Abstract
Naegleria fowleri, a ubiquitous pathogenic free-living amoeba, is the most virulent species and causes primary amoebic meningoencephalitis in laboratory animals and humans. The parasite secretes various inducing molecules as biological responses, which are thought to be involved in pathophysiological and immunological events during infection. To investigate what molecules of N. fowleri excretory–secretory proteins (ESPs) are related with amoebic pathogenicity, N. fowleri ESPs fractionated by two-dimensional electrophoresis were reacted with N. fowleri infection or immune sera. To identify immunodominant ESPs, six major protein spots were selected and analyzed by N-terminal sequencing. Finally, six proteins, 58, 40, 24, 21, 18, and 16 kDa of molecular weight, were partially cloned and matched with reference proteins as follow: 58 kDa of exendin-3 precursor, 40 kDa of secretory lipase, 24 kDa of cathepsin B-like proteases and cysteine protease, 21 kDa of cathepsin B, 18 kDa of peroxiredoxin, and 16 kDa of thrombin receptor, respectively. These results suggest that N. fowleri ESPs contained important proteins, which may play an important role in the pathogenicity of N. fowleri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naegleria fowleri is a pathogenic free-living amoeba which causes primary amoebic meningoencephalitis (PAME) in experimental animals and humans. The organisms are commonly found in soil and in warm bodies of fresh water such as lakes, rivers, hot springs, and unchlorinated swimming pools and in warm water discharge pools from industrial plants (Culbertson 1970). PAME is a fulminant infection that typically leads to death with 1 to 2 weeks from the onset of symptoms. The trophozoites, active stages of N. fowleri, infect the brain through the olfactory bulb by penetrating nasal epithelium (Ma et al. 1990). Cloning of pathogenicity-related agents of PAME by N. fowleri is important in the exploration to determine the mechanisms of parasite–host interaction. The factors that determine the pathogenicity of N. fowleri have not been fully established. The pathogenicity-related agents of the N. fowleri depend on a contact mechanism and non-contact mechanism with host cell. In a contact mechanism of N. fowleri infection, Nfa1 protein had been characterized (Shin et al. 2001a; Jeong et al. 2004). In non-contact mechanism, excretory–secretory proteins (ESPs) of parasites are involved, and their pathogenicity may be related to the properties of ESP components. In fact, it was the amount of evidence that several studies have shown that ESPs contributed to parasitic disease. In secreted factors of helminthes, which affect the function of host cells, Echinococcus ganulosus secretes a protein, which inhibits neurtophil chemotaxis (Shepherd et al. 1991).
Darcy et al. (1988) reported that excreted/secreted antigens of Toxoplasma gondii could play a major role in the immune response and demonstrated the key role played by excreted/secreted antigens in the protective immune response. Thus, they suggested that these antigens should be of value for the development of new strategies for immunization against toxoplasmosis. It is apparent that the ability of Candida albicans to transport proteins onto the cell surface via the secretion pathway and to secrete degradative enzyme out of the cell is required for virulence and pathogenesis (Naglik et al. 2004).
At present, the role of ESPs in immunity against N. fowleri infection and the mechanisms by which N. fowleri produce cell damage were poorly understood. Several attempts have been made to induce protective immunity against experimental N. fowleri amoebae in mice, but the study of immunity in experimental and natural infections has been limited to the analysis of serum antibody responses (Bush and John 1988).
In a preliminary study, ESPs of N. fowleri showed the in vitro cytotoxicity against target Chinese hamster ovary (CHO) cells. In the present study, we would like to explain pathogenecity-related agents, which contribute to N. fowleri diseases and found pathogenicity-related proteins from N. fowleri ESPs using proteomics-based approaches such as sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), two-dimensional electrophoresis (2-DE), and Western blot analysis with anti-ESP/infection sera obtained from infected BALB/c mice. Also, this study pursued the possible role of ESPs on the penetrating of N. fowleri into host cells.
Materials and methods
Parasite and ESPs
N. fowleri trophozoites (ATCC no. 30215) were axenically cultured at 37°C in Nelson’s medium containing 10% fetal bovine serum (Willaert 1971). For ESP collection, N. fowleri trophozoites (1 × 108) were incubated at 37°C for 1 h with phosphate-buffered saline (PBS). After centrifugation at 1,300 rpm for 5 min, supernatants were saved as ESPs. The protein concentration of ESPs was adjusted to 1.0 mg/ml per the batches.
Production of anti-ESPs and infection sera
Anti-ESPs and infection sera were prepared according to a method described in a previous paper (Shin et al. 2001b). For the production of infection sera, N. fowleri trophozoites (1 × 105) were infected intranasally into 6-week-old female BALB/c mice (purchased from the Korea Institute of Science and Technology, Daegeon, Korea) under anesthesia. Blood were collected from mice showing the behavior of death due to PAME. For the production of anti-ESP sera, ESPs (50 µg/mouse) were mixed with an equal volume of Freund’s complete adjuvant (Sigma, St Louis, MO, USA) and injected intraperitoneally into a mouse, and then the mouse received biweekly booster injections with ESPs (25 µg/mouse) containing equal volume of incomplete Freund’s adjuvant (Sigma Chemical Co., St. Louis, USA) for another 4 weeks. After third booster injection, ESPs (5 µg/mouse) without adjuvant were injected intravenously. Four days later, anti-ESP polyclonal sera were collected from mice blood by centrifugation at 12,000 rpm and 4°C for 10 min. An enzyme-linked immunosorbent assay (ELISA) was performed with ESPs (5 µg/mouse) and rabbit antimouse whole immunoglobulin (1:10,000 dilution) conjugated with alkaline phosphate (Sigma Chemical Co.). Western blot analysis against ESP protein was performed according to a method described in a previous paper (Laemli 1970). These sera were stored −20°C until use.
In vitro cytotoxicity of N. fowleri ESPs on CHO cells
CHO cells are useful in observing in vitro cytotoxicity of amoeba (Song et al. 2008). CHO cells were cultured as monolayer using 24-well culture flask (Nunc A/S, Roskilde, Denmark) in Earle’s minimal essential medium (EMEM; Gibco BRL, Gaithersburg, MD, USA) at 37°C. In control group, 3 × 104 CHO cells were cultured only in 500 µl of EMEM, 3 × 104 CHO cells cultured with various concentration N. fowleri ESPs in experimental groups. The total volume per well was 500 µl with EMEM. CHO cells were observed at cultured intervals using an inverted microscope. To measure in vitro cytotoxicity of ESPs, lactate dehydrogenase (LDH) released from lysed cells was assayed as previously described (Kim et al. 2008). For LDH assay, 50 µl of reacted supernatant in each well was transferred on 96-well assay plates (Nunc A/S, Roskilde, Denmark). After 50 µl of the reconstituted substrate mix, buffer in CytoTox96® Non-radioactive Cytotoxicity Assay Kit (Promega, Madison, WI, USA) for LDH release assay was added, the plate was incubated 30 min at room temperature, and then 50 µl of stop solution was added. The reactants were read at 490 nm with ELISA reader. The formula of in vitro cytotoxicity was as follows:
SDS–polyacrylamide gel electrophoresis and 2-DE
ESP samples were analyzed by 15% SDS-PAGE using reducing sample buffer (62.2 mM Tris pH 6.8; 10% glycerol; 10% 2-mercaptoethanol; 3% SDS and 0.1% bromophenol blue). ESP samples (100 µg) were applied for analytical run (pattern comparison). Rehydration, isoelectric focusing (IEF), and equilibration were performed as previously described (Lee et al. 2006). The ESP samples, containing 100 µg for 2-DE SYPRO Ruby-stained gels, were diluted in 125 µl of rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 50 mM DTT, 0.2% IPG buffer of the respective pH gradient and bromophenol blue). The samples were allowed to mix gently for 1 h at room temperature before centrifugation at 18,000×g over 30 min to remove all particulate material. The supernatants were applied to the IPG strips by in-gel rehydration at 20°C for at least 12 h, after which IEF was run for a total of 20,000 V/h. Following IEF, the strips were reduced in equilibration buffer (6 M urea, 0.05 M Tris pH 8.8, 2% SDS, and 20% glycerol) containing 2% DTT over 15 min and then alkylated in equilibration buffer containing 2.5% iodoacetamide for 10 min. The second dimension was performed on 4–20% gradient SDS-polyacrylamide gels (Amersham Biosciences, Uppsala, Sweden). Running proceeded at 80 V/gel for the first 15 min and then at 130 V/gel. After running, 2-DE gels were either stained or transferred onto polyvinylidene difluoride membrane (PVDF; Millipore, Bedford, MA, USA) for N-terminal sequencing and Western blot analysis.
Western blot analysis
ESP proteins were separated by SDS-PAGE and transferred to PVDF membrane for 2 h at 250 mA post 2-DE. Following transfer, the membranes were incubated in PBST containing 5% skimmed milk overnight at 4°C. After washing with 0.05% PBST three times, primary anti-ESP (1:500 dilution) or infection (1:500 dilution) sera were applied to the membranes overnight at 4°C. After washing with 0.05% PBST, the antimouse Ig G secondary antibody conjugated with alkaline phosphatase (Sigma Chemical Co.) was applied to the PVDF membrane at a dilution of 1:1,000 in PBS for 2 h. After washing with 0.05% PBST three times, the membranes were developed using AP conjugate Substrate Kit (Bio-Rad Laboratories, Hercules, CA, USA).
N-Terminal sequencing analysis and data searching
Selected ESP spots were requested for N-terminal sequencing to Korea Basic Science Institute (Seoul, Korea). Unidentified proteins separated by 2-DE PAGE were electrotransferred onto a PVDF membrane, and selected protein spots were excised and subjected to N-terminal sequencing using a Procise 492 cLC Model 610A Protein sequencer (Applied Biosystems, Hong Kong, China). Amino acid sequences obtained were searched against either the Protein DataBank (PDB) by BLAST. Settings for querying short sequences for nearly exact matches of peptides were used.
Results
In vitro cytotoxicity N. fowleri ESPs on CHO cells
To evaluate the cytotoxicity of N. fowleri ESPs on CHO target cells, LDH release assay was carried out. When CHO cells were treated with ESPs of N. fowleri, the cytotoxicity was gradually increased by a time- and dose-dependent manner (Fig. 1). It suggested that N. fowleri ESPs affected target cells as cytotoxic agents.
Protein profiles and Western blot analysis of N. fowleri ESPs
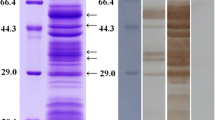
SDS-PAGE (15%) analysis of N. fowleri ESPs revealed different protein profiles patterns in comparison with N. fowleri lysate. The N. fowleri ESP bands corresponding to 91, 73, 60, 58, 49, 45, 40, 36, 28, 24, 20, 21, 18, and 16 kDa of molecular weight were observed as major proteins by Coomassie brilliant blue (Fig. 2a). To investigate the antigenicity of the N. fowleri ESPs, Western blot analysis was performed with anti-ESPs or infection sera. The results of Western blot analysis with anti-ESPs, 58, 48, 40, 30, 26, 24, 21, 18, and 16 kDa bands, showed immunodominance (Fig. 2b). In the Western blot analysis with infection sera, two major immunodominant bands, 24 and 18 kDa, were detected (Fig. 2c).
SDS-PAGE band patterns and Western blots of N. fowleri ESPs and lysates. a N. fowleri ESPs and lysates were electrophoresed under reducing conditions and stained with Coomassie blue. b, c, and d Western blots analysis of the N. fowleri ESPs with anti-ESP or infection sera and normal mouse sera for negative control. M molecular size marker, lane 1 N. fowleri ESPs, lane 2 N. fowleri lysates. Arrowheads indicate major antigenic ESPs
Protein profiles and Western blot analysis of N. fowleri ESPs post 2-DE
To select immunodominant proteins in the N. fowleri ESPs, 2-DE and Western blot analysis with ESPs sera or infection sera were carried out (Fig. 3). Most spots are localized in the low pI region (data not shown), so we separated the ESPs with low pI IEF strip (pI 4–7; Fig. 3a). In the results of Western blotting with the anti-ESPs sera, six antigenic spots, 58, 40, 30, 25, 24, 21, 18, 16, and 15 kDa, were immunodominant (Fig. 3b). In the case of the infection sera, two antigenic spots, 24 and 18 kDa, were immunodominant (Fig. 3c).
N-Terminal sequencing of candidate antigen-related proteins
Immunodominant 2 proteins (24 and 18 kDa), which commonly reacted with the two kinds of antibodies, and four proteins (58, 40, 21, and 16 kDa), which reacted only anti-ESPs sera, were requested for N-terminal sequencing (Fig. 4). As results of search with the PDB by BLAST, 58 and 40 kDa of protein were matched as secreted exendin-3 precursor protein and as secretory lipase. In turns, 24 and 21 kDa of protein were matched as cathepsin B-like protease and as cathepsin B. In addition, 18 and 16 kDa protein were matched as peroxiredoxins and as thrombin receptor (Table 1).
Discussion
To understand the pathogenesis of PAME induced by N. fowleri infection, the identification of pathogenecity-related agents of N. fowleri is important. Previous studies reported that in relation with pathogenicity, parasites secreted or excreted various proteins (Shin et al. 2001a). Parasites employ a variety of strategies to evade and/or modify host immune responses. ESPs released by parasitic helminths have been shown to both induce host antiparasite immune responses as well as modify the function of host immune cells (Lightowlers and Rickard 1988). In this present study, the distinct patterns of the ESPs were observed by 2-DE and Western blotting with anti-ESP or infection sera. Despite the absence of a N. fowleri genome sequencing project, this global investigation of the components from N. fowleri ESPs by a proteomic approach has been shown that it was possible to identify a number of the prominent proteins found using 2-DE.
The results revealed that N. fowleri ESPs contained various pathogenic proteins, which function in organism entering into host cell and various dominant antigenic proteins, such as secreted effector protein, cathepsin B-like protease, cysteine protease, secretory lipase, peroxiredoxins, and thrombin receptor.
Christel and DeNardo (2006) have recently demonstrated that release of exendin-4, an exendin-3 analog, is controlled by mechanical action in glia monsters, Heloderma suspectum, and suggest that exendin-4 is released from the salivary glands in response to mechanical stimulation and not detected in food either by smell, taste, or distention of gut. In this present study, 58 kDa secreted exendin-3 precursor protein of N. fowleri ESPs may play an important role in the digestion and absorption-related events of uptake molecules through food-cup structure, but further study is needed to elucidate the functional role of exendin-3 precursor protein. Both cathepsin B and cathepsin B-like protease are cysteine proteases, which are secreted into the hosts, and each of these has been proposed to facilitate degradation of ingested host proteins including hemoglobin (Brindley et al. 1997; Klinkert et al. 1989; Caffrey and Ruppel 1997; Weinbauer et al. 2001). Kong et al. (1994) showed that 27-kDa cysteine proteases of Spirometra mansoni has been found to be most important in tissue invasion and nutrient uptake, and its biochemical and structural nature has well been characterized. The cysteine proteases also modulate host immune response by cleaving immunoglobulins or by provoking IgE antibody responses. The roles and developmental modulation of cysteine proteases have been known in many parasites (Chung et al. 1997; Skuce et al. 1999). In this study, two cysteine proteases secreted by N. fowleri, 21 and 24 kDa of molecular weight, have been shown to be major enzymes in the N. fowleri ESPs. We suggest that the functional roles of the cysteine protease secreted by N. fowleri may be involved in an invasion to the blood–brain barrier of host.
Peroxiredoxins are ubiquitous peroxidases that reduce reactive oxygen and nitrogen species such as hydrogen peroxide, alkyl hydroperoxides, and peroxinitrate using an active-site cysteine residue. The reducing equivalents required for these reactions are provided by thioredoxin, glutaredoxin, alkylhydroperoxide reductase, glutathione, or cyclophilin. Donnelly et al. (2005) reported that thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Hence peroxiredoxins contained within the ESPs may be inhibited macrophages, which activated by a specific signal dependent pathway.
Bacterial lipases have been implicated in a variety of pathogenic processes, including the inhibition of phagocyte function (Aragon et al. 2002). Thrombin is concentrated at vascular injury sites within fibrin clots. Fibrin-binding thrombin is protected against inhibitors and gradually released into a wound area, in order to regulate tissue repair (Coughlin 1999). Thrombin interaction with cells is mediated by thrombin binding to a seven transmembrane domain, G-protein-coupled cell surface receptor, which belongs to the so-called protease activated receptors family (Cocks and Moffatt 2000). We suggest that the role of thrombin receptor in N. fowleri ESPs may be to activate inflammation, but the exact role of secreted thrombin receptor of N. fowleri would be elucidated with further studies.
In this study, we identified several candidate proteins, which should be related with pathogenicity of N. fowleri. These results suggest that antigenic proteases in N. fowleri ESPs may play a part in host cell invasion by N. fowleri and the cleaving ability of host immunoglobulins, which may contribute as one of the immune evasion mechanisms for parasite survival in the host. Peroxiredoxins, cysteine protease, and thrombin receptor secreted by N. fowleri have been implicated in host cell invasion and facilitated immunoresponse suppression of hosts. Finally, N. fowleri ESPs appear to play a vital role, with the proteolytic activity being preferentially expressed during the period in which the parasite actively invades or migrates through the tissue/organs of the host. Also the above several protein findings prompted us to observe what other composing proteins exist in N. fowleri ESPs and to confirm what other antigenic proteins are secreted and what functions exist in ESPs.
References
Aragon V, Rossier O, Cianciotto NP (2002) Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiol 148:2223–2231
Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JY, Smythe ML, McManus DP (1997) Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol 89:1–9
Bush LE, John DT (1988) Intranasal immunization of mice against Naegleria fowleri. J Protozool 35:172–176
Caffrey CR, Ruppel A (1997) Affinity isolation and characterization of the cathepsin B-like proteinase Sj31 from Schistosoma japonicum. J Parasitol 21:327–329
Christel CM, DeNardo DF (2006) Release of exendin-4 is controlled by mechanical action in Glia Monsters, Heloderma suspectum. Comp Biochem Physiol 143:85–88
Chung YB, Kong Y, Yang HJ, Kang SY, Cho SY (1997) Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol 83:902–907
Cocks TM, Moffatt JD (2000) Protease-activated receptors: sentries for inflammation? Trends Pharmacol Sci 21:103–108
Coughlin SR (1999) How the protease thrombin talks to cells. Proc Natl Acad Sci USA 96:11023–11027
Culbertson CG (1970) Pathogenic Naegleria and Hartmannella (Acanthamoeba). Ann N Y Acad Sci 74:1018–1022
Darcy F, Deslee D, Santoro F, Charif H, Auriault C, Decoster A, Duquesne V, Capron A (1988) Induction of a protective antibody-dependent response against toxoplasmosis by in vitro excreted/secreted antigens from tachyzoites of Toxoplasma gondii. Parasite Immunol 10:553–567
Donnelly S, O’Neill SM, Sekiya M, Mulcahy G, Dalton JP (2005) Thioredoxin peroxidase secretd by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun 73:166–173
Jeong SR, Kang SY, Lee SC, Song KJ, Im KI, Shin HJ (2004) Decreasing effect of an antiNfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri. Korean J Parasitol 42:35–40
Kim JH, Kim DS, Shin HJ (2008) Contact-independent cell death of human microglial cells due to pathogenic Naegleria fowleri trophozoites. Korean J Parasitol 46:217–221
Klinkert MQ, Felleisen R, Link G, Ruppel A, Beck E (1989) Primary structure of Sm31/32 diagnostic proteins of Schistosoma mansoni and their identification as proteases. Mol Biochem Parasitol 33:113–122
Kong Y, Chung YB, Cho SY, Kang SY (1994) Cleavage of immunoglobin G by excretory–secretory cathepsin S-like proteases of Spirometra mansoni plerocercoid. Parasitology 109:611–621
Laemli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee EG, Na BK, Bae YA, Je KSH, EY JuJW, Cho SH, Kim TS, Kang SY, Cho SY, Kong Y (2006) Identification of immunodominant excretory–secretory cysteine proteases of adult Paragonimus westermani by proteome analysis. Proteomics 6:1290–1300
Lightowlers MW, Rickard MD (1988) Excretory–secretory products of helminth parasites: effects on host immune responses. Parasitology 96:S123–S166
Ma P, Visvesvara GS, Martinez AJ, Thedore FH, Daggett PM, Sawyer TK (1990) Naegleria and Acanthamoeba infection: review. Rev Infect Dis 12:490–513
Naglik J, Albrecht A, Bader O, Hube B (2004) Candida albicans proteinases and host/pathogen interactions. Cell Microbiol 6:915–926
Shepherd JC, Aitken A, McManus DP (1991) A protein secreted in vivo by Echinococcus granulosus inhibits elastase activity and neutrophil chemotaxis. Mol Biochem Parasitol 44:81–90
Shin HJ, Cho MS, Jung SY, Kim HI, Park S, Kim HJ, Im KI (2001a) Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of Naegleria fowleri. J Euk Microbiol 48:713–717
Shin MH, Kita H, Park HY, Seoh JY (2001b) Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect Immun 69:1599–1604
Skuce PJ, Redmond DL, Liddell S, Stewart EM, Newlands GF, Smith WD, Knox DP (1999) Molecular cloning and characterization of gut-derived cysteine proteinases associated with a host protective extract from Haemonchus contortus. Parasitology 119:405–412
Song KJ, Song KH, Kim JH, Sohn HJ, Lee YJ, Park CE, Shin HJ (2008) Heat shock protein 70 of Naegleria fowleri is important factor for proliferation and in vitro cytotoxicity. Parasitol Res 103:313–317
Weinbauer GF, Aslam H, Krishnamurthy H, Brinkworth MH, Einspanier A, Hodges JK (2001) Quantitative analysis of spermatogenesis and apoptosis in the common marmoset (Callithrix jacchus) reveals high rates of spermatogonial turnover and high spermatogenic efficiency. Biol Reprod 64:120–126
Willaert E (1971) Isolation and in vitro culture of the amoeba of the genus Naegleria. Ann Soc Belg Med Trop 51:701–708
Acknowledgments
This work was supported by a grant from National Institute of Health, Korea Centers for Disease Control and Prevention (2007), and partially supported by the Korean Research Foundation Grant funded by the Korea Government (MOEHRD, Basic Research Promotion Fund; R03-2004-000-10003-0).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Kyoung-Ju Song and Ho-Joon Shin equally contributed to this work.
Rights and permissions
About this article
Cite this article
Kim, JH., Yang, AH., Sohn, HJ. et al. Immunodominant antigens in Naegleria fowleri excretory–secretory proteins were potential pathogenic factors. Parasitol Res 105, 1675–1681 (2009). https://doi.org/10.1007/s00436-009-1610-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1610-y