2008) Paragonimiasis: an important food-borne zoonosis in China. Trends Parasitol 24:318–323
Maruyama F, Kenzaka T, Yamaguchi N, Tani K, Nasu M (2003) Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification. Appl Environ Microbiol 69:5023–5028
Miyazaki I (1978) Two types of lung flukes which has been called Paragonimus westermani (Kerbert, 1878). Med Bull Fukuoka Univ 5:251–263
Miyazaki I (1982) Geographical distribution of Paragonimus westermani and P. pulmonalis in Asia. Med Bull Fukuoka Univ 9:11–22
Na BK, Kim SH, Lee EG, Kim TS, Bae YA, Kang I, Yu JR, Sohn WM, Cho SY, Kong Y (2006) Critical roles for excretory–secretory cysteine proteases during tissue invasion of Paragonimus westermani newly excysted metacercariae. Cell Microbiol 8:1034–1046
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16:223–229
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63
Okafuji T, Yoshida N, Fujino M, Motegi Y, Ihara T, Ota Y, Notomi T, Nakayama T (2005) Rapid diagnostic method for detection of mumps virus genome by loop-mediated isothermal amplification. J Clin Microbiol 43:1625–1631
Poon LL, Wong BW, Ma EH, Chan KH, Chow LM, Abeyewickreme W, Tangpukdee N, Yuen KY, Guan Y, Looareesuwan S, Peiris JS (2006) Sensitive and inexpensive molecular test for falciparum malaria: detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin Chem 52:303–306
Sohn BS, Bae YJ, Cho YS, Moon HB, Kim TB (2009) Three cases of paragonimiasis in a family. Korean J Parasitol 47:281–285
Sugiyama H, Morishima Y, Kameoka Y, Kawanaka M (2002) Polymerase chain reaction (PCR)-based molecular discrimination between Paragonimus westermani and P. miyazakii at the metacercarial stage. Mol Cell Probes 16:231–236
Tandon V, Prasad PK, Chatterjee A, Bhutia PT (2007) Surface fine topography and PCR-based determination of metacercaria of Paragonimus sp. from edible crabs in Arunachal Pradesh, Northeast India. Parasitol Res 102:21–28
Thekisoe OM, Kuboki N, Nambota A, Fujisaki K, Sugimoto C, Igarashi I, Yasuda J, Inoue N (2007) Species-specific loop-mediated isothermal amplification (LAMP) for diagnosis of trypanosomosis. Acta Trop 102:182–189
Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM (2009) Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int J Parasitol 40:327–331
Zhao QP, Moon SU, Na BK, Kim SH, Cho SH, Lee HW, Kong Y, Sohn WM, Jiang MS, Kim TS (2007) Paragonimus westermani: biochemical and immunological characterizations of paramyosin. Exp Parasitol 115:9–18
Zhao GH, Li J, Zou FC, Liu W, Mo XH, Lin RQ, Yuan ZG, Weng YB, Song HQ, Zhu XQ (2009) Heterogeneity of class I and class II MHC sequences in Schistosoma japonicum from different endemic regions in mainland China. Parasitol Res 106:201–206
Zhou P, Chen N, Zhang RL, Lin RQ, Zhu XQ (2008) Food-borne parasitic zoonoses in China: perspective for control. Trends Parasitol 24:190–196
Acknowledgements
This work is supported in part by the Program for National S&T Major Program (grant no. 2008ZX10004-011), National Key Technology R&D Program (grant no. 2008BAI56B03) and the State Key Laboratory of Veterinary Etiological Biology, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. X. Chen and L. Ai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, M.X., Ai, L., Zhang, R.L. et al. Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop-mediated isothermal amplification (LAMP). Parasitol Res 108, 1193–1198 (2011). https://doi.org/10.1007/s00436-010-2162-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2162-x