Abstract
In several mountainous regions of Northeastern India, foci of Paragonimus infection reportedly involving species that are known to prevail in China have been identified. The present study was undertaken to demonstrate the surface fine topography and sequence analysis of the ribosomal deoxyribonucleic acid (rDNA; second internal transcribed spacer, ITS2) of the metacercarial stages of the lung fluke collected from a mountain stream of the area (Miao, Changlang District in Arunachal Pradesh). The encysted metacercariae were oval in shape and had a smooth surface. The newly excysted metacercaria had a ventral sucker larger than the oral; the body surface was covered with numerous single-pointed and thorn-like tegumentary spines, of which those on the anterior part of the body were bigger in size and showed a gradual reduction in length and number towards the posterior end; dome-shaped papillae in variable numbers were seen around the rim of the oral sucker and were sparsely distributed all over the body surface. The polymerase chain reaction-amplified rDNA ITS2 sequences of the metacercariae were aligned with known sequences for the various species of Paragonimus, and the expectation value was found to be most significant with P. westermani, revealing an absolute match. The surface topography including the number and distribution of papillae and spination patterns and the ITS2 sequences of the metacercariae strongly suggest that the Paragonimus species, prevalent in the region of India, is in fact P. westermani.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lung flukes of the genus Paragonimus have been known as one of the most important zoonotic parasites causing paragonimiasis, also known as endemic haemoptysis, in man. It is estimated that more than 20 million people are infected worldwide because of several species of Paragonimus (review, Toscano et al. 1995). More than 40 species are known to infect the lung of different mammalian hosts throughout the world (Bunnag and Harinasuta 1985), and approximately 15 species are known to infect humans. The parasite can migrate to several other vital tissues including the brain (Kusner and King 1993). The best-known species is P. westermani (Kerbert 1878) Braun 1899—a human parasite that can undergo development in as many as 16 different snail species and 50 crustacean species. Beside P. westermani, P. pulmonalis (Baelz 1880) Miyazaki 1978; P. ohirai Miyazaki 1939; P. iloktsunensis Chen 1940; P. skrjabini Chen 1959; P. miyazaki Kamo et al. 1961 and P. heterotremus Chen and Hsia 1964, all reported to be occurring in Asia, P. africanus and P. uterobilateralis Voelker and Vogel 1965 in Africa and P. mexicanus Miyazaki and Ishii 1968 in America are considered pathogenic to man. While P. westermani is distributed mostly in Asia, P. heterotremus is the predominant causative agent of paragonimiasis in Thailand (Blair et al. 1999a, b).
In the context of India, Chandler and Read (1961) indicated Bengal, Assam and some other parts of the country as endemic foci of human paragonimiasis. In recent years, this infection has been reported in a sizeable human population of Manipur, a northeastern state of the country (Razaque et al. 1991; Singh et al. 1993). Although the fluke is known to parasitize a wide range of mammalian hosts representing as many as 11 families, the status of its prevalence and host range in India is not well documented. Very recently, in Manipur and Arunachal Pradesh (Northeast India), the suspected foci of human infection where consumption of crustacean intermediate hosts is of regular practice, the Chinese species, P. hueitungensis and P. heterotremus, respectively, were identified as etiological agents of paragonimiasis (Singh 2002; Narain et al. 2003). However, no or scanty information is available about the prevalence of the parasite among its molluscan and crustacean intermediate hosts as even in the suspected foci of human infection.
Morphology of the encysted and excysted metacercariae, which occur as the infective stage in the muscle tissue of the crustacean second intermediate host, has been conventionally used in the identification of the species of Paragonimus. The external appearance of the newly excysted metacercariae has also been studied for various species of the genus Paragonimus using scanning electron microscopy (Higo and Ishii 1984, 1987; Tongu et al. 1985, 1987, 1995; Sugiyama et al. 1990).
The identification of closely related species based on morphological characters can be difficult. This is particularly the case of soft-bodied animals such as digenean trematodes. However, recent advances in molecular biology, in particular the amplification of specific deoxyribonucleic acid (DNA) regions via the polymerase chain reaction (PCR) and improved sequencing techniques, have been employed to resolve taxonomic issues related to various helminth parasites by comparing their DNA. The ribosomal DNA cluster (rDNA), which codes for structural components of ribosomes, is particularly useful for genetic studies because it is highly repeated and contains variable regions flanked by more conserved regions (Hillis and Dixon 1991). PCR-based techniques utilizing the rDNA second internal transcribed spacer (ITS2) sequences, which occur between the 5.8S and 28S coding regions, have proven to be a reliable tool to identify the helminth species and their phylogenetic relationships (Morgan and Blair 1995; Blair et al. 1999a,b; Leon-Regagnon et al. 1999; Iwagami et al. 2000; Tkach et al. 2000; Kostadinova et al. 2003; Scholz et al. 2004). The nuclear ribosomal DNA ITS2 sequences, which occur between the 5.8S and 28S coding regions, have proven useful for diagnostic purposes at the level of species. Fasciola spp. and isolates of Fascioloides magna from different geographical regions were discriminated on the basis of ITS sequences (Adlard et al. 1993). Studies on phylogeny and/or intra-specific variation in Paragonimus species have also been done using ITS2 region in recent years (Blair et al. 1996, 1997), and the usefulness of the method for species discrimination has also been demonstrated in nematodes (Campbell et al. 1994; Hoste et al. 1995; Samson-Himmelstjerna et al. 1997).
During an exploratory survey of edible crab species, undertaken to ascertain the prevalence of crustacea-borne trematodiasis in the region, stream crabs from Miao, Changlang District, of Arunachal Pradesh were found to be heavily infected with metacercariae of Paragonimus species. The present study was aimed at identifying the Paragonimus species implicated in infection in the region using surface fine topography of the metacercariae and molecular markers as the identifying tools.
Materials and methods
Parasite material
Naturally infected freshwater edible crabs (Barytelphusa lugubris) were collected from a mountain stream of the suspected focal area Miao, Changlang District, in Arunachal Pradesh (altitude = 213 m above sea level, longitude = 96°15′N and latitude = 27°30′E). Metacercariae were isolated from the muscles of the crustacean host by digestion technique. The crabs were cut into small pieces with the help of scissors, minced and digested by overnight incubation at 37°C in the artificial gastric juice. The digested materials were filtered through mesh wire sieves, and the filterable sediments were washed repeatedly with tap water to get a clearer supernatant. The sediments were examined for Paragonimus metacercariae under a dissecting stereoscopic microscope. A few specimens were duly processed for whole-mount preparation and subsequent light microscopy observations.
Scanning electron microscopy
The isolated metacercariae were fixed in 10% neutral-buffered formalin at 4°C for 24 h, washed in phosphate-buffered saline and dehydrated with ascending grades of acetone to pure dried acetone. The specimens were then treated with Tetra methyl Silane (boiling point 23.3°C, surface tension 10.2 dynes/cm at 20°C) following Roy and Tandon (1991). The dried specimens mounted on brass stubs were coated with a thin (300 Å) layer of gold vapour. The gold-coated specimens were observed using LEO 435 VP scanning electron microscope at electron-accelerating voltages ranging between 10 and 20 kV.
DNA isolation
The 70% alcohol-fixed metacercariae were further processed for DNA extraction and PCR amplification. For the purpose of extraction, metacercariae recovered from one single host were pooled together, DNA was extracted from metacercariae in the FTA card by using Whatman’s FTA Purification Reagent and amplified by PCR. After applying the tissue homogenate on FTA cards, the latter were allowed to dry for 1 h at room temperature before punching. Two to three sample discs of 1.2 mm size were taken from the desired spot using a coring device assuming a 25-μl reaction volume and placed in a PCR amplification tube. The discs were then washed with FTA purification reagent and Tris–ethylenediamine tetraacetic acid (EDTA) buffer and allowed to dry at room temperature for 1 h to make them ready for PCR.
DNA amplification and sequencing
The rDNA region spanning the ITS2 was amplified from metacercarial DNA by PCR. As primers, we used the following:
-
1.
3S (forward): 5′-GGTACCGGTGGATCACTCGGCTCGTG-3′
-
2.
A28 (reverse): 5′-GGGATCCTGGTTAGTTTCTTTTCCTCCGC-3′
which were designed based on the conserved sequences of the 5.8S and 28S genes of Schistosoma species (Bowles et al. 1995). The PCR amplification was performed following the standard protocol (White 1993) with minor modifications in 100 mM Tris–HCl (pH 9.0), 500 mM KCl, 1.5 mM MgCl2 and 0.2 mM deoxynucleotide triphosphates each of deoxyadenosine triphosphate, deoxyguanosine triphosphate, deoxycytidine triphosphate and deoxythymidine triphosphate, 0.25 mM of each primer and 2.5 U of Taq polymerase (Bangalore Genei, India). DNA was pre-heated at 94°C for 5 min and added to each PCR reaction. The PCR cocktail (final reaction volume, 25 μl) was amplified with the following conditions: 26 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 38 s and extention at 72°C for 42 s followed by a final extention at 72°C for 10 min. The resultant PCR products were separated by electrophoresis through 1.6% (w/v) agarose gels in Tris–acetate–EDTA buffer, stained with ethidium bromide, transilluminated under ultraviolet light and then photographed. The known size fragments of Phi X 174 DNA/HaeIII Digest in agarose gel were used as marker.
For DNA sequencing, the PCR products were purified using Genei Quick PCR purification Kit and sequenced in both directions using PCR primers A28 and 3S on an automated sequencer.
Molecular phylogenetic analysis using bioinfomatic tools
The DNA sequences were put to further analysis with the usage of bioinfomatics tools including a similarity search using Basic Local Alignment Search Tool (BLAST) provided at http://www.ncbi.nlm.nih.gov/blast and phylogentic prediction using ClustalW provided at http://www.ebi.ac.uk/clustalw for DNA sequence query. Phylogenetic tree-building methods presume particular evolutionary models. Therefore, while interpreting the results obtained, different tree building models were considered to entertain possible explanations. Only unique sequences were used in tree construction. ITS sequences arranged with the MEGA format were entered in the MEGA for construction of the phylogenetic trees that were inferred using distance methods like neighbor joining, minimum evolution, unweighted pair group method with arithmetic mean and character state method like maximum parsimony. Test of phylogenetic accuracy was done by bootstrap for the neighbor-joining and minimum evolution trees.
Results
Morphology
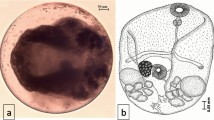
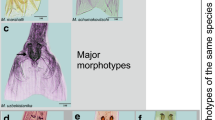
The newly excysted metacercaria has an elongate body (815.91 × 492.79 μm) in size; the ventral sucker situated somewhat pre-equitorally, is larger than the oral; the intestinal caeca are long and extend up to the posterior end of the body; the conspicuous excretory bladder extends medially in the intercaecal space (Fig. 1a–d). The scanning electron microscopy observations revealed that the encysted metacercaria is oval in shape and has a smooth surface. The whole body surface of the excysted metacercaria is covered with numerous single-pointed and thorn-like tegumentary spines; those covering the anterior part of the body are bigger in size and show a gradual reduction in size towards the posterior end. The tegument in the circum-oral region has a dense aggregation of small spines that are arranged in several circular rows. A few dome-shaped papillae abound on the rim of the oral sucker and the adjacent area in a random fashion. The tegument of the ventral sucker and its surrounding region and that of the general body surface also has a dense spination. The surface fine topography in the area reveals the presence of many papillate protuberances, but the latter are randomly distributed and do not exhibit a definite pattern of distribution and numbers (Fig. 2a–i).
SEM views. a, b Encysted and excysted metacercaria (scale bar = 100 μm). c–i Scale bar = 10 μm c Oral sucker region. Note the presence of a few domed papillae on and around the rim of the sucker. Frontal view. d Another view of the oral end, depicting spination pattern on circum oral tegument. Several rows of minute spines arranged in circular fashion are conspicuous. e Ventral sucker region. A few randomly distributed papillate protuberances are present. f Ventral sucker region in another specimen. g Spination in anterior region of body. h Single-pointed, backwardly directed spines in the mid-body region. i Smaller spines in the posterior part of the body
PCR amplification of ITS region and its analysis
The PCR amplified products of ITS2 of rDNA were successfully obtained using the primers as mentioned above (Fig. 3). The nucleotide sequences obtained were compared with other sequences of trematode species from Genbank. The fragments of amplified DNA were estimated to be ∼500 bp long. Sequence analysis of the ITS2 PCR products revealed that the alignments of the rDNA region spanning the ITS2 were 496 bp for the forward primer and 494 bp for the reverse primer, respectively. No intra-specific variations in length or composition of the sequence were observed, and all the ITS2 sequences of the metacercariae were found to be identical in all the samples.
The BLAST hit results show that the query ITS2 Paragonimus metacercariae forward and reverse sequences are closer and more similar to the sequences of the species P. westermani, P. mexicanus, P. siamensis, P. miyazakii and Euparagonimus cenocopiosus with maximum similarity being with P. westermani.
Construction of phylogenetic trees
Phylogenetic trees were obtained by comparing the ITS2 sequences of Paragonimus species from different geographical isolates. Phylogenetic analyses using the various distance methods and character state method like maximum parsimony show that the topology is similar among the trees obtained (Fig. 4a,b). The values of 70% and above in the bootstrap test of phylogenetic accuracy indicates reliable grouping.
Discussion
Surface fine topography of encysted and newly excysted metacercariae has been described in respect of several species of Paragonimus, e.g. P. skrjabini, P. iloktsuensis, P. ohirai, P. pulmonalis, P. westermani (diploid type), P. miyazaki (triploid types of P. westermani), P. mexicanus, P. heterotremus and P. westermani (Miyazaki 1974; He et al. 1982; Li et al. 1987; Higo and Ishii 1987; Aji et al. 1984; Tongu et al. 1987; Sugiyama et al. 1990; Jiang and Xia 1993; Sugiyama et al. 2001). Characters such as the number and distribution pattern of tegumental papillae around the oral and ventral suckers of the newly excysted metacercariae have been used as the morphological taxonomic tools for differentiating the various species of Paragonimus. For example, the number and size of the domed papillae in metacercariae of Paragonimus spp. in Japan seems to vary with the species (Higo and Ishii 1984, 1987), although geographical differences do not supposedly exist with regards to the morphology of the excysted metacercariae of P. westermani, in which the number of papillae ranges between 5 and 13 (Sugiyama et al. 2001). However, these morphological characters are prone to variations and thus not absolutely reliable.
The metacercariae under the present study revealed a ventral sucker larger than the oral unlike P. heterotremus (in which the oral sucker is larger than the ventral), a species that has earlier been reported from the same region in Northeast (Narain et al. 2003). The surface fine topography, including the number and distribution of papillae and spination pattern of our material, suggests more closeness and resemblance with P. westermani. In the latter species, although Sugiyama et al. (2001) reported the occurrence of dome-shaped papillae as evenly distributed over the whole body and in circular fashion around the suckers, as per our observations, the papillae were fewer in number and revealed to be randomly scattered across the general body surface.
In the sequence analysis of the rDNA ITS2 comparing with the known sequences of the other lung fluke species, the present study revealed that the sequence of ITS2 (plus flanking regions) show close resemblance with the sequences of P. westermani, both of metacercarial and adult origins. The results corroborate that the ITS2 sequences are not stage specific and are conserved through different stages of the development of the fluke (Sugiyama et al. 2002). In phylogenetic analysis, as a general rule, if the bootstrap value for a given interior branch of a phylogenetic tree is 70% or higher, then the topology at that branch is considered ‘correct.’ Our results showed a bootstrap value to be greater than 70% among the trees obtained, and the ITS2 sequence resembled P. westermani. Thus, on the basis of surface fine topography features and supplemented by absolute matching of ITS2 sequence that could be used as one of the species markers, it can be concluded that Paragonimus species prevailing in Miao region of Arunachal Pradesh is indeed P. westermani and not P. heterotremus as reported by earlier workers.
References
Adlard RD, Barker SC, Blair D, Cribb TH (1993) Comparison of the second internal transcribed spacer (ribosomal DNA) from populations and species of Fasciolidae (Digenea). Int J Parasitol 23:423–425
Aji T, Oh H, Tongu Y, Inatomi S, Hata H, Kobayashi M, Yokogawa M, Miranda H, Ibanz N (1984) Ultrastructure of tegumental surface of the metacercaria of Paragonimus peruvianus. Jpn J Parasitol 33:15–21
Blair D, Campos A, Cummings MP, Laclette JP (1996) Evolutionary biology of parasitic platyhelminths: the role of molecular phylogenetics. Parasitol Today 12:66–71
Blair D, Agatsuma T, Watanobe T, Okamoto M, Ito A (1997) Geographical genetic structure within the human lung fluke, Paragonimus westermani, detected from DNA sequences. Parasitology 115:411–417
Blair D, Xu ZB, Agatsuma T (1999a) Paragonimiasis and the Genus Paragonimus (Review). Adv Parasitol 42:113–222
Blair D, Wu B, Chang ZS, Gong X, Agatsuma T, Zhang YN, Chen SH, Lin JX, Chen MG, Waikagui J, Guevara AG, Feng Z, David GM (1999b) A molecular perspective on the genera Paragonimus Braun, Euparagonimus Chen and Pagumogonimus Chen. J Helminthol 74:295–299
Bowles J, Blair D, McManus DP (1995) A molecular phylogeny of the human schistosomes. Mol Phylogenet Evol 4:103–109
Bunnag D, Harinasuta T (1985) Opisthorchiasis, clonorchiasis and paragonimiasis. In: Warren KS, Mahmoud AAF (eds) Tropical and geographical medicine. McGraw-Hill, New York, pp 461–470
Campbell AJD, Gasser RB, Chilton NB (1994) Differences in a ribosomal DNA sequence of Strongylus species allows identification of single eggs. Int J Parasitol 25:359–365
Chandler AC, Read CP (1961) Introduction to parasitology. Wiley, New York, pp 299–331
Chen HT (1940) Morphological and developmental studies of Paragonimus iloktsuenensis with some remarks on other species of the genus (Trematoda: Troglotrematidae). Lingnan Sci J 19:429–530
Chen HT (1959) The occurrence of a new type of Paragonimus and some clinical problems related to lung fluke in China. 1958 Annual Report. Chung Shang Medical College, China, pp 192–193 (in Chinese)
Chen HT, Hsia TK (1964) A prelimnary report on a new species of Paragonimus. 1. Paragonimus heterotremus sp.nov. Acta Zhongshan Univ 2:236–238 (in Chinese, English abstract)
He YX, Lin YG, Zhong WY (1982) Scanning electron microscopy of Paragonimus skrjabini, a pathogenic lung fluke in china. Acta Zool Sin 28:146–148 (in Chinese, English abstract)
Higo H, Ishii Y (1984) Scanning electron microscopy of the newly excysted juveniles Paragonimus westermani (Kerbert, 1878) Braun, 1899 (parthenogenetic type) and P. miyazakii Kamo, Nishida, Hatsushika and Tominura, 1961. Jpn J Parasitol 33:421–427
Higo H, Ishii Y (1987) Comparative studies on surface ultrastructure of newly excysted metacercariae of Japanese lung flukes. Parasitol Res 73:541–549
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453
Hoste H, Chilton NB, Gasser RB, Beveridge I (1995) Differences in the second internal transcribed spacer (ribosomal DNA) between five species of Trichostrongylus (Nematoda: Trichostrongylidae). Int J Parasitol 25:75–80
Iwagami M, Lo Y, Su K, Lai PF, Fukushima M, Nakano M, Blair D, Kawashima K, Agatsuma T (2000) Molecular phylogeographic studies on Paragonimus westermani in Asia. J Helminthol 74:315–322
Jiang JX, Xia DG (1993) Scanning electron microscopic observation on the encysted and excysted metacercaria of Paragonimus heterotremus (in Chinese, English abstract). Chin J Parasitol Parasit Dis 11:132–134
Kamo H, Nishida H, Hatsushika R, Tomimura T (1961) On the occurrence of a new lung fluke, Paragonimus miyazakii n. sp. In Japan (Trematoda: Troglotrematidae) (in Japanese). Yonaga Acta Med 5:43–52
Kerbert C (1878) Zur Trematoden-Kenntnis. Zool Anz 1:271–273
Kostadinova A, Herniou EA, Barrett J, Littlewood DT (2003) Phylogenetic relationships of Echinostoma rudolphi, 1809 (Digenea: Echinostomatidae) and related genera re-assessed via DNA and morphological analysis. Syst Parasitol 54:159–176
Kusner DJ, King CH (1993) Cerebral paragonimiasis. Semin Neurol 13:201–208
Leon-Regagnon V, Brooks DR, Perez-Ponce G (1999) Differentiation of Mexican species of Haematoloechus Looss, 1899 (Digenea: Plagiorchiformes): molecular and morphological evidence. J Parasitol 85:935–946
Li GY, Zhan XM, Liang JM (1987) Scanning electron microscopy of Paragonimus hueitungensis and Paragonimus bangkokensis (in Chinese). Annu Bull Soc Parasitol Guangdong Prov 8–9:113–114
Miyazaki I (1939) A new lung fluke Paragonimus ohirai n. sp.. Fukuoka Acta Med 32:1247–1252 (in Japanese, German abstract)
Miyazaki I (1974) Lung flukes in the world. Morphology and life history. In: Sasa M (ed) A symposium on epidemiology of parasitic diseases. International Medicine Foundation of Japan, Tokyo, pp 101–135
Miyazaki I (1978) Two types of lung fluke, which has been called Paragonimus westermani (Kerbert, 1878). Med Bull Fukuoka Univ 5:251–263
Miyazaki I, Ishii Y (1968) Studies on the Mexican lung fluke, with special reference to a description of Paragonimus mexicanus sp.nov. (Trematoda: Troglotrematidae). . Jpn J Parasitol 17:445–453
Morgan JAT, Blair D (1995) Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: an aid to establishing relationships within the 37-collar spine group. Parasitology 111:609–615
Narain K, Devi KR, Mahanta J (2003) Paragonimus. and paragonimiasis—a new focus in Arunachal Pradesh, India. Curr Sci 84:985–987
Razaque MA, Mutum SS, Singh TS (1991) Recurrent haemoptysis? Think of paragonimiasis. Trop Doct 21:153–155
Roy B, Tandon V (1991) Usefulness of Tetramethylsilane in the preparation of helminth parasites for scanning electron microscopy. Riv Parassitol 8:405–413
Samson-Himmelstjerna GV, Woidtke S, Epe C, Schnieder T (1997) Species- specific polymerase chain reaction for the differentiation of larvae from Dictyocaulus viviparus and Dictyocaulus eckerti. Vet Parasitol 68:119–126
Scholz T, Skerikova A, Shimazu T, Grygier MJ (2004) A taxonomic study of species of Bothriocephalus Rudolphi, 1808 (Cestoda: Pseudophyllidea) from eels in Japan: morphological and molecular evidence for the occurrence of B. claviceps (Goeze, 1782) and confirmation of the validity of B. japonicus Yamaguti, 1934. Syst Parasitol 57:87–96
Singh TS (2002) Occurrence of the lung fluke Paragonimus hueitungensis in Manipur, India. Zhonghua Yi Xue Za Zhi (Taipei) 65:426–429
Singh TS, Mutum S, Razaque MA, Singh YI, Singh EY (1993) Paragonimiasis in Manipur. Indian J Med Res 97:247–252
Sugiyama H, Horiuchi T, Tomimura T, Shibahara T, Agatsuma T, Habe S, Ketudat P, Thaithong S (1990) Surface ultrastructure of newly excysted metacercariae of Paragonimus heterotremus. South Asian J Trop Med Public Health 21:109–113
Sugiyama H et al (2001) Surface ultrastructure of newly encysted metacercaria of Paragonimus westermani from Malaysia and the Philippines. Jpn J Trop Med Hyg 29:375–378
Sugiyama H, Morishima Y, Kameoka Y, Kawanaka M (2002) Polymerase chain reaction (PCR)-based molecular discrimination between Paragonimus westermani and P. miyazakii at the metacercarial stage. Mol Cell Probes 16:231–236
Tkach VV, Pawlowski J, Sharpilo VP (2000) Molecular and morphological differentiation between species of the Plagiorchis vespertilionis group (Digenea, Plagiorchiidae occurring in European bats, with a re-description of P. vespertilionis group (Muller, 1780). Syst Parasitol 47:9–22
Tongu Y, Aji T, Oh H, Ishii A, Yokogawa M, Hata H, Ito J, Lamothe-Argumedo R (1985) Surface ultrastructure of Paragonimus mexicanus Miyazaki et ishii, 1968. Jpn J Parasitol 34:441–447
Tongu Y, Iwanaga Y, Hata H, Tsuji M, Yokogawa M, Morera P, Conejo M (1987) Morphological features of Paragonimus metacercariae from Costa Rica. Jpn J Parasitol 36:236–241
Tongu Y, Hata H, Orido Y, Pinto MR, Lamothe-argumedo R, Yokogawa M, Tsuji M (1995) Morphological observations of Paragonimus mexicanus from Guatemala. Jpn J Parasitol 44:365–370
Toscano C, Yu SH, Nunn P, Mott KE (1995) Paragonimiasis and tuberculosis, diagnostic confusion: a review of the literature. Trop Dis Bull 92:R1–R26
Voelker J, Vogel H (1965) Zwei neue Paragonimus-arten aus West-Afrika: Paragonimus africanus und Paragonimus uterobilateralis (Troglotrematidae: Trematoda). Z Tropenmed Parasitol 16:125–148 (English abstract)
White BA (1993) PCR Protocols, current methods and applications, vol 15. Humana, Totowa, NJ
Acknowledgements
This study was carried out under the ‘All India Co-ordinated Project on Capacity Building in Taxonomy: Research on Helminths,’ sanctioned to VT by Ministry of Environment and Forests, GOI; DBT Project to VT and AC and DSA programme of the University Grants Commission, GOI, in the Department of Zoology, NEHU, Shillong. We thank the co-ordinator, Bioinformatics Centre, NEHU, for allowing access to its facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
The nucleotide sequence data reported in this paper have been submitted to the Genbank data with the accession number DQ351845.
Rights and permissions
About this article
Cite this article
Tandon, V., Prasad, P.K., Chatterjee, A. et al. Surface fine topography and PCR-based determination of metacercaria of Paragonimus sp. from edible crabs in Arunachal Pradesh, Northeast India. Parasitol Res 102, 21–28 (2007). https://doi.org/10.1007/s00436-007-0715-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0715-4