Abstract
During 15 months (01 April 2003–31 July 2004), 20,389 women showing positive pregnancy tests were included in a serological evaluation of toxoplasmosis prevalence using automated immunoenzymatic assays. The women’s serum samples were tested for the presence of IgG and/or IgM antibodies. Overall, 53.03% of the women were positive for IgG and 3.26% were positive for IgM; the analysis used a chi-square adherence test and a significance level of 0.05 (χ 2 = 14,720.35; p = 0.00). To discriminate between recent and past infection, IgG avidity tests (n = 166) were carried out, of which 28.3% (n = 47) presented low avidity. The seroconversion index observed in this study was 0.44%. The seroprevalence results obtained were similar to other serology data found in other regions of Brazil. These data demonstrate the importance of continuous regional and national seroepidemiological inquiries to define public health strategies that can revert and reduce serological prevalence, as described in other countries where toxoplasmosis monitoring is mandatory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasmosis is a cosmopolitan parasitic disease caused by Toxoplasma gondii (Nicole and Manceaux 1909; Dubey and Beatie 1988). Exposure to T. gondii can occur by the ingestion of raw, undercooked meat, or unpasteurized milk; organ and tissue transplantation; blood transfusion; or by contact with oocysts in water or food contaminated with cat feces. Vertical transmission allows T. gondii to cross the placenta (Warnekulasuriya et al. 1998; Tenter et al. 2000; Martino et al. 2000). The disease is usually asymptomatic in immunocompetent individuals, but in immunocompromised patients and fetuses at early stages of pregnancy, it can lead to severe symptoms and sequelae (Sabin’s tetrad; Petersen et al. 2001; Jones et al. 2003; Khurana et al. 2005; Boyer et al. 2005). Infection can be diagnosed with several non-automated methods or, as in most cases, with automated immunoassays such as direct parasitological methods, cell culture and mouse inoculation, and, more recently, polymerase chain reaction (PCR); PCR techniques also allow the characterization of specific genotypes (Filisetti et al. 2003; Morris and Croxson 2004; Chabbert et al. 2004; Spalding et al. 2005). Despite the high prevalence of toxoplasmosis throughout the world (20%–90%) and the high resistance and persistence of the parasite in a broad spectrum of biological matrixes, there is no consensus concerning the clinical significance of antenatal or postnatal screening methods. Nor are there specific laws that compel the administration of serological tests or conventional or other molecular tests for the detection of toxoplasmosis in pregnant women, immunocompromised patients, and blood donors, except in a few European Union countries like France (Cantos et al. 2000; Pelloux et al. 2002).
The assessment of gestational toxoplasmosis is complex and requires interdisciplinary teams working toward better clinical follow-up, laboratory diagnosis, and treatment. It is essential to observe the clinical and laboratory parameters of pregnant woman from the prenatal period to the postnatal period in order to determine which diagnostic method(s) is safer; this must constitute the basis of an algorithm for clinical- and laboratory-based decision making in monitoring pregnant women (Boyer et al. 2005; Mazzola et al. 2007; Mioranza et al. 2008).
Considering the facts presented above, the aims of the present work were to determine the seroprevalence of anti-T. gondii antibodies among pregnant women served by the Health Units of the public service system in the city of Curitiba, Brazil and evaluate the serological profile of that population.
Materials and methods
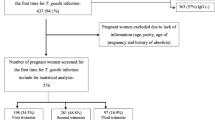
Blood sample collection and serological tests were carried out at the Curitiba County Public Laboratory (LMC-SMS-PMC) over 15 months (01 April 2003–31 July 2004) as well as at the Public Health Units (US) associated with the Gynecology Clinical Offices at the University of Parana Clinical Hospital (HC-UFPR). All the results were analyzed at the Molecular Parasitology Laboratory of the Federal University of Parana (LPM-UFPR). Prior to the initiation of this study, the project was submitted and approved by the Ethics Committee of the Federal University of Parana Clinical Hospital and also by the Curitiba County Health Department (CES-SMS-PMC). All serum samples (n = 20,389) were randomly selected from among women with positive pregnancy tests (TIG+) out of 225,052 women of child-bearing age (15–49 years old) who used the Curitiban Mother Assistance Program of the Curitiba Public Health System (Programa Mãe Curitibana).
Sample processing
The blood was drawn by venopunction and preserved in a hemogram tube (EDTA, Becton and Dickinson) in order to obtain the plasma or in a dry tube (neutral gel, Becton and Dickinson) to obtain the serum after centrifugation at 3,000 rpm for 10 min. All plasma and serum samples were analyzed by automated immunoassays to detect anti-T. gondii antibodies (IgM and IgG avidity). The assays used for the serological analysis were MEIA-AxSym® (ABBOTT) and CLIA/LIASON® (DiaSorin; IgM and IgG). The commercial avidity tests used were as follows: ELIFA/VIDAS® (Biomerieux) and CLIA/LIASON® (DiaSorin). All the samples (plasma and/or serum) were cryopreserved at −80°C to comply with quality control and legal requirements.
Validation of the results
All the commercial kits used were tested from 01 April 2003 to 18 February 2004 (MEIA-AxSym®, ABBOTT(IgG/IgM), and ELFA-VIDAS®, Biomerieux (avidity)) and from 19 February 2002 to 31 July 2004 (CLIA-LIASON®, DiaSorin (IgG/IgM avidity)). To evaluate inter-assay variability and concordance, a K test (kappa) was applied, and the hypothesis was tested using the chi-square adherence test and a significance level of 0.05 (p < 0.05).
Results
Validation of the immunoenzymatic methods
The results of the variability and global concordance assays comparing the three automated immunoenzymatic tests used in the present study, as evaluated by the non-weighted kappa test (K), were as follows: 0.75 to 1.00 (excellent) for MEIAXCLIA-IgG/IgG, IgM/IgM and >0.75 (good to excellent) for CLIA X ELIFA, avidity.
Seroprevalence study
During the period of the serological analysis (01 April 2003 to 31 July 2004), 530,119 people were served by the Public Health System of Curitiba county. Out of this total number of patients, 29% (n = 153,734) were male and 71% (n = 376,385) were female. A total of 225,052 (59.8%) women of reproductive age (between 15 and 49 years old, average 25.8 ± 7.0 years old) were preliminarily included in the study. Only female patients with a positive immunological pregnancy test (TIG+) were analyzed and subjected to seroprevalence tests for anti-T. gondii antibodies (n = 20,389; Tables 1 and 2). Serological results were analyzed according to a distribution standard: IgG+/IgM+, IgG+/IgM−, IgG−/IgM+, and IgG−/IgM−. The results showed that the frequency distribution was not equal between different serological patterns (χ 2 = 14,720.35; p = 0.00). The results defined the serological profile of the pregnant women as follows: immune (IgG+/IgM−), susceptible (IgG−/IgM−), and acute phase (IgM+/IgG−). Overall, 53.03% of the women were immune (n = 8,740, chronic phase of the disease, IgG+/IgM−), and 3.26% were at risk of acute/recent infection (n = 536, IgG−/IgM+, IgG+/IgM+; Fig. 1).
Avidity tests
Avidity tests (n = 166) were carried out on dually immunoreactive samples (IgM+/IgG+) in order to define the specific clinical phase. Low avidity (seroconversion) was observed in 28.3% (n = 47) of the tests; 58.4% (n = 97) showed high avidity (past infection) and 13.3% (n = 22) were considered borderline (intermediate avidity)
Seroconversion Index
A seroconversion index (SCI) of 0.44% was calculated based on serological results for IgM reactivity (n = 24, 0.16%) and serological results indicating recent infection as indicated by low avidity (n = 47, 0.28%; Fig. 2). However, the SCI was 1.19% when all 536 pregnant women were considered regardless of infection phase including those with either IgM+ or IgM+/IgG+ results (in seroconversion or not; n = 151, 0.91%) in addition to the 47 women who had low avidity.
Discussion
The seroprevalence of anti-T. gondii antibodies of the IgG class observed in the present study was 53.03%; patients with these antibodies were considered immune. These data are within the range of the average seroprevalence (43.7%) of European countries such as Austria (37%), Belgium (53%), Spain (38.8%), France (55%), Italy (40%), Poland (36%), and Switzerland (46.1%; Lappalainen et al. 1992, 1993; Pelloux et al. 2002). However, it is higher than the average reported rates for Scandinavian countries, especially those of Finland (20%), or for other countries in the New World, such as Australia and New Zealand (4%).
The prevalence of antibodies of the IgM class, which is related to the acute phase of infection, was 0.16% (n = 24) if we consider only IgM+ results. Only 3.10% of pregnant women presented IgG+/IgM+ serology (n = 512), which indicates a diagnostic suspicion of recent infection or not. The percentage of pregnant women with results that fit the aim of the present study (IgM+ and IgM+/IgG−) was 3.26% (n = 536). The distinction between recent and late infection was established using the avidity test, which was carried out in 166 pregnant women; these women returned to receive the test and 28.3% (n = 47) presented low avidity (recent infection), whereas 58.4% (n = 97) presented high avidity (late infection) and 13.3% (n = 22) presented intermediate avidity.
The seroconversion rate observed in data from patients in the acute phase (n = 24, 0.16%) and in patients in the recent infection phase (n = 47, 0.28%) was 0.44%. However, if we consider all 536 pregnant women, the seroconversion rate would be 1.19% (n = 151, 0.91%). The data related to IgM+ prevalence in the present study show that our rates are close to rates of incidence of acute toxoplasmosis during pregnancy throughout the world, which vary from 0.06% to 1.4% (Remington 1990; Cantos et al. 2000; Remington et al. 1994). The results of this study are similar to the results obtained in some countries that carried out acute phase studies by detecting Acs IgM; for instance, results were similar in Spain (1.2% or 1.7%; Jaqueti et al. 1991; Jacquier et al. 1995), in Florianopolis, SC (0.87%; Cantos et al. 2000), and in Campo Grande, MS (0.42%; Figueiro-Filho et al. 2005).
There are no previous studies from Brazil presenting average values obtained in a continuous way (day-to-day, month-to-month, year-to-year), which makes it difficult to monitor or establish projects related to the prevention and treatment of toxoplasmosis in pregnant women and risk groups in the various regions of Brazil. However, according to Pelloux et al. (2002), the worldwide average incidence of congenital toxoplasmosis is one to two per 1,000 births, which would represent approximately 750,000 annual births in the world. Our work shows that the situation in the city of Curitiba, PR is similar to the global rate.
Our results emphasize the importance of monitoring pregnant women throughout pregnancy, especially those women with serological profiles compatible with acute or recent infection; monitoring should aim at minimizing the risks of prenatal infection and the sequelae, from the least to the most severe in the peri-conceptional period, as well as late sequelae that can be observed in the postnatal period and even after birth. Importantly, the compulsory serological monitoring of toxoplasmosis in pregnant women, as a public prevention program, reversed the seroprevalence trends in countries like France and Austria; the average incidence of fetal toxoplasmosis was reduced from 40% to 7% (Warnekulasuriya et al. 1998; Lopez et al. 2000; Spalding et al. 2005). Thus, it is important that measures be taken to avoid trivial infections that result in severe consequences for the susceptible population.
Conclusions
Of all the pregnant women evaluated for the presence of anti-T. gondii antibodies, 53.03% showed IgG+/IgM− results, which indicates an immune profile (past infection/chronic phase), and thus, these women do not need further clinical or laboratory monitoring. Another 43.71% were seronegative for both immunoglobulins (IgM−/IgG−), representing a population at risk of developing toxoplasmosis throughout the gestational period. Finally, 0.16% and 3.10% had IgM+/IgG− and IgM+/IgG+ results, respectively; they were subsequently tested for avidity. Low avidity was found in 28.3% of the women tested, indicating recent and/or acute disease; 58.4% showed high avidity, which is indicative of a past infection (chronic phase; Fig. 2). The seroprevalence results for IgG and IgM allowed us to define the serological profile of the pregnant women included in this study and also revealed the similarity between our results and other serology data found in other regions of Brazil; this demonstrates the importance of continuous regional and national seroepidemiological inquiries to better define public health strategies and policies aiming to revert and reduce the prevalence of serology, as described in other countries where toxoplasmosis monitoring is mandatory (Cantos et al. 2000; Pelloux et al. 2002; Morris and Croxson 2004; Spalding et al. 2005).
References
Boyer KM, Holfels E, Roizen N, Swisher C, Mack D, Remington J, Withers S, Meier P, McLeod R, and the Toxoplasmosis Study Group (2005) Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: implications for prenatal management and screening. Am J Obst Gynecol 192:2557–2564
Cantos GAA, Prando MD, Siqueira MV, Teixeira RM (2000) Toxoplasmose: ocorrência de anticorpos anti-Toxoplasma gondii e diagnóstico. Rev Assoc Med Bras 46:335–341
Chabbert E, Lachaud L, Crobu L, Bastien P (2004) Comparison of two widely used pcr primer systems for detection of Toxoplasma in amniotic fluid blood and tissues. J Clin Microbiol 42:1719–1722
Dubey JP, Beatie CP (1988) Toxoplasmosis of animals and man. CRC, Boca Raton, pp 1–220
Figueiró Filho EA, Lopes AHA, Senefonte FRA, Souza VG, Botelho CA, Figueiredo MS, Duarte G (2005) Toxoplasmose aguda: estudo da freqüência. taxa de transmissão vertical e relação entre os testes diagnósticos materno-fetais em gestantes em estado da Região Centro-Oeste do Brasil. Rev Bras Ginecol Obstet 27:442–449
Filisetti D, Gorcii M, Pernot-Marino E, Villard O, Candolfi E (2003) Diagnosis of congenital toxoplasmosis comparison of targets for detection of Toxoplasma gondii by PCR. J Clin Microbiol 41:4826–4828
Jacquier P, Nadal D, Zuber P, Eckert J (1995) The status of infection with Toxoplasma gondii in the Swiss population: contribution of a seroepidemiologic study from the Zurich canton. Schweiz Med Wochenschr Suppl 65:23S–28S
Jaqueti J, Hernandez-Garci R, Nicolas D, Martinez-Hernandez D, Navarro-Gallar F, Garcia-Esteban RJ (1991) Serology against Toxoplasma gondii in pregnant women. Development of prevalence rates in the course of 4 years. Rev Clin Esp 188:278–280
Jones JL, Kruszon-Moran D, Wilson M (2003) Toxoplasma gondii infection in the United States, 1999–2000. Emerg Infect Dis 9:1371–1374
Khurana S, Dubey ML, Malla N (2005) Association of parasitic infections and cancers. Ind J Med Microbiol 23:74–79
Lappalainen M, Koskela P, Hedman K, Teramo K, Ammälä P, Hiilesmaa V, Koskiniemi M (1992) Incidence of primary Toxoplasma infections during pregnancy in southern Finland: a prospective cohort study. Scand J Infect Dis 24:97–104
Lappalainen M, Koskela P, Koskiniemi M, Ammala P, Hillemaa V, Terame K (1993) Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity and IgG. Infect Dis 167:691–697
Lopez A, Dietz VJ, Wilson M, Navin TR, Jones JL (2000) Preventing congenital toxoplasmosis. Morb and Mort Weekly Rep—Recommendations and Reports 31:57–75
Martino R, Maertens J, Bretagne S, Rovira M, Deconinck E, Ullmann AJ, Held T, Cordonnier C (2000) Toxoplasmosis after hematopoietic stem cell transplantation. Clin Infect Dis 31:1188–1194
Mazzola A, Casuccio A, Romano A, Schimmenti MG, Titone L, Di Carlo P (2007) Diagnostic problems and postnatal follow-up in congenital toxoplasmosis. Minerva Pediatrica 59:207–213
Mioranza SL, Meireles LR, Mioranza EL, Andrade Júnior HF (2008) Serological evidence of acute Toxoplasma gondii infection in pregnant women in Cascavel, Paraná. Rev Soc Bras Med Trop 41:628–629
Morris A, Croxson M (2004) Serological evidence of Toxoplasma gondii infection among pregnant women in Auckland. N Zeal Med J 117:1189
Nicole C, Manceaux L (1909) Sur un protozoaire nouveau du gondii. Acad Sci 147:763–766
Pelloux H, Fricker-Hidalgo H, Pons JC, Bost-Brut C, Brenier-Pinchart MP, Jouk PS, Ambroise-Thomas P (2002) Congenital toxoplasmosis: prevention in the pregnant woman and management of the neonate. Arch Pediatr 9:206–212
Petersen E, Pollak A, Reiter-Owona I (2001) Recent trends in research on congenital toxoplasmosis. Intl J Parasitol 31:115–144
Remington JS (1990) The tragedy of toxoplasmosis. Ped Infect Dis J 9:762–763
Remington JS, Thulliez P, Montoya JG (1994) Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol 42:941–945
Spalding SM, Amendoeira MRR, Klein CH, Ribeiro LC (2005) Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev Soc Bras Med Trop 38:173–172
Tenter AM, Heckeroth AR, Weiss LM (2000) Toxoplasma gondii: from animals to humans. Intl J Parasitol 30:1217–1258
Warnekulasuriya MR, Johnson D, Holliman RE (1998) Detection of Toxoplasma gondii in cured meats. Intl J Food Microbiol 45:211–215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaz, R.S., Thomaz-Soccol, V., Sumikawa, E. et al. Serological prevalence of Toxoplasma gondii antibodies in pregnant women from Southern Brazil. Parasitol Res 106, 661–665 (2010). https://doi.org/10.1007/s00436-009-1716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1716-2