Abstract
We propose maslinic acid (2-α, 3-β-dihydroxiolean-12-en-28-oic acid), found in the leaves and fruit of the olive tree (Olea europaea L.), as a new natural coccidiostatic product against Eimeria tenella. Its action in infected animals has been compared with animals treated with sodium salinomycin. The lesion index (LS), the oocyst index (OI) and the anticoccidial index (ACI) were studied with regard to the weight of the chicks. The ACI for maslinic acid was 210.27 and for sodium salinomycin 173.09. Similarly, both LS and OI decreased in the groups treated with maslinic acid. A considerable increase in weight was found in the chicks treated with maslinic acid compared with those in the control group. Histopathological studies of the caecum at 120 h post-infection showed that the infection rate decreased significantly in chicks treated with maslinic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The group of diseases known as coccidiosis, which can seriously affect farm animals, is considered to be one of the parasitic infections responsible for the greatest economic impact on poultry production. The estimated annual cost deriving directly or indirectly from diseases caused by Eimeria species in the raising of fowls is estimated to be some US $800 million (Williams 1998) including the expense involved in medication, losses due to mortality, and damages occasioned by the poor food conversion of the surviving chicks. The use of coccidiostatic products is widespread in the poultry industry. Their modes of action vary but the most widely used are polyether ionophores, including sulphanamides, pyrimidine derivatives, triazinetrions and benzenacetonitryls, all of which alter ion transport and interrupt osmotic balance. Although these compounds are effective, resistance to them appears to be growing (Allen and Fetterer 2002; Chapman 1997; Williams 2002; Abbas et al. 2008) and regulations are tightening in the EU against the use of coccidiostatics and histomonstatics in food because of the accumulation of residues building up during their passage through the food chain, with possible eventual risks to the consumer. These regulations establish maximum levels of these substances in non-target feed (European Council Regulation, EC 2009), thus obliging poultry farmers to search for alternatives. Within this context new natural compounds are being studied for the control of coccidiosis in chicks (Allen and Fetterer 2002; Dalloul and Lillehoj 2005; Jang et al. 2007; Naidoo et al. 2008), including those with antioxidant properties, which, according to (Allen et al. 1998), reduce the infection rate of Eimeria tenella and lower the degree of lipid peroxidation in the intestinal mucosa. Maslinic acid (2-α, 3-β-dihydroxiolean-12-en-28-oic acid) is a triterpenoid compound deriving from oleanolic acid (3-β-hydroxiolean-12-en-28-oic acid), which is present in numerous plants (Numata et al. 1989; Hou et al. 2009; Lee et al. 2008; Cheng et al. 2008; Kim et al. 2008; Jovel et al. 2007), but above all in considerable quantities in the leaves and fruit of Olea europaea L. (Bianchi et al. 1994; Bianchi 2003). It is obtained from the solid waste of olive-oil production through a profitable extraction technique that produces large quantities of chemically pure acid (Parra et al. 2009).
The biological activity of maslinic acid has been demonstrated in vitro to be antiviral (Xu et al. 1996), antitumoral (Reyes et al. 2006; Juan et al. 2006; Juan et al. 2008) and antioxidant (Montilla et al. 2003).
We describe here the coccidiostatic activity of maslinic acid in vivo against the experimental infection of Gallus domesticus chicks with E. tenella.
Materials and methods
Maintenance of the chicks, infection and treatment
In our experimental design we followed the recommendations of the World Association for the Advancement of Veterinary Parasitology (WAAVP) (Holdsworth et al. 2004). A total of 240 seven-day-old chicks, weighing 25 g, were divided into four groups of 60 individuals. Each of these groups was subdivided into six cages of ten individuals, with the following treatments: uninfected-untreated (control); infected-untreated; infected and treated with the coccidiostatic sodium salinomycin (Sigma); and infected and treated with maslinic acid. Both the maslinic acid group and the sodium salinomycin group were supplied ad libitum with food mixed with 90 ppm of maslinic acid or 60 ppm of sodium salinomycin, beginning two days before infection. The maslinic acid was purified from industrially produced olive oil by extraction with solvents of medium polarity. This extract was then subjected to physical procedures to separate the fats and polar products, with some 95% richness in maslinic acid, as described elsewhere (Parra et al. 2009). The solid was ground and mixed at the desired proportion with the feed meal for the chicks. The chicks, with ad libitum access to water and feed, were penned under the proper conditions for animal rearing in terms of illumination and constant temperature. The food consumption of each cage was recorded daily.
Ten days later the chicks were infected orally with a suspension of 5 × 104 oocysts/chick from a stock of E. tenella oocysts in PBS, provided by Professor JM Alunda of the Department of Animal Health at the Faculty of Veterinary Medicine of the Complutense University (Madrid, Spain). Prior to infection, in order to activate their virulence the initial stock of oocysts was used to infect two lots of chicks. The faeces of these chicks were collected and the new oocysts were purified, suspended in 2% potassium dichromate solution and kept in darkness at 4°C before sporulation for no more that 7 days. Sporulation took place at 28°C according to the procedure described by Long and Joyner (1976).
Evaluation of the effectiveness of the treatment
To evaluate the effectiveness of the treatment we followed the recommendations of Chapman (1998), who describes histopathological observations and the different indices as oocystindex (OI), body weight gain (BWG), relative weight gain (RWG), lesion scores (LS), and anticoccidial index (ACI). The faeces of the different groups were collected everyday between days 5 and 10 after infection. The total faecal material from each cage was collected, mixed with 20 ml of water, shacked, filtered and the oocysts counted in a McMaster chamber according with the method described by Long and Joyner (1976).
BWG and RWG (RWG = BWG × 100/untreated group BWG) were scrutinised by weighing the chicks 21 days post-infection. For the lesion scores two chicks from each cage were chosen at random and killed 10 days post-infection. The end of the intestine and caecum of each bird was examined and the gravity of the lesions was scored between 0 and 4 following the method described by Johnson and Reid (1970). Histopathological studies were made with 2-μm cuts made in the caecum of two randomly chosen chicks per group killed 120 h post infection. The caecum was removed and fixed in 10% formaldehyde in cacodylate buffer, pH 7.2, for 24 h before being embedded in paraffin. The thin slices were stained with toluidine blue, mounted and examined under a light microscope.
To analyse the effectiveness of the treatment at the end of the experiment we calculated the anticoccidial index using the formula: \( \left( {\hbox{ACI}} \right) = \left( {\% {\hbox{S}} + \% {\hbox{RGW}}} \right) - \left( {{\hbox{IL}} + {\hbox{OI}}} \right) \), where ACI is the anticoccidial index, %S the percentage of survival, %RGW the percentage of relative weight gain, IL the lesion index and OI the oocyst index. We considered a product to lack anticoccidian activity when values were lower than 120, partially effective at values of 120–160, and very effective at values higher than 160.
The data were statistically analysed by Bonferroni's test to estimate the significance of the difference between means. The results are indicated as mean values ± SEM of the different cages of the different groups at different times during the experiments. All the experiments were repeated three times. p ≤ 0.001 was considered to be extremely significant. GraphPad Instat v 3.05 software was used for the statistical test.
Results and discussion
We have looked into the effectiveness as a coccidiostatic drug of maslinic acid (2-α, 3-β-dihydroxiolean-12-en-28-oic acid), a triterpenoid compound derived from oleanolic acid, which is present in considerable proportions (38%) in the fruit and leaves of O. europaea (Bianchi et al. 1994; Bianchi 2003).
The values found for the ACI (Table 1) imply that E. tenella is highly susceptible to treatment with maslinic acid, the release of oocysts being 80.1% lower in chicks treated with maslinic acid than in the untreated controls and 55.3% lower than in those treated with sodium salinomycin (Fig. 1). The BWG value for the group treated with maslinic acid was higher than that of both the control group and the group treated with salinomycin, indicating that maslinic acid not only diminished parasitism by E. tenella but also enhanced weight-gain in the treated animals (Fig. 2). Given that there was no significant difference in the quantity of food consumed by the treated groups (Table 2), it would seem reasonable to suppose that these weight increases were due to the reduction in parasitisation in the group treated with maslinic acid, without ruling out some sort of complementary beneficial effect of the maslinic acid itself.
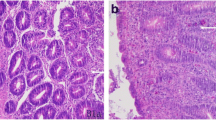
Histological sections of the caeca of the infected and untreated chicks revealed that the parasites were located in the epithelial cells, inducing inflammation and haemorrhages as a consequence of ulcerous lesions, with the appearance of pseudopolypoids and a loss of mucosecretory cells in the epithelium of the intestinal mucosa, lesions characteristic of this disease. These lesions were less evident in the treated groups, which is reflected in the LS (Table 1).
Our group has recently observed that maslinic acid affects the gliding of Toxoplasma gondii (unpublished results), possibly by inhibiting the serin proteases of the protozoan, a necessary mechanism for the entry of tachyzoites into the cytoplasm of the host cell. This activity, together with anti-inflammatory (Aladedunye et al. 2008) and antioxidant properties (Montilla et al. 2003), could be responsible for the anticoccidian properties observed in vivo in this present work.
Maslinic acid, a natural product present in the leaves and fruit of O. europaea, used in animal and human foodstuffs for millennia, not only reduces parasitemia in G. domesticus chicks infected with E. tenella but also increases the weight of the treated birds.
In future studies we intend to investigate the mode of action of this compound against parasites of the phylum Apicomplexa.
References
Abbas RZ, Iqbal Z, Sindhu ZD, Khan MN, Arshad M (2008) Identification of cross resistance and multiple resistance in Eimeria tenella field isolates to commonly used anticoccidials in Pakistan. J Appl Poult Res 17:361–368
Aladedunye FA, Okorie DA, Ighodaro OM (2008) Anti-inflammatory and antioxidant activities and constituents of Platostoma africanum P. Beauv. Nat Prod Res 22:1067–1073
Allen PC, Danforth HD, Augustine PC (1998) Dietary modulation of avian coccidiosis. Int J Parasitol 7:1131–1140
Allen PC, Fetterer RH (2002) Recent advances in biology and immunobiology of Eimeria species and in diagnosis and control of infection with these coccidian parasites of poultry. Clin Microbiol Rev 15:58–65
Bianchi G, Pozzi N, Vlahov G (1994) Pentacyclic triterpene acids in olives. Phytochemistry 37:205–207
Bianchi G (2003) Lipids and phenol sintable olives. Eur J Lipid Sci Technol 105:229–242
Chapman HD (1997) Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol 26:221–244
Chapman HD (1998) Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int J Parasitol 28:1141–1144
Cheng K, Zhang P, Liu J, Xie J, Sun H (2008) Practical synthesis of bredemolic acid, a natural inhibitor of glycogen phosphorylase. J Nat Prod 11:1877–1880
Dalloul RA, Lillehoj HS (2005) Recent advances in immunomodulation and vaccination strategies against coccidiosis. Avian Dis 49:1–8
European Council Regulation (EC) (2009) Regulation (EU) No 124/2009. J Eur Un 40:7–11
Holdsworth PA, Conway DP, McKenzie ME, Dayton AD, Chapmand HD, Mathis GF, Skinner JT, Mundtg HC, Williams RB (2004) World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating theefficacy of anticoccidial drugs in chicks and turkeys. Vet Parasitol 121:189–212
Hou W, Li Y, Zhang Q, Wei X, Peng A, Chen L, Wei Y (2009) Triterpene acids isolated from Lagerstroemia speciosa leaves as alpha-glucosidase inhibitors. Phytother Res 23:614–618
Jang SI, Jun MH, Hyun S, Lillehoj HS, Dalloul RA, Kong IK, Kim S, Min W (2007) Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet Parasitol 144:172–175
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chicks. Exp Parasitol 28:30–36
Jovel EM, Zhou XL, Ming DS, Wahbe TR, Towers GH (2007) Bioactivity-guided isolation of the active compounds from Rosa nutkana and quantitative analysis of ascorbic acid by HPLC. Can J Physiol Pharmacol 85:865–871
Juan ME, Wenzel U, Ruiz-Gutiérrez V, Daniel H, Planas JM (2006) Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J Nutr 136:2553–2557
Juan ME, Planas JM, Ruiz-Gutierrez V, Daniel H, Wenzel U (2008) Antiproliferative and apoptosis-inducing effects of maslinic and oleanolic acids, two pentacyclic triterpenes from olives, on HT-29 colon cancer cells. Br J Nutr 100:36–43
Kim JM, Jang DS, Lee YM, Yoo JL, Kim YS, Kim JH, Kim JS (2008) Aldose-reductase- and protein-glycation-inhibitory principles from the whole plant of Duchesnea chrysantha. Chem Biodivers 5:352–356
Lee IK, Kim do H, Lee SY, Kim KR, Choi SU, Hong JK, Lee JH, Park YH, Lee KR (2008) Triterpenoic acids of Prunella vulgaris var. lilacina and their cytotoxic activities in vitro. Arch Pharm Res 31:1578–1583
Long PL, Joyner LP (1976) A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat 6:201–217
Montilla MP, Agil A, Navarro MC, Jiménez MI, García-Granados A, Parra A, Cabo MM (2003) Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Panta Med 69:472–474
Naidoo V, McGawa LJ, Bisschop SPR, Duncan N, Eloff JN (2008) The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chicks. Vet Parasitol 153:214–219
Numata A, Yang P, Takahashi C, Fujiki R, Nabae M, Fujita E (1989) Cytotoxic triterpenes from a Chinese medicine, Goreishi. Chem Pharm Bull (Tokyo) 37:648–651
Parra A, Rivas F, Lopez PE, Garcia-Granados A, Martinez A, Albericio F, Marquez N, Muñoz E (2009) Solution- and solid-phase synthesis and anti-HIV activity of maslinic acid derivatives containing amino acids and peptides. Bioorg Med Chem 17:1139–1145
Reyes FJ, Centelles JJ, Lupiáñez JA, Cascante M (2006) (2Alpha, 3beta)-2, 3-dihydroxyolean-12-en-28-oic acid, a new natural triterpene from Olea europaea, induces caspase dependent apoptosis selectively in colon adenocarcinoma cells. FEBS Lett 580:6302–6310
Williams RB (1998) Epidemiological aspects of the use of live anticoccidial vaccines for chicks. Int J Parasitol 28:1089–1098
Williams RB (2002) Fifty years of anticoccidial vaccines for poultry (1952–2002). Avian Dis 46:775–802
Xu HX, Zeng PQ, Wan M, Sim KY (1996) Anti-HIV triterpene acids from Geum japonicum. J Nat Prod 59:643–645
Acknowledgements
This study was supported in part by a grant FQM1228 from the Regional Government of Andalucia (Spain) and by the companies: Granjas Avelino Góngora and BIOMASLINIC S.L. L.M. De Pablos received a fellowship from the Regional Government of Andalucia (Spain). The authors thank Dr. J. Trout of the Scientific Translation Service of the University of Granada for revising their text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Pablos, L.M., dos Santos, M.F.B., Montero, E. et al. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol Res 107, 601–604 (2010). https://doi.org/10.1007/s00436-010-1901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1901-3