Abstract
Three specimens of Caudisoma durissa terrifica infected with Hepatozoon spp. were studied. One was parasitized by one type of gamont and the other two were each infected by two morphologically different gamonts. Utilizing morphology and morphometry analysis, we concluded that three types of gamonts were very similar and may represent the same Hepatozoon species, but at least three different Hepatozoon species were infecting the C. durissa terrifica snakes in this study. Some of this species caused erythrocyte modifications. The sporogonic development of Hepatozoon sp. was observed from 12 h to the 20th day after female Culex quinquefasciatus blood meals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatozoon spp. are the most common intracellular protozoa found in snakes (Wozniak et al. 1994). The Hepatozoon spp. life cycle is characterized by sporogonic development in a hematophagous arthropod that results in the formation of large oocysts, each containing sporozoite-filled sporocysts. Merogonic and gamontogonic development occur in the internal organs of a vertebrate host after ingestion of an infected definitive host (Smith 1996). Mosquitoes have been used as experimental vectors for Hepatozoon spp. of snakes with success (Pessoa et al. 1974; Sloboda et al. 2007; Telford et al. 2008, 2010). Bashtar et al. (1991) monitored the developmental stages of Hepatozoon melhorni sp. nov. in the viper Echis carinatus and the mosquito Culex pipiens. It was observed that the formation of microgamonts, which produced four uniflagellate microgametes, and the fertilization of the macrogamete with the formation of the zygote were at 3 days post-infection (p.i.). About the 16th day p.i., the maturation of the oocysts was completed. Lowichichik et al. (1993) studied the sexual life cycle of Hepatozoon mocassini in Aedes aegypti. By day 17, the mature oocysts were observed.
There are two described species of Hepatozoon from Caudisoma durissa terrifica: Hepatozoon romani and Hepatozoon capsulata (Phisalix 1931). H. romani measured 17–18 μm in length and 8.4 μm in width, with a non-condensed nucleus that measured 2–3 μm. H. capsulata measured 16.8 μm in length and 6.3 μm in width, with a condensed nucleus at 4.2 μm (Phisalix 1931). In addition, two other Hepatozoon spp. infecting C. durissa terrifica were reported in Brazil (Moço et al. 2002; O’Dwyer et al. 2004). The former species had an elongated and slender shape, with the cytoplasm lightly stained and homogeneous, measuring 33.2 ± 4.1 μm2 in area, 14.7 ± 0.6 μm in length, and 2.4 ± 0.4 μm in width. The nucleus was homogeneous, dense, central or lightly displaced, and measured 8.5 ± 1.7 μm2 in area, 5.1 ± 0.8 μm in length, and 1.7 ± 0.4 μm in width (Moço et al. 2002). The other presented a small and short body, measuring 8.1 ± 0.5 μm long and 3.8 ± 0.4 μm wide, with round extremities and a large nucleus (O’Dwyer et al. 2004). There are no descriptions of the sporogonic stages of these species.
The aim of the present investigation was to describe the gamontogonic and sporogonic stages of Hepatozoon sp. from three C. durissa terrifica specimens.
Materials and methods
Snakes
Three male specimens of C. durissa terrifica were studied. The animals were donated to the Center for the Study of Venoms and Venomous Animals from São Paulo State University (CEVAP), Botucatu, São Paulo, Brazil, and were naturally infected with Hepatozoon spp. For this study, the snake specimens were named as Cdt 1, Cdt 2, and Cdt 3. The Cdt 1 snake refused to eat and died. This animal was necropsied, and touch impression smears were prepared from all organs, fixed with methanol, and stained with Giemsa.
Morphologic and morphometric analyses of the Hepatozoon gamonts
Blood was collected from the snakes by ventral tail venipuncture, and blood smears were prepared immediately upon collection. Smears were air-dried, fixed with absolute methanol for 3 min, and stained with 10% Giemsa for 30 min. The shape of the gamonts, the presence of pigment in the cytoplasm, and the format and position of its nucleus were observed. Morphometric analysis was performed using the computerized image analysis system (Qwin Lite 2.5—Leica) at ×1,000 magnification. The analyzed variables were area, length, and width of the parasite and area, length, and width of the parasite nucleus. Whenever possible, at least 100 parasites were measured for each infected snake. Also, 100 normal erythrocytes and 100 infected erythrocytes had the following variables measured: area, length, and width of the erythrocyte and area, length, and width of the erythrocyte nucleus.
Sporogonic development of Hepatozoon spp. in mosquitoes
Laboratory-reared Culex quinquefasciatus were raised from eggs, and the adults were maintained at 25°C in a mosquito cage previously prepared to permit the introduction of the infected snakes. The infected snakes were introduced in the mosquito cages and made to stay through the night to allow the females to feed on. In the morning, the snakes were removed from the cage. The blood-fed female mosquitoes were maintained at 25°C and supplied with water supplemented with 10% sucrose. The females were periodically dissected as described by Consoli and Oliveira (1994) in order to follow the development of the oocysts in fresh coverslip preparations. Briefly, the female mosquito was introduced in the freezer for 1 min to be anesthetized. It was transferred to a drop of saline solution on a microscope slide for dissection. The wings and legs were removed. To remove the gut from the body, one needle was placed on the thorax and the other on the tip of the abdomen and gently pulled. The midgut and hindgut were removed intact as well the free oocysts. To observe the initial development, smears from the gut were prepared, 12 and 20 h after repletion, fixed with methanol, and stained with Giemsa. Afterward, females were dissected daily from the 5th to the 20th day after engorgement. The immature and mature oocysts, the mature and immature sporocysts, and the sporozoites were described and measured. Morphometric analysis was performed using the computerized image analysis system (Qwin Lite 2.5—Leica) at ×1,000 magnification. The analyzed variables were area, length, and width of the parasites.
Statistical analysis
Data obtained for the parasites were analyzed by analysis of variance, and the difference among the groups was verified by the Student–Newman–Keuls test. Data of infected and non-infected erythrocytes were compared by t test. All statistical tests were performed using Sigma Stat 2.0. Differences were considered significant when the level of significance was lower than 5% (p < 0.05).
Results
Description of the intraerythrocytic gamonts
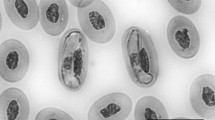
The Cdt 1 possessed a Hepatozoon sp. gamont that presented an elongated shape. The cytoplasm was deeply stained, with no granulations. The nucleus was homogeneous, dense, and parallel to the longer axis of the parasite and displaced towards one of the extremities. In the majority of cases, the parasites were attached to the erythrocyte nucleus (Fig. 1a). Free gamonts were also observed (Fig. 1b).
Two types of gamonts were identified in the second specimen (Cdt 2). The first one (Cdt 2-T1) was slender with pointed extremities, with a rose, vacuolated cytoplasm. The nucleus was central or slightly displaced and not condensed (Fig. 1c). The Cdt 2-T2 was shorter and wider, with round extremities and a rose and uniform cytoplasm. Its nucleus was compact and displaced to one of the extremities (Fig. 1d). The Cdt 3 possessed also two types of gamonts. The Cdt 3-T1 had an elongated curved shape, with round extremities and a uniform cytoplasm. The nucleus was elongated, condensed, and displaced to one of the extremities. The parasites were always adhered to the erythrocyte nucleus (Fig. 1e). The Cdt 3-T2 was shorter and larger, with a uniform cytoplasm and round extremities, and longitudinally adhered to the erythrocyte nucleus. The gamont nucleus was short, not condensed, and centrally located (Fig. 1f). The morphometry of the gamonts is shown in Table 1.
Changes of the morphology of the infected erythrocyte
The results obtained by erythrocyte analysis showed that Cdt 1 and Cdt 2-T1 gamonts changed all variables of infected erythrocytes (p < 0.05). The Cdt 2-T2 gamonts only changed the nucleus of the erythrocytes. The Cdt 3-T1 gamonts changed the same variables from erythrocytes and also from its nucleus, while the Cdt 3-T2 changed only one variable of the erythrocyte nucleus (Table 2).
Sporogonic development within experimentally infected C. quinquefasciatus
The infection of C. quinquefasciatus with Hepatozoon spp. of Cdt 1 was monitored as follows: free gamonts were observed within the gut, with or without the capsule on the first 20 h after feeding (Fig. 2a, b). Immature oocysts were observed from the 5th to the 9th day free in the mosquitoes’ hemocoel. The immature oocysts were round to oval and had double membrane and a sporont that could be central or displaced to one side. Initially, the sporont was irregular in shape, but with the oocyst development the sporont became oval and well defined (Fig. 2c, d). The immature oocysts became larger as maturation occurs. From the 10th to the 18th day, all the dissected mosquitoes were found negative. Mature oocysts as well as sporocysts were observed free in the hemocoel on the 19th and 20th day. Two kinds of oocysts were observed: one that was small with a few large sporocysts (Fig. 2e) was the main oocyst found. The other one, large with numerous sporocysts (Fig. 2f), measured 293 × 254 μm and the sporocysts 33.3 × 22.2 μm. Only two large oocysts were detected and measured. The sporocysts were round or oval, with numerous sporozoites and a residual sporont (Fig. 3a). The sporozoites were typically banana-shaped with a more tapered pole, and the nucleus was displaced to this pole and measured 24.8 × 5.2 μm (Fig. 3b). The morphometry of the sporogonic stages of Hepatozoon from Cdt 1 is shown in Table 3.
Sporogonic development of Hepatozoon sp. from C. durissa terrifica snake within the mosquito C. quinquefasciatus. a Gamonts within a capsule in the digestive tract, 12 and 20 h after repletion; b free gamonts in the digestive tract, 12 and 20 h after repletion; c immature oocyst with an irregular sporont (×100); d immature oocyst with a regular sporont (×40); e mature oocyst (×40); f mature oocyst (×20)
a, b Sporogonic development of Hepatozoon sp. from C. durissa terrifica snake (Cdt 1) within the mosquito C. quinquefasciatus: a mature sporocyst (×100); b sporozoites (×100) (fresh preparation); c, d squizogonic stages of Hepatozoon sp. from Crotalus durissa terrificus snake within the spleen (touch impression smear, Giemsa, ×1,000): c free gamont; d young squizont
All attempts to infect mosquitoes with the Hepatozoon spp. of Cdt 2 and Cdt3 failed. The majority of the females died after feeding and the ones that survived were all negative.
Necropsy of the Cdt 1
The necropsy of Cdt 1 revealed that the liver was yellowish and the spleen, the heart, and the lungs had some nodules on the superficies. The analysis of touch impression smears showed that numerous intraerythrocytic gamonts were observed in all the organs (Fig. 3c). Empty capsules were also detected. Nevertheless, the only developmental stage detected was an exemplar of an immature schizont (Fig. 3d).
Discussion
The intraerythrocytic gamonts studied were morphologically and morphometrically different from the already described species of Hepatozoon from C. durissa terrifica and may represent new Hepatozoon species, expanding the universe of Hepatozoon of this snake species. Analyzing only the morphology of the gamonts (Fig. 1), it is possible to note that the Cdt 1 gamonts, Cdt 2-T2 gamonts, and Cdt3-T2 gamonts were very similar. So, utilizing morphology and morphometry analysis, we concluded that the three types of gamonts were very similar and may represent the same species of Hepatozoon, with light differences among the different hosts, but at least three Hepatozoon species were infecting the C. durissa terrifica snakes in this study. Interestingly, Cdt 2-T2 and Cdt 3-T2 caused minor changes in the erythrocytes; on the contrary, the Cdt 2-T1 and Cdt 3-T1 produced large modifications on the host cells, reinforcing the differences from the groups. It is difficult to explain the large modification of the cells parasitized by Cdt 1 gamonts if we considered them morphologically and morphometrically similar to the Cdt 2-T2 and Cdt 3-T2 gamonts. This modification could be associated to the snake Cdt 1’s sensibility.
The studies of sporogonic cycle within the C. quinquefasciatus were very difficult. With two snakes, the various trials of infecting the mosquitoes failed, with high mortality rates. Even the infection of the mosquitoes that fed on the first snake was very difficult. Although the mosquitoes engorged after feeding on the snakes, few mosquitoes became infected. The mortality rate was also very high. In one of the tentative stages of infection, all the mosquitoes died within 24 h after repletion. The accomplishment of the development of sporogonic stages was incomplete since we did not observe any developmental stages from the 10th to 18th days. The occurrence of two kinds of mature oocysts may indicate that the snake could be infected with two different Hepatozoon species, although we could detect only one type of gamonts by smear examination. Nevertheless, Desser et al. (1995) stated that the mature oocysts of one same species highly vary in size and therefore their dimensions would have low taxonomic value.
Adham et al. (2003) studied the effects of laboratory Hepatozoon gracilis infection on the mosquito Culex pipiens and concluded that the infection leads to a reduction in the fecundity of the females, although it had no effect on the mortality. Ebrahemm et al. (2006) also described that infection with Hepatozoon sp. did not changed the longevity of the mosquitoes but caused a significant reduction in the number of eggs deposited. Rashdam and El-Sebaii (2006) observed that the temperature of maintenance of the infected mosquito females affects the longevity and mortality of the mosquitoes and the development of the oocysts.
Although several authors have been successful in experimentally infecting mosquitoes (Pessoa et al. 1974; Wozniak and Telford 1991; Sloboda et al. 2007; Telford 2010), the results obtained in the present study suggest that mosquitoes are not the natural hosts of these protozoans of C. durissa terrifica. Ball et al. (1967) found a relationship between high parasitemia and mortality of infected mosquitoes. Wozniak and Telford (1991) also observed a high mortality of infected mosquitoes and considered hemogregarins as pathogenic to these dipterans. The above-cited articles were performed with different Hepatozoon species from different snake species, and it is impossible to compare their results with ours. The results of our experiments make it possible to conclude that the Hepatozoon species of C. durissa terrifica may not be well adapted to the C. quinquefasciatus, hence explaining the high mortality rate of the mosquitoes.
Although we did not achieve all the biological stages of the Hepatozoon species of the studied snakes, we believe that in this study we worked with at least three different Hepatozoon species from C. durissa terrifica, demonstrating that the universe of these parasites is very complex. There are many questions that must be answered: how many species of Hepatozoon infect C. durissa terrifica? Can the same Hepatozoon species parasitize more than one snake species? Are there variations of the Hepatozoon morphology within the snake specimens? Are the mosquitoes the natural vectors of Hepatozoon of snakes? More studies, including genetic studies, should be conducted to answer these and other questions about Hepatozoon from snakes.
References
Adham FK, Refaat MG, Tahany HA, Galal FH (2003) The effects of laboratory Hepatozoon gracilis infection on the fecundity, mortality and longevity of Culex (Culex) pipiens Linneaus (Diptera: Culicidae) in Egypt. J Egypt Soc Parasitol 33:353–360
Ball HG, Chao J, Telford SR Jr (1967) The life history of Hepatozoon rarefaciens (Sambon & Seligman, 1907) from Drymarchon corais (Colubridae), and its experimental transfer to Constrictor constrictor (Boidae). J Parasitol 53:897–909
Bashtar AR, Abdel-Ghaffar FA, Shazly MA (1991) Lyfe cycle of Hepatozoon melhorni sp. nov. in the viper Echis carinatus and the mosquito Culex pipiens. Parasitol Res 77:402–410
Consoli RA, Oliveira RL (ed) (1994) Principais mosquitos de importância sanitária no Brasil. FIOCRUZ, Rio de Janeiro, Brazil
Desser SS, Hong H, Martin DS (1995) The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae N. Comb., an apicomplexan parasite of the bullfrog, Rana catesbeiana and the mosquito, Culex territans in Algonquin Park, Ontario. J Parasitol 81:212–222
Ebrahemm MH, Rashdan NA, Fayed HM, Galal FH (2006) Laboratory studies on the possibility of Culex (Culex) pipiens L. to harbour and transmit Hepatozoon matruhensis to the Egyptian snake Psammophis schokari. J Egypt Soc Parasitol 36:241–250
Lowichichik A, Lanners HN, Lowrie RC Jr, Meiners NE (1993) Gametogenesis and sporogony of Hepatozoon mocassini (Apicomplexa: Adeleina: Hepatozoidae) in an experimental mosquito host, Aedes aegypti. J Eukaryot Microbiol 40:287–297
Moço TC, O’Dwyer LH, Vilela FC, Barrella TH, Silva RJ (2002) Morphologic and morphometric analysis of Hepatozoon spp. (Apicomplexa, Hepatozoidae) of snakes. Mem Inst Oswaldo Cruz 97:1169–1176
O’Dwyer LH, Moço TC, Silva RJ (2004) Description of the gamonts of a small species of Hepatozoon sp. (Apicomplexa, Hepatozoidae) found in Crotalus durissus terrificus (Serpentes, Viperidae). Parasitol Res 92:110–112
Pessoa SB, De Biasi P, Puorto G (1974) Transferência do Hepatozoon tupinambis parasita do lagarto Tupinambis teguixin, para a serpente cascavel (Crotalus durissus terrificus) por intermédio de mosquito Culex fatigans. Mem Inst Oswaldo Cruz 72:295–299
Phisalix M (1931) Les hémogrégarines de Crotalus terrificus Lau. Bull Soc Pathol Exot 24:190–194
Rashdam NA, El-Sebaii SE (2006) Culex neavei Theobold, as a possible transmitter of Hepatozoon matruhensis to the Egyptian snake Psammophis schokari. J Egypt Soc Parasitol 36:1–6
Sloboda M, Kamler M, Bulantová J, Votýpka J, Modrý D (2007) A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J Parasitol 93:1189–1198
Smith TG (1996) The genus Hepatozoon (Apicomplexa: Adeleina). J Parasitol 82:565–585
Telford SR Jr, Moler PE, Butler JF (2008) Hepatozoon species of the timber rattlesnake in northern Florida: description of a new species, evidence of salivary gland oocysts, and a natural cross-familial transmission of an Hepatozoon species. J Parasitol 94:520–523
Telford SR Jr (2010) Three new Hepatozoon species (Apicomplexa: Hepatozoidae) infecting the Florida kingsnake, Lampropeltis getula floridana. J Parasitol 96:162–169
Wozniak EJ, Telford SR (1991) The fate of possible two Hepatozoon species naturally infecting Florida black racers and watersnakes in potential mosquito and soft tick vectors: histological evidence of pathogenicity in unnatural host species. J Parasitol 21:511–516
Wozniak EJ, Telford SR, McLaughlin GL (1994) Employment of the polymerase chain reaction in the molecular differentiation of reptilian hemogregarines and its application to preventative zoological medicine. J Zoo Wildl Med 23:538–547
Acknowledgements
Financial support was provided by FAPESP (process 01/02070-9). All the presented experiments complied with the currents laws of research in Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Dwyer, L.H., da Silva, R.J. & Madeira, N.G. Description of gamontogonic and sporogonic stages of Hepatozoon spp. (Apicomplexa, Hepatozoidae) from Caudisoma durissa terrifica (Serpentes, Viperidae). Parasitol Res 108, 845–851 (2011). https://doi.org/10.1007/s00436-010-2124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2124-3