Abstract
Proper diagnosis of toxoplasmosis is very important for proper treatment in manifested cases and to prevent congenital toxoplasmosis in case of pregnancy. Screening of 180 pregnant females by automated immune assay for Toxoplasma IgG and IgM Abs was done first then positive cases and some of negative cases were evaluated for the Toxoplasma IgG avidity test versus Toxoplasma IgM and IgA Abs using ELISA technique for the detection of active toxoplasmosis during gestation using IHA rising titer as reference test. Assessment of Toxoplasma IgM by ELISA compared with rising titer of IHA as a reference test revealed that the sensitivity, specificity, PPV, and NPP of IgM by ELISA were 66.67, 88.73, 42.86, and 95.45%, respectively. Assessment of Toxoplasma IgA Abs by ELISA compared with rising titer IHA as a reference test showed that the sensitivity, specificity, PPV, and NPP of IgA Abs by ELISA were 44.4, 92.96, 44.4, and 92.96%, respectively. Additionally, by assessment of Toxoplasma IgG avidity compared with rising titer of IHA as a reference test, the sensitivity, specificity, PPV, and NPP of Toxoplasma IgG avidity were 100, 98.59, 90%, and 100%, respectively. Detection of specific Toxoplasma IgM antibodies by ELISA is not always sufficient in the diagnosis of early and late Toxoplasma gondii infection during pregnancy, because Toxoplasma-specific IgM antibodies may persist as long as 18 months after acute acquired infection. Thus, the specific IgG avidity test should be used as more or less low cost tool to detect acute toxoplasmosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma is an obligate intracellular protozoal parasite inside all nucleated cells such as macrophages, lymphocytes, and muscle fibers. It is widely spread between human and all warm-blooded animals (Elsheikha 2008). Toxoplasma causes pathological changes in affected organs such as the reticuloendothelial system, eye, brain, muscle fibers, heart, lung, and placenta most likely caused by the strong T-helper 1 (Th1) response causing focal areas of necrosis surrounded by cellular inflammatory infiltrate (Roldan et al. 2014). Transmission of infection during pregnancy causes either abortion, still birth, or full-term baby having many dangerous congenital abnormalities such as microcephaly, hydrocephaly, convulsions, retinochoroiditis, and many other abnormalities; however, the full-term baby may not have any manifestations but in some cases may develop any symptoms in later decades of life (Werre et al. 2002). Proper diagnosis of toxoplasmosis is very important to give proper treatment in manifested cases and to prevent congenital toxoplasmosis in case of pregnancy (Flori et al. 2009). The diagnostic approach to toxoplasmosis depends on the clinical presentation and laboratory tests (Montoya and Liesenfeld 2004). Direct tests such as isolation of the parasite, demonstration in tissue sections by histopathology, and by detection of parasite nucleic acid by PCR is commonly performed in immunocompromised patients. While, indirect serological tests are commonly used in immunocompetent patients (Roldan et al. 2014). A variety of serological tests including the Sabin–Feldman dye test, the immunofluorescent antibody test, enzyme-linked immunosorbent assay (ELISA), and indirect hemagglutination test (IHA) test are used for the detection of antibodies against Toxoplasma in patients serum (Abu-Madi et al. 2008). IgG that arise within 1–2 weeks after infection and persist through life, while IgM antibodies that rise rapidly within the first week of infection, thus later on decrease and disappear at variable rates. A negative IgM test result essentially indicates old infection. However, presence of IgM does not indicate recent infection as it may persist for variable time in patients serum may be more than 1 year (Abu-Madi et al. 2008). Tests for the avidity (functional affinity) of IgG antibodies have become standardized to determine the time of infection (recent or old). IgG avidity is based on protein-denaturing reagent, including urea, are used as a dissociating agent to break up weak antigen–antibody complex by destruction of hydrogen bonds in recent infection, as the host immune response develops; there is increase in strength of hydrogen bonds between T. gondii- IgG antibody and the antigen over time. This leads to strong bonds difficult to be dissociated by urea (Montoya et al. 2002). A low avidity index (<35–40%) being a marker of an acute phase of infection indicating weak overall strength of interaction between antibody and antigen, while a high avidity index (>35–40% avidity) indicates chronic infection (Lappalainen and Hedman 2004). So that, this test has become supplementary tool in the discrimination between recent and chronic asymptomatic infection using a single serum sample in pregnancy (Flori et al. 2008). This study is based on assessment of role of IgG avidity and IgA, IgM by ELISA in the diagnosis of active toxoplasmosis in pregnant females attending the antenatal care clinic in Kasr Al-Ainy Hospital and Preventive Medicine Hospital versus rising titer of IHA as standard test.
Patients and methods
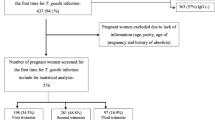
A total of 180 women in antenatal period from antenatal outpatient clinic in Kasr Al-Ainy Hospital and Preventive Medicine Hospital, Cairo University in the period from September 2015 to March 2016. All pregnant women with severe other comorbid disease(s) or pregnant women who received Toxoplasma treatment before sampling were excluded.
Written informed consent was obtained from individual participants. The study was approved by the local ethics committee and conforms to the 1995 Helsinki declaration. The screening of 180 samples (screened group) was done by Automated Immunoassay [ADVIA XPT (SIEMENS) USA] to detect IgG and IgM of Toxoplasma. The results showed 38 positive samples for either IgG, IgM or both, and 142 negative samples for both IgG and IgM. Random sample selection test program was performed to randomly select 42 negative samples considered as negative random sample selection group ultimately; the study included 80 samples (test group) which contain 38 positive sample group and 42 negative random sample selection group. These two groups were subjected to Toxoplasma detection by IHA [TOXO-HAI FUMOUZE France], (IgG, IgM, and IgA) by ELISA [Nova LISA Germany], and IgG avidity [NovaTec Germany], all according to the manufacturer’s instructions. Two weeks later, resampling from the 38 positive women was done to evaluate the rising titer of T.gondii infection by using IHA which was introduced as a reference test for significance.
Results
All risk factors including contact with cats, eating raw not clean vegetables, eating undercooked meat, pregnant women having low socioeconomic standards, threatened abortion, congenital anomalies, history of Toxoplasma, fever, palpable lymph nodes, and spleen were statistically significant related to Toxoplasma infection as (p value <0.05). While, age, rural residence, education, occupation, contact with soil, trimester of pregnancy, diabetes in recent pregnancy, hypertension in recent pregnancy, primary gravida, abortion in previous pregnancies, congenital anomalies in previous pregnancies, premature labor, diabetes in previous pregnancies, hypertension in previous pregnancies, liver enlargement, retinal problems, autoimmune disease, diabetes, and hypertension have not any statistically significant relationship with percentage of Toxoplasma infection (p value >0.05) (Table 1).
The statistical relationship between each variable in data collection sheet to the number of acute infection measured by IHA rising titer (9 active infection group and 71 non-active control group) revealed that threatened abortion, fever, and palpable lymph nodes were significantly related and more specific to acute Toxoplasma infection as (p value <0.05) (Table 2).
IHA was used as a reference test in comparing ELISA test and automated immunoassay for detection of both IgG and IgM in diagnosis of Toxoplasma infection. Out of 80 samples, 38 were positive detected by IHA; there were three negative cases by automated immunoassay, and two were negative by ELISA. However, among 42 negative detected by IHA there were three positives by automated immunoassay and seven were positive by ELISA (Tables 3 and 4).
Assessment of both IgG and IgM using automated immunoassay compared with IHA as a reference test, the sensitivity, specificity, PPV, and NPP of automated immunoassay were 92.11, 92.86, 92.11, and 92.88%, respectively, and kappa degree of agreement was 0.85 which indicates almost perfect agreement. As regards, assessment of both IgG and IgM using ELISA compared with IHA as a reference test, the sensitivity, specificity, PPV, and NPP of ELISA were 94.74, 83.33, 83.72, and 94.59%, respectively, and kappa degree of agreement was 0.766 which indicates substantial agreement (Fig. 1).
Automated immunoassay is more specific and less sensitive than ELISA in detection of Toxoplasma antibodies (IgG, IgM) with higher kappa degree which is in automated immunoassay 0.85 indicating almost perfect agreement than in ELISA which is substantial agreement 0.766, respectively, which mean more validity of automated immunoassay than ELISA (Fig. 1).
Out of 80 samples, 38 were positive by IHA, nine were negative by automated immune assay, and seven were negative by ELISA. However, among 42 negative cases of IHA there were three positive cases of automated immunoassay and five were positive by ELISA. Using assessment of IgG using ELISA compared with IHA as a reference test, the sensitivity, specificity, PPV, and NPP of ELISA were 81.58, 88.10, 86.11, and 84.09%, respectively, and kappa degree of agreement was 0.698 which indicates substantial agreement. As regards, assessment of IgG using automated immunoassay compared with IHA as a reference test, the sensitivity, specificity, PPV, and NPP of automated immune assay were 76.32, 92.86, 90.62, and 81.25%, respectively, and kappa degree of agreement was 0.697 which indicates substantial agreement (Table 5 and Fig. 2).
Out of 80 samples included in this study, nine were positive by IHA rising titer in comparison to ELISA IgM, three were negative, ELISA IgA five were negative, whereas IgG avidity no negative cases were detected. However, among 71 negative cases of IHA rising titer eight were positive ELISA IgM, five were positive by ELISA IgA, and one positive case by avidity. Assessment of IgM ELISA compared with rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP IgM ELISA were 66.67, 88.73, 42.86, and 95.45%, respectively, and the kappa degree of agreement was 0.446 which is indicative of moderate agreement. As regards, assessment of IgA ELISA compared with rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP IgA ELISA were 44.4, 92.96, 44.4, and 92.96%, respectively, and the kappa degree of agreement was 0.374 which is indicative of fair agreement. Additionally, by assessment of IgG avidity compared with rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP of IgG avidity were 100, 98.59, 90, and 100%, respectively, and the kappa degree of agreement was 0.94 which is indicative of perfect agreement. Conclusively, IgG avidity is the most sensitive and specific in comparison to ELISA IgM, and IgA with the degree of agreement by kappa test for IgG avidity was perfect whereas, for ELISA IgM and IgA which were moderate and fair degrees, respectively, for detection the proper method in diagnosis of acute toxoplasmosis (Tables 6 and 7).
Discussion
Studies on T. gondii infection during pregnancy in different governorates in Egypt (Hussein et al. 2001; ElDeeb et al. 2012; Tammam et al. 2013) revealed higher percentage than the results in the present study. On the other hand, in Al Minia (Kamal et al. 2015), lower results (8.3%) were reported. The variation between the present study and other studies is related to various factors concerned with the socioeconomic standard, health care and hygienic measures, epidemiologic and geographical aspects, and presence of pregnancy complications as well as routine screening during pregnancy.
In the present study regarding the serological tests, assessment of Toxoplasma IgM by ELISA compared to rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP for IgM ELISA were 66.67, 88.73, 42.86, and 95.45%, respectively, and kappa degree of agreement was 0.446 which indicates moderate agreement. Assessment of Toxoplasma IgA by ELISA compared with rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP IgA ELISA were 44.4, 92.96, 44.4, and 92.96%, respectively, and kappa degree of agreement was 0.374, which indicates fair agreement.
Additionally, assessment of IgG avidity compared with rising titer IHA as a reference test, the sensitivity, specificity, PPV, and NPP of IgG avidity were 100, 98.59, 90, and 100%, respectively, and kappa degree of agreement was 0.9 4 which indicates perfect agreement. Using the rising titer of IHA as a reference test for detection of the best method in diagnosis of active toxoplasmosis, IgG avidity test was more sensitive and specific than IgM, IgA by ELISA with perfect degree of agreement by kappa test in comparison to IgM, IgA ELISA that were moderate and fair degrees, respectively. Evaluating the role of IgA and IgG avidity, a study was in agreement with the present study. The author concluded that IgA is not adequate for diagnosis of active toxoplasmosis. However, testing the avidity of IgG is more reliable for the diagnosis of active toxoplasmosis; this results agreed with the present study except in higher sensitivity of IgG avidity100% and lower sensitivity of IgA ELISA 44.4% in the present work (Gutierrez et al. 2000).
Determination of the avidity of T. gondii-specific IgG in patients with detectable IgM antibodies defines the most accurate detection tool to active infection than IgA (Suzuki et al. 2001) which is aligned with the present results. A confirmative study (Bahar et al. 2005), determining the serological status of pregnant women in Turkey who were suspected of having active or old toxoplasmosis as well as the relationship of a specific anti-Toxoplasma IgG, IgM and IgA antibodies, and specific IgG avidity in their sera, revealed that IgM levels showed a good correlation with the IgG avidity results, whereas, positivity of IgA was limited in diagnosis of active toxoplasmosis which is similar to the present study. Another study (Candolfi et al. 2007) was in agreement with the present study results in case of avidity test. However, as for IgA test, the sensitivity is lower in the present results (44.4%) but with slightly higher specificity (92.96%).This may be due to using a different reference test indirect immune florescent antibody test (IFAT) test or using different trade mark in commercial kits (PlateliaToxo from BIO-RAD (Marnes La Coquette, France).
IgG avidity test was evaluated also in a reported study (Kaul et al. 2004) with Toxoplasma IgM Abs by ELISA. The sensitivity of IgG avidity test was 56%, and specificity was 94% which is much lower sensitivity than the present study results. This variation could be due to the fact that the IgM has a high half-life. In another study (Hashoosh and Majeed 2014) which investigated the IgG-avidity ELISA test and reverse transcriptase (RT)-PCR for the identification of recent T. gondii infection among pregnant women in reference to IgM by ELISA proved that RT-PCR provides a rapid, sensitive, automated, and quantitative way of detecting Toxoplasma DNA. It can detect the infection very early and can be used in follow-up of the treatment. However, it is very expensive and needs technical skills especially in developing countries, besides that it is difficult to be implemented as a routine diagnostic tool; in addition, the DNA of parasite in blood disappear after early period of parasitaemia. So that IgG avidity test is a good screening test, cost effective, and can be used to diagnose recent infection in pregnancy.
Conclusively, Toxoplasma IgA Abs detection by ELISA is not an adequate method for diagnosis of active toxoplasmosis as this test has lower sensitivity and specificity than other tests such as IgG avidity and IgM ELISA. Detection of specific IgM antibodies by ELISA test is not always sufficient in the diagnosis of early and late T.gondii infection during pregnancy, because Toxoplasma-specific IgM antibodies may persist as long as 18 months after acute acquired infection. Thus, the specific IgG avidity test should be used as a low cost tool to detect acute toxoplasmosis. The test can be used to rapidly distinguish between chronic and acute infection in a single serum sample from a pregnant woman, for early detection and treatment of disease preventing any complication to the fetus provided that there is no underlying immunosuppression and no ongoing anti-toxoplasmic treatment to the pregnant woman.
References
Abu-Madi MA, Al-Molawi N, Behnke JM (2008) Sero-prevalence and epidemiological correlates of Toxoplasma gondii infections among patients referred for hospital-based serological testing in Doha. Qatar Parasit Vectors 1(1):39–43
Bahar, I. H., Karaman, M., Kirdar, S., Yilmaz, O., Celıloğlu, M and Mutlu, D. (2005): IgA antibodies The importance and validity of anti-Toxoplasma gondii IgG, IgM and IgG avidity tests in the diagnosis of Toxoplasmosis infection during pregnancy Turkey. 29(2): 76–79.
Candolfi E, Pastor R, Huber R, Filisetti D, Villard O (2007) IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Korean J Parasitol 50(2):99–102
ElDeeb HK, Salah-Eldin H, Khodeer S, Allah AA (2012) Prevalence of Toxoplasma gondii infection in antenatal population in Menoufia governorate. Egypt Acta Trop 124(3):185–191
Elsheikha HM (2008) Congenital toxoplasmosis: priorities for further health promotion action. Public Health 122:335–353
Flori P, Bellete B, Crampe C, Maudry A, Patural H, Chauleur C, Hafid J, Raberin H, Sung R (2008) A technique for dating toxoplasmosis in pregnancy and comparison with the VIDAS anti-toxoplasma IgG avidity test. Clin Microbiol Infect 14(3):242–249
Flori P, Chene G, Varlet MN, Sung RT (2009) Toxoplasma gondii serology in pregnant woman: characteristics and pitfalls. Ann Biol Clin (Paris) 67(2):125–133
Gutierrez, J., Rodriguez, M., Piedrola, G and Maroto, M.C. (2000) Detectionof IgA and low-avidity IgG antibodies for the diagnosis of recent active toxoplasmosis. 3(6).
Hashoosh, D.A and Majeed, I.A. (2014): Comparisonoftwoassaysinthediagnosisoftoxoplasmosis: immunologicalandmolecular. 20(1).
Hussein AH, Ali AE, Saleh MH, Nagaty IM, Rezk AY (2001) Seroprevalence of Toxoplasma infection in Qualyobia governorate, Egypt. J Egypt Soc Parasitol 31:355–363
Kamal AM, Ahmed AK, Abdellatif MZM, Tawfik M, Hassan EE (2015) Seropositivity of toxoplasmosis in pregnant women by ELISA at Minia University Hospital. Egypt Korean J Parasitol 53(5):605–610
Kaul, R., Chen, P and Binder, S.R. (2004):Detection of ImmunoglobulinM antibodies specific for Toxoplasma gondii with increased selectivity for recently acquired infections.; 42 (12):5705–5709.
Lappalainen M, Hedman K (2004) Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity. Ann Ist Super Sanita 40(1):81–88
Montoya JG, Liesenfeld PO (2004) Toxoplasmosis Lancet 363(9425):1965–1976
Montoya JG, Liesenfeld O, Kinney S, Press C, Remington JS (2002) VIDAS test for avidity of Toxoplasma-specific immunoglobulin G for confirmatory testing of pregnant women. J Clin Microbiol 40:2504–2508
Roldan, M. M., Heimesaat, M. M and Liesenfeld, L. (2014): Toxoplasmosis Manson tropical disease 23rd edition chapter 48 Elseivirsaunders.
Suzuki AL, Rocha RNJ, Rossi CL (2001) Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis Sa-o Paulo, Brazil J. Med Microbiol 50:62–70
Tammam AE, Haridy MA, Abdellah AH, Ahmed SR, Fayed HM, Sammani MA (2013) Seroepidemiology of Toxoplasma gondii infection in women with first trimester spontaneous miscarriage in Qena governorate, Egypt. J Clin Diagn Res 7:2870–2873
Werre SR, Jacobson RH, Bowman DD, Dubey JP, Mohammed HO (2002) Evaluation of kinetics and single-read enzyme-linked immunoassays for detection of Toxoplasma gondii antibodies in sheep. J Vet Diagn Investig 14:225–230
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamed, A.S.M.R., Omar, S.H., Basyoni, M.M.A. et al. Comparative and analytical study on active toxoplasmosis to assess the IgG avidity in correlation to serological profile in a cohort of Egyptian patients. Comp Clin Pathol 26, 1157–1163 (2017). https://doi.org/10.1007/s00580-017-2501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2501-8