Abstract
For the first time, the process of encapsulation of acanthocephalans in a paratenic host has been investigated experimentally. It was shown that the formation of a leukocytal capsule around the acanthocephalan Corynosoma strumosum (Rudolphi, 1802) (Luhe, 1904) in Middendorffʼs eelpout Hadropareia middendorffii (Schmidt, 1904) occurs in three stages. In the first days after the penetration of the acanthocephalan into the body cavity of the host, a migration of leukocytes to the parasite, including macrophages, is observed. Two weeks later, a few fibroblasts and collagen fibers are included in the capsule, the number of which further increases. Fifty days after the start of encapsulation, a significant number of fibroblasts/fibrocytes and bundles of collagen fibers are found in the composition of the capsule, together with leukocytes, and in its cellular composition it does not differ from that described from Middendorffʼs eelpout infected naturally. In response to the hostʼs cellular response, the parasite forms a typical thick glycocalyx layer on its surface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the first communication, we presented the results of a study of the histological and ultrafine organization of the capsules surrounding Corynosoma strumosum in Middendorff’s eelpout, naturally invaded. It was shown that these capsules belong to the leukocyte type, which is characterized by the presence of a significant or even predominant number of leukocytes. In response, C. strumosum forms a thick layer of glycocalyx on its surface, the formation of which we consider as protection against the host’s cellular response. Both of these features, in our opinion, are signs of one of the two currently known strategies for the relationship of acanthocephalans with the paratenic hosts (Nikishin and Skorobrekhova, 2018).
The possibility of infecting paratenic hosts with acanthocephalans under experimental conditions has been repeatedly demonstrated in the studies of V.P. Sharpilo (1965, 1971); however, the process of acanthocephalan encapsulation in such cases remained unexplored. In order to study the process of capsule formation around Corynosoma strumosum in natural paratenic hosts, an experimental infection of Middendorff’s eelpouts was carried out. Such experiments were performed for the first time with marine fish. This report provides preliminary results of this study.
MATERIALS AND METHODS
The choice of Middendorff’s eelpouts for experiments was made for two reasons. Firstly, during low tide, these fish often remain under stones and in littoral baths, which made it possible to create conditions close to natural in the aquarium. Secondly, in Nagaevo Bay, a littoral area was found in which the eelpouts had a low infestation: during the years 2011–2013, in different periods from June to September, 146 fish of this species caught in this area were dissected, and the extension of their natural invasion was less than 5%, with the intensity varying from one to three specimens.
The experimental fish were caught during low tide under stones or in littoral baths. Some of the fish were dissected immediately (control group), the rest were used in the experiment (experimental group). The experiments were carried out in laboratory conditions in June and September–November 2012.
The experimental group of fish was kept in an aquarium with seawater (volume 80 L). At the bottom of the aquarium, coarse soil taken from the natural biotope of experimental animals was placed. Heaps of large stones were erected as shelters, and hollow ceramics were installed. The water in the aquarium was constantly enriched with oxygen through an aerating filter. In order to maximize the imitation of natural conditions, the aquarium was set up in an unheated room. Before the experiments, the caught fish were kept at rest for a week. Two to three days after capture the fish began to eat. They were fed once a day with frozen feed.
The first experiment was carried out in June 2012 to investigate the initial stages of the encapsulation process for the acanthocephalans, so all experimental fish were dissected three days after infection. The experimental group of fish consisted of 12 eelpouts with a length of 8.2 to 11.8 cm (Table 1). Simultaneously with the experimental group of fish, a control group of 14 fish was examined; autopsy results showed no natural infestation with acanthocephalans. The air temperature in the room with the aquarium ranged from 12 to 18°С. Experimental fish were infected by the oral method with encapsulated acanthocephalans with pieces of adjacent tissues extracted from other natural paratenic hosts (viviparous blennies and Middendorff’s eelpouts) caught in an area with a high fish infestation with this acanthocephalan. A total of 32 acanthocephalans were fed.
The second experiment was carried out in September–November of the same year with the aim of studying the later stages of the process of encapsulation of worms. The experimental group of fish consisted of 11 individuals from 6.3 to 18.2 cm long. The control group consisted of 26 fish; the autopsy showed that they were all uninfected. The conditions of keeping the experimental fish and the method of their infection were similar to those described above. In the room where the aquarium was placed, the air temperature from the beginning of the experiment to its end decreased from 12 to –3°С. As the fish preferred to stay in certain parts of the aquarium, each of them was individually infected. Three fish were fed 11 acanthocephalans each, four fish were fed five acanthocephalans, and the remaining four, from 5 to 11 acanthocephalans each (Table 1). In the latter case, both regular and larger capsules were used for infection, which contained more than one acanthocephalan (we found capsules containing from two to six acanthocephalans). The autopsies were performed 14 (two fish), 30 (two fish), and 50 (seven fish) days after the infection.
Treatment of preparations and light microscopic and electron microscopic studies were carried out according to the methods described in the first communication. A total of 58 acanthocephalans were examined by light microscopic examination, and seven by electron microscopic examination.
RESULTS
The results of experiments on infection of Middendorff’s eelpouts with acanthocephalans are presented in Table 1.
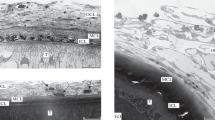
The infection period is three days. Five of the 12 dissected fish were infected. All the acanthocephalans that penetrated the body cavity of the host showed signs of incipient encapsulation. The emerging capsule was either represented by a discontinuous cell layer (Figs. 1a, 1b), or small clusters of cells (Fig. 1c), or almost completely surrounded the parasite (Fig. 1d).
General view of Corynosoma strumosum on (a, b) the intestinal mesentery, (c) the abdominal wall, and (d) the liver of Middendorff’s eelpout Hadropareia middendorffii three days after infection. (a) Longitudinal and (b) transverse sections of the worm, partially surrounded by a thin forming capsule (arrows); (c) the evaginated proboscis of the parasite has penetrated into the muscles of the abdominal wall, the capsule is not formed, only separate clusters of host cells are visible (arrows); (d) the acanthocephalan is almost completely surrounded by a capsule, which is the thickest in the area adjacent to the liver, and in the fold of the helminth. (IM) intestinal mesentery, (IW) intestinal wall, (T) tegument, and (P) proboscis. Scale: 200 µm.
Structural features of the tegument of the acanthocephalan. By light microscopy, a homogeneous light-colored layer of glycocalyx was found on the surface of the tegument of all three-day-old acanthocephalans. Electron microscopy showed that the glycocalyx is formed by fibrillar and granular material of low electron density and has a thickness of 1.5–2.1 µm (Figs. 2a–2d). Its outer border is uneven. Pseudopodia of host cells are often introduced into it, however, they do not reach the tegument surface (Fig. 2a). The narrow inner part of the glycocalyx is characterized by a denser organization of the material forming it and has an increased electron density. In some areas at the base of the glycocalyx, clusters of small vesicles, 18–23 nm in diameter, surrounded by an envelope resembling a membrane, were observed (Fig. 2c). In the thickness of the glycocalyx, formations were often found in the form of tubules, 21–26 nm in diameter, oriented more or less perpendicularly to the surface of the parasite; the wall of these tubes was similar to the wall of the vesicles, but its exact nature was not determined (Fig. 2d). In some areas, the glycocalyx was separated from the tegument, but at the same time retained its continuity (Fig. 2b). In these cases, fragments of host cells were often observed in the spaces formed between it and the surface of the tegument.
Ultrastructure of the surface of the tegument and glycocalyx of Corynosoma strumosum on the intestinal mesentery of Hadropareia middendorffii three days after infection. (a) Processes of the host macrophage (*) are immersed in the glycocalyx layer; the cleared cytoplasm and swollen mitochondria indicate the destruction of the macrophage; (b) detachment of the glycocalyx layer from the tegument of the ridge; (c) accumulations of small vesicles in the inner part of the glycocalyx; the submerged parts of the “canals” (*) of the tegument forming its vesicular layer are expanded; (d) the inner part of the glycocalyx includes numerous tubular formations. The mouths of the “channels” (arrows) of the tegument are widened. (G) Glycocalyx, (M) macrophage, (Mt) mitochondria, (N) neutrophils, (T) tegument, and (Nu) macrophage nucleus.
The tegument of three-day-old acanthocephalans is generally similar in structure to that of encapsulated acanthocephalans from natural paratenic hosts (see Communication 1) (Figs. 3a–3c). On semithin sections, the striated, felt, and radial layers are clearly distinguished. Six acanthocephalans had a vesicular layer, the elements of which were expanded to 0.5 μm and contained unclear flocculent material or vesicular formations similar to vesicles noted in the glycocalyx (Fig. 2c). In these cases, as shown by electron microscopy, the orifices of the channels of the striated layer connecting these vesicles with the external environment were also expanded to 30–60 nm, which may indicate an increased secretory activity of the tegument (Fig. 2d).
Microstructure of the tegument and capsule of Corynosoma strumosum on (a) the gonads, (b) the abdominal wall, and (c) the liver of Middendorff’s eelpout Hadropareia middendorffii three days after infection. (a) Eosinophils, macrophages, erythrocytes, neutrophil, and large lipid drops are observed in the capsule; (b) the forming capsule is represented by two or three layers of host cells; (c) in the composition of the capsule in the intercellular spaces, a dense homogeneous material of unknown origin is visible (arrows). (L) Lipid drops, (M) macrophages, (N) neutrophil, (T) tegument of the acanthocephalan, (E) eosinophils, and (Er) erythrocytes. Scale: 20 µm.
The structure of the capsule. Six acanthocephalans were found on the intestinal mesentery; all of them had invaginated probosces. Around two of them, the capsule was completely absent, and only a few host cell clusters were observed at the surface of the helminths. Around the rest of the acanthocephalans, the capsules were of an intermittent nature and uneven thickness (Figs. 1a, 1b). In the areas in which the acanthocephalans were in contact with the mesentery, the thickness of the capsule reached the greatest values; with distance from them, its thickness decreased, it became intermittent and was interspersed with areas free of cells. The cells in the capsule were located loosely with noticeable intercellular spaces. In the inner part of the capsule, they were characterized by numerous pseudopodia directed toward the parasite and often submerged in the upper part of the glycocalyx layer on its surface (Fig. 2a).
Four acanthocephalans were attached to the wall of the abdominal cavity with an evaginated proboscis. Moreover, three of them lacked a capsule, and on their surface there were only small clusters of cells (Fig. 1c). Near the posterior part of one of these acanthocephalans, a large cellular infiltrate was observed, consisting of several dozen (on one section) erythrocytes. Signs of encapsulation of this acanthocephalan were observed only in the area of its contact with the infiltrate; there were no host cells above the rest of the parasite surface. In the fourth acanthocephalan, the capsule was intermittent, but some parts of its surface were not covered with host cells. The capsule consisted of two or three layers of cells, and its thickness varied from 20 to 60 µm (Fig. 3b). Some of the innermost cells formed fingerlike processes directed toward the surface of the acanthocephalan.
Two acanthocephalans were found on the surface of the fish liver. Both helminths contained an invaginated proboscis, and most of their body was surrounded by a capsule (Figs. 1d, 3c); in other areas it was completely absent. The thickness of the capsule varied from 16 to 35 μm and reached the highest values at the points of contact between the acanthocephalans and the liver, as well as in the bends of the helminth bodies.
One partially encapsulated acanthocephalan with a retracted proboscis was found on the gonads. The capsule, 35 to 129 μm thick, covered the anterior part of the acanthocephalan, while its posterior end was free, and only in some places on its surface were single accumulations of cells observed. In the capsule, the cells were located loosely, with wide intercellular spaces (Fig. 3a).
In the composition of all capsules detected, several types of cells were identified, among which macrophages, neutrophils, and erythrocytes predominate, eosinophils and lymphocytes were less numerous. Many cells are characterized by signs of destruction, most clearly manifested in the clarification of the peripheral cytoplasm and swelling of mitochondria (Fig. 2a). In terms of their characteristics, the cells as a whole do not differ from those described in capsules from fish infested naturally. Fibroblasts were not found in the three-day-old capsules.
The diameter of macrophages varies from 8 to 14 μm (Figs. 2a, 3a). Their nuclei are light, round or bean-shaped, and located eccentrically. The cytoplasm includes short tubules of the granular endoplasmic reticulum, small dark phagosomes, light vacuoles, and lipid droplets.
Neutrophils are rounded cells, 6–9 µm in diameter, characterized by an eccentrically located nucleus (Fig. 3a); cells with rounded and rod-shaped nuclei predominate, and less often nuclei consist of 2‒3 segments. The nuclei are characterized by coarse accumulations of heterochromatin along the membrane, and small specific granules of a rounded, less often elongated shape are visible in the cytoplasm. The peripheral part of the granules contains dense homogeneous contents, and their central part appears to be “empty.”
Eosinophils more often have an elongated shape, their width is 6–7 µm, and their length is 12–15 µm (Fig. 3a, 3b); their nuclei are located eccentrically. Unlike neutrophils, eosinophils are characterized by larger specific granules with dark, homogeneous contents.
Lymphocytes differ from other cells by their small size (~5 μm) and a dense nucleus that occupies most of their body.
A dense homogeneous material was observed in the intercellular spaces; on semi-thin sections, it was stained in dark blue (Fig. 3c), and on histological sections stained by Mallory’s trichrome method it is an intense red color. By electron microscopy, this material consists of chaotically oriented thin fibrils without transverse striation. In addition, in the capsules of most of the worms studied, large lipid droplets with a diameter of 4–5 µm, in some cases up to 20 µm in diameter, were present in the intercellular spaces (Fig. 3a).
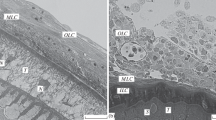
The period of infection is 14 days. Two fish were opened 14 days after the start of the experiment. In the first of them (no. 13), six acanthocephalans were found; in the second (no. 14), 5. All parasites were located on the mesentery of the hosts’ intestines; acanthocephalans were not found in the intestinal lumen. In the first fish, two parasites were almost completely devoid of capsules, and rare groups of cells were observed near their surface. The rest of the helminths were covered with a thin intermittent capsule. In the second fish, one acanthocephalan was also free of the capsule; three parasites were surrounded by a thin intermittent capsule. The last acanthocephalan was enclosed in an almost continuous multilayer capsule, interrupted only in separate short sections (Figs. 4a–4c).
The acanthocephalan Corynosoma strumosum on the intestinal mesentery of Middendorff’s eelpout Hadropareia middendorffii 14 days after infection. (a) Tegument of the worm with expanded elements of the vesicular layer (arrows); the surrounding capsule is multi-layered and formed by leukocytes and macrophages; (b) collagen fibers in the capsule (arrows), stained blue according to Mallory; (c) intercellular material in the capsule (arrows), stained red according to Mallory; (d, e) tegument of the worm with expanded elements of the vesicular layer (*) and the orifices of the “canals” (arrows) of the striated layer; the glycocalyx layer on the tegument surface is formed by an amorphous material (d) and includes microtubules (e). (G) Glycocalyx, (C) capsule, (T) tegument, and (N) tegument nuclei.
Structural features of the tegument. On the light-microscopic and electron-microscopic preparations, three acanthocephalans showed dilated elements of the vesicular layer of the tegument similar to those described in some acanthocephalans at the age of three days (Fig. 4a); the mouths of the channels connecting these vesicles with the external environment were also expanded to 40–60 nm (Figs. 4d, 4e). In other acanthocephalans, the vesicular layer had a “normal” appearance (Figs. 4b, 4c). On the surface of the tegument, in all cases, a loose layer of glycocalyx formed by a feltlike material was clearly visible (Figs. 4d, 4e). In some areas, tubules were observed in this material, morphologically similar to those observed in the glycocalyx of three-day-old parasites, but in a significantly smaller amount (Fig. 4e).
The structure of the capsule. Macrophages and neutrophilic leukocytes formed the basis of capsules in the worms studied; eosinophils, lymphocytes, and erythrocytes were present in smaller amounts (Fig. 4a). In addition, a few fibroblasts were noted, distinguished by their large size, round or elongated shape, relatively high nuclear-plasma ratio, and a light nucleus with a distinct nucleolus. The intercellular material of the capsule was represented by two fiber modifications. The fibers of the first modification form rather large clusters, which are stained by the Mallory’s trichrome method in a bright red color (Fig. 4c). The material of the second modification, in contrast to the first, is represented by a network of collagen fibers, as evidenced by their blue coloration by the Mallory method (Fig. 4b).
The period of infection is 30 days. On the thirtieth day of the experiment, two fish were opened: in the first (no. 15), 11 acanthocephalans were found, in the second (no. 16), 6. All acanthocephalans found were located on the intestinal mesentery and were surrounded by a continuous multilayer capsule, from 32 to 160 µm thick. As in the previous stages of the experiment, an increase in the thickness of the capsule was observed in the areas in which the acanthocephalans were most closely located to the mesentery (Fig. 5a).
The acanthocephalan Corynosoma strumosum on the intestinal mesentery of Middendorff’s eelpout Hadropareia middendorffii 30 days after infection. (a) General view of the worm in the capsule, which is the thickest in the area facing the mesentery tissue; (b) a fragment of a capsule formed not only by leukocytes and macrophages, but also by fibroblasts; a glycocalyx layer is visible on the tegument surface (arrow); (c) glycocalyx formed by fine fibrous material; elements of the vesicular layer of the tegument are expanded (*); (d) tubules in the glycocalyx. (G) Glycocalyx, (C) capsule, (M) macrophages, (T) tegument, (MT) mesenteric tissue, and (F) fibroblasts.
Structural features of the tegument. The light-microscopic tegument of the investigated acanthocephalans did not differ in structure from that of the acanthocephalans from naturally infested fish (Fig. 5b). The glycocalyx layer on the tegument surface was clearly observed in cases when there was a lumen between the acanthocephalan and the capsule (Fig. 5b).
Electron microscopic examination was carried out on two acanthocephalans. In both, the elements of the vesicular layer were expanded (Fig. 5c). The glycocalyx of the acanthocephalans reached 1.5–2.0 µm in thickness and was formed by fine fibrillar material (Fig. 5c) with the inclusion of microtubules in some areas (Fig. 5d). In these areas, the mouths of the “canals” of the striated layer appear to be widened, in other areas they have a “normal” appearance.
The structure of the capsule. Numerous macrophages, neutrophils, and fibroblasts were found in the capsules; eosinophils, lymphocytes, and erythrocytes were observed in a much smaller amount. The density of the cells in the capsule varies from dense in its inner part to loose in the middle and surface layers (Fig. 5b). The structure of cellular elements is similar to that described in the previous stages of the experiment. Fibroblasts are large (17.7–24.0 × 3.3–6.6 µm) elongated cells with an oval nucleus including 1–2 nucleoli. The intercellular material in these capsules is represented only by collagen fibers organized in the form of bundles that are thicker than in 14-day-old capsules, which are detected clearly on histological preparations. Their number increases markedly in areas in which the capsule is adjacent to the host’s mesentery.
The infection period is 50 days. Fifty days after infection, seven eelpouts were opened. In two of them, one acanthocephalan was found; in three, three; in one, two; and in one, four. Most of the acanthocephalans were located on the mesentery of the intestine, one acanthocephalan was found on the liver (no. 22) and spleen (no. 19) and five acanthocephalans, on the abdominal wall. In one case (no. 21), one capsule contained two acanthocephalans and the other (no. 23), three.
Structural features of the tegument. Microscopically and electron microscopically, two acanthocephalans were studied. No noticeable differences in the structure of the tegument of these acanthocephalans were found in comparison with worms from naturally infested eelpouts. However, differences were observed in the structure of the glycocalyx. In the worm from fish no. 17, the glycocalyx had a thickness of at least 2 µm and was formed by delicate intertwining filaments with the inclusion of clusters of small bubbles up to 50 nm in diameter (Fig. 6d). At the base of the glycocalyx layer, at a distance of no more than 0.1 µm from the tegument surface, a continuous strip of moderately dense material was observed (Fig. 6d). The shape of the strip was wavy, probably repeating the outline of the tegument surface. Its maximum thickness was 0.3 µm. Another investigated acanthocephalan, taken from fish no. 22, was not covered with a thick layer of glycocalyx, and the capsule cells were in direct contact with the surface of its tegument (Fig. 6c).
The acanthocephalan Corynosoma strumosum on the intestinal mesentery of Middendorff’s eelpout Hadropareia middendorffii 50 days after infection. (a) General view of the worm in the capsule; (b) a fragment of a capsule in which leukocytes predominate; (c) a parasite on the surface of which the typical glycocalyx layer is absent, and the cells of the capsule are in contact with the surface of the tegument; (d) a typical thick layer of glycocalyx on the surface of the acanthocephalan, which also includes accumulations of bubbles; at the base of the glycocalyx there is a strip of electron-dense material (arrows). (G) Glycocalyx, (C) capsule, and (T) tegument.
The structure of the capsule. All acanthocephalans examined had an invaginated proboscis and were surrounded by a solid thick capsule, the cellular composition of which was generally similar to that of 30-day-old capsules and differed visually in a slightly larger number of collagen fibers (Figs. 6a–6c). The thickness of the capsule on different organs was approximately the same and amounted to 87‒116 µm. The cells in the capsules are either tightly packed, or small intercellular spaces are visible between them (Fig. 6b). Like 30-day-old capsules, 50-day-old capsules are formed predominantly by macrophages, leukocytes, and fibroblasts (Fig. 6c); the intercellular substance is represented only by collagen fibers.
DISCUSSION
We have previously shown that the tegument of Corynosoma strumosum in natural paratenic hosts of many species is covered with a thick layer of glycocalyx (Nikishin and Skorobrekhova, 2007; Skorobrechova and Nikishin, 2011, 2013). The acanthocephalans from eelpouts studied in the first report are no exception and are also characterized by the presence of the same glycocalyx layer. The results obtained in the experiment described in this paper indicate that this glycocalyx is formed already during the first three days of invasion. If we take into account the time required for the penetration of the parasite from the intestine into the body cavity of the paratenic host, the period of formation of this glycocalyx on the surface of the helminth should be even shorter.
The fact of glycocalyx reforming in the discussed experiment seems to be the most likely. It is known that it contains mainly acidic and neutral mucopolysaccharides, as well as glycoproteins (Bennett, 1963; Ito, 1969, 1974). Therefore, when the worm enters the paratenic host, the glycocalyx covering the acanthocephalan in the previous host must be destroyed under the influence of digestive enzymes. Consequently, in our experiment, a thick layer of glycocalyx on the surface of the parasite is most likely formed anew, rather than being preserved from the previous stage of development. This assumption is also supported by the facts of the absence of glycocalyx in the acantocephalans during their migration through the intestinal wall of the paratenic host (Skorobrechova et al., 2012), as well as its partial loss during sequential modeling of the media of the stomach and duodenum in cases with cysticercoids of cestodes (our unpublished data). A more detailed discussion of the fact of the neoplasm of the glycocalyx was carried out by us earlier (Nikishin, 2018).
The mechanism of glycocalyx formation was previously studied using radioautography on the rat intestinal epithelium (Bennett, 1970; Bennett and Leblond, 1970). In particular, these authors have shown that after an injection of tritium-labeled carbohydrates into an animal, after some time they accumulate in the Golgi complex, from which they enter the apical cell membrane in the form of vesicles, and are then observed among microvilli in the glycocalyx layer. We observed a similar process of movement of vesicles along the cytoplasmic bridges from the cytons of the tegument to its distal part and then to the surface of the parasite in the cyst wall of cysticcercoids of some cestodes (Krasnoshchekov and Nikishin, 1979). Similar small vesicles were found in the present study on the third day of the experiment in the glycocalyx layer on the surface of the parasite, as well as in the terminal extensions of the “channels” of the striated layer of the tegument. These facts, as well as the nature of the arrangement of these vesicles in the form of relatively small clusters, indicate their probable formation in the tegument of the acantocephalan and release onto its surface through the “channels” of the striated layer. Indirect confirmation of these assumptions is the noticeable widening of the mouths of the “canals” of the surface layer of the tegument. By analogy with the above assumption (Bennett, 1970; Bennett and Leblond, 1970), it can be assumed that these vesicles, both in the case of the acanthocephalans and in the case of cysticercoids, may be associated with the formation of a glycocalyx layer on the surface of the parasite.
Unusual is the fact of detection of extensive accumulations of tubules in the glycocalyx of the same acanthocephalans. The data are clearly insufficient for its interpretation at present. It can be noted, however, that, firstly, these tubules hardly differ in their diameter from typical microtubules present in the cytoplasm of many cells (Chentsov, 2005), and secondly, the diameters of microtubules and the above-mentioned vesicles are almost identical, which suggests their genetic similarity. We assume that these tubules, like vesicles, are isolated from the “channels” of the striated layer of the tegument and are somehow related to the formation of a thick layer of the glycocalyx.
The interpretation of three other features in the organization of the glycocalyx in the worms studied is even more difficult. First, the aforementioned accumulations of tubules were found not only at the very beginning of the experiment (in three-day-old acanthocephalans), but also at all other stages studied. If we take into account the supposed participation of tubules in the formation of a thick layer of glycocalyx on the surface of the tegument (and it is very likely), the discussed feature suggests the continuation of the process of glycocalyx formation throughout the entire period of the experiment.
The second difficult to interpret feature of the glycocalyx is its partial separation from the tegument surface in some three- and fourteen-day-old acanthocephalans; at the same time, its layer retained its continuity. This process could be considered as an expression of some pathology, especially since one of the studied fifty-day-old acanthocephalans had a thick layer of glycocalyx with the tegument surface completely absent. However, since one of the most likely putative functions of the glycocalyx is the protective one (Nikishin, 2016, 2018), in our opinion, it is more acceptable to assume a periodic change (partial or complete) of the glycocalyx at the initial stage of invasion of the paratenic host. This assumption is in good agreement with the possible participation of the aforementioned vesicles and tubules in the formation of this glycocalyx, which were observed in worms at all periods of the experiment. In addition, in this way the parasite can get rid of the negative material accumulated in the glycocalyx as a result of counteracting the host’s immune response to the invasion.
Finally, the third feature of the glycocalyx, noted for the first time, is a wavy strip of material with increased electron density, found at its base near the surface of the tegument in fifty-day-old worms. In appearance, it slightly resembles the inner layer of the cyst, secreted one time by late acanthellae from the “canals” of the striated layer of the tegument (Nikishin, 1992), or it can be a detached inner layer of the glycocalyx, which usually adjoins the tegument closely (Nikishin, 2016, 2018) and which is clearly visible in Fig. 3a in the first message. Further research is needed to verify these versions.
The results obtained indicate that the process of encapsulation of Corynosoma strumosum in Middendorff’s eelpout begins with intensive migration of leukocytes and macrophages to the parasite. This result is similar to most of the previously obtained data on the encapsulation of helminths in second intermediate hosts. Thus, the basis of the young capsules surrounding the plerocercoids of the cestodes Ligula intestinalis in the fry of naturally invaded roach were inflammatory cells (Hoole and Arme, 1982, 1983a). Fibroblasts and bundles of collagen fibers were also not observed during implantation, including crossover, of plerocercoids of this species into roach or minnows (Hoole and Arme, 1983b). In experimentally infected goldfish, metacercariae of the trematodes Ribeiroia marini at an early stage of encapsulation were surrounded by macrophages, granulocytes, and lymphocytes, and fibroblasts were included in the capsule only on the third day of the experiment (Huizinga and Nadakavukaren, 1997).
At the same time, the encapsulation of Posthodiplostomum cuticola metacercariae, parasitizing in the vobla, begins with the deposition of one or two layers of fibroblasts (Berezantsev and Dobrovol’skii, 1968), while the presence of leukocytes in the capsule composition was not noted. This difference can be explained by the fact that the capsule surrounding these flukes is possibly fibroblastic, the formation process of which may differ from that of the leukocyte capsule. In our study, a few fibroblasts were first observed in the capsule only on the fourteenth day of the experiment, and then their number increased. The number of collagen fibrils also increased, and most of them were observed in the distal half of the capsule, which corresponds to the pattern of collagen arrangement in capsules from naturally infected eelpouts.
Thus, the results obtained suggest the following scheme for the encapsulation of acanthocephalans in Middendorff’s eelpout. During the first three days of infection, the parasite penetrates the intestinal wall of the host, localizes on any of its organs, and becomes covered with a layer of glycocalyx. At the same time, macrophages and leukocytes migrate from the host tissues surrounding the acantocephalan and come into contact with the glycocalyx layer on the surface of the worm. In the future, some of them, obviously, die as a result of this interaction, and their remnants form an inner layer found in older capsules. Two weeks after the infection, fibroblasts migrate into the forming capsule, but the migration of leukocytes also continues. By the 50th day of the experiment, the migration of leukocytes and fibroblasts into the capsule continues, but the cells in the thickness of the capsule are denser, and the remaining intercellular spaces are filled with collagen fibrils synthesized by fibroblasts.
No noticeable differences in the nature of the formation of capsules localized on different host organs were found. At the same time, the areas of the worms, which are closer to the adjacent tissue of the host, are covered with its cells faster than the distant ones, and the capsule in these areas, as a rule, has a greater thickness.
CONCLUSIONS
Experimental study of the process of encapsulation of acanthocephalan in marine fish—natural paratenic hosts of the worm Corynosoma strumosum—was carried out for the first time. It was found that, already on the third day, a typical thick layer of glycocalyx forms on the surface of the acantocephalan. This process is most likely associated with small vesicles and microtubules found in the glycocalyx and, probably, secreted through the dilated orifices of the “channels” of the striated layer of the tegument. The phenomenon of detachment of glycocalyx regions from the surface of the tegument is possibly a consequence of its interaction with the host macrophages.
In the process of encapsulating the parasite, three stages can be distinguished. At the first stage, macrophages and leukocytes migrate to the acantocephalan, some of which die, apparently as a result of interaction with the worm’s glycocalyx. Two weeks later, fibroblasts are added to the migrating macrophages and leukocytes and the first bundles of collagen fibers appear. After fifty days of invasion, the number of collagen fibers in the composition of the capsule increases significantly, the organization of the cellular elements in it becomes denser, and in its structure it approaches the capsules from the naturally invaded eelpouts.
REFERENCES
Bennett, H.S., Morphological aspects of extracellular polysaccharides, J. Histochem. Cytochem., 1963, vol. 11, no. 1, pp. 14–23. https://doi.org/10.1177/11.1.14
Bennett, G., Migration of glycoprotein from Golgi apparatus to cell coat in the columnar cells of the duodenal epithelium, J. Cell Biol., 1970, vol. 45, no. 3, pp. 668–673. https://doi.org/10.1083/jcb.45.3.668
Bennett, G. and Leblond, C.P., Formation of the cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with L-fucose-3H, J. Cell Biol., 1970, vol. 46, no. 2, pp. 409–416. https://doi.org/10.1083/jcb.46.2.409
Berezantsev, Yu.A. and Dobrovol’skii, A.A., The processes of encapsulation of metacercariae of the trematodes Posthodiplostomum cuticola (Nordmann, 1832) (Dubois, 1936) in fish, Tr. Astrakhan. Zapov.: Sb. Gel’mintolog. Rabot, 1968, no. 11, pp. 7–12.
Chentsov, Yu.S., Vvedenie v kletochnuyu biologiyu (Introduction to Cell Biology), Moscow: Akademkniga, 2005.
Hoole, D. and Arme, C., Ultrastructural studies on the cellular response of roach, rutilus rutilus l., to the plerocercoid larva of the pseudophyllidean cestode, ligula intestinalis, J. Fish Diseases, 1982, vol. 5, no. 2, pp. 131–144. https://doi.org/10.1111/j.1365-2761.1982.tb00466.x
Hoole, D. and Arme, C., Ligula intestinalis (Cestoda, Pseudophyllidea): an ultrastructural study of the cellular response of roach fry Rutilus rutilus, Int. J. Parasitol., 1983a, vol. 13, no. 4, pp. 359–365. https://doi.org/10.1016/S0020-7519(83)80041-1
Hoole, D. and Arme, C., Ultrastructural studies on the cellular response of fish hosts following experimental infection with the plerocercoid of Ligula intestinalis (Cestoda, Pseudophyllidea), Parasitology, 1983b, vol. 87, no. 1, pp. 139–149. https://doi.org/10.1017/S0031182000052483
Huizinga, H.W. and Nadakavukaren, M.J., Cellular response of goldfish, Carassius auratus (L.), to metacercarie of Ribeiroia marini (Faust and Hoffman, 1934), J. Fish Dis., 1997, vol. 20, no. 6, pp. 401–408. https://doi.org/10.1046/j.1365–2761.1997.00311.x
Ito, S., Structure and function of the glycocalyx, Fed. Proc., 1969, vol. 28, pp. 12–25.
Ito, S., Form and function of the glycocalyx on free cell surfaces, Philos. Trans. R. Soc., B, 1974, vol. 268, no. 891, pp. 55–66. https://doi.org/10.1098/rstb.1974.0015
Krasnoshchekov, G.P. and Nikishin, V.P., The ultrastructure of the cyst wall of metacestodes of Aploparaksis polystictae (Schiller, 1955) and A. furcigera (Cestoda, Cyclophyllidea), Parazitologiya, 1979, vol. 13, no. 3, pp. 250–256.
Nikishin, V.P., Formation of the capsule around Filicollis anatis (Acanthocephala) in its intermediate host, J. Parasitol., 1992, vol. 78, no. 1, pp. 127–137.
Nikishin, V.P., Morphofunctional diversity of the glycocalyx in tapeworms, Usp. Sovrem. Biol., 2016, vol. 136, no. 5, pp. 506–526.
Nikishin, V.P., Glycocalyx modifications in acanthocephalans, Biol. Bull. (Moscow), 2018, vol. 45, no. 1, pp. 35–46. https://doi.org/10.1134/S1062359018010090
Nikishin, V.P. and Skorobrechova, E.M., Encapsulation of acanthocephalans Corynosoma sp. in two reservoir host species, Dokl. Biol. Sci., 2007, vol. 417, pp. 462–464. https://doi.org/10.1134/S0012496607060154
Nikishin, V.P. and Skorobrechova, E.M., Two strategies of acanthocephalan interrelations with paratenic hosts, Biol. Bull. (Moscow), 2019, vol. 46, no. 8, pp. 814–822. https://doi.org/10.1134/S1062359019080090
Sharpilo, V.P., On the ability of acanthellae of the genus Centrorhynchus (Acanthocephala, Giganthorhynchidae) to passaging through reservoir hosts, in Materialy nauchnoi konferentsii Vsesoyuznogo obshchestva gel’mintologov (Proceedings of Scientific Conference of the All-Union Society of Helminthologists), Moscow: B.I., 1965, part 4, pp. 312–317.
Sharpilo, V.P., The use of reservoir hosts for the accumulation and storage of invasive helminth larvae, Parazitologiya, 1971, vol. 5, no. 1, pp. 88–91.
Skorobrechova, E. and Nikishin, V., Structure of capsule surrounding acanthocephalans Corynosoma strumosum in paratenic hosts of three species, Parasitol. Res., 2011, vol. 108, no. 2, pp. 467–475. https://doi.org/10.1007/s00436-010-2088-3
Skorobrechova, E., Nikishin, V., and Lisitsyna, O., Structure of the capsule around acanthocephalan Corynosoma strumosum from uncommon paratenic hosts—lizards of two species, Parasitol. Res., 2012, vol. 110, no. 1, pp. 459–467. https://doi.org/10.1007/s00436-011-2512-3
Skorobrechova, E.M. and Nikishin, V.P., Dependence of the structure of the capsule surrounding the acanthocephalan Corynosoma strumosum on the species of its natural paratenic host, Biol. Bull. (Moscow), 2014, vol. 41, no. 4, pp. 333–348. https://doi.org/10.1134/S1062359013050166
Funding
This work was carried out under the government contract “Taxonomic, Morphological, and Ecological Diversity of Helminths of Vertebrates in North Asia,” project no. AAA-A17-117012710031-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by N. Smolina
Rights and permissions
About this article
Cite this article
Skorobrekhova, E.M., Nikishin, V.P. Structure and Formation of a Capsule around the Acanthocephalan Corynosoma strumosum in a Natural Paratenic Host, the Fish Hadropareia middendorffii: 2. Experimental Study of the Encapsulation Process (Preliminary Results). Biol Bull Russ Acad Sci 48, 588–600 (2021). https://doi.org/10.1134/S1062359021050149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062359021050149