Abstract
Serodiagnosis of human strongyloidiasis is a practical alternative to parasitological methods due to its high sensitivity. However, cross-reactivity with other helminth infections limits its utility, and this problem is due to the use of homologous or heterologous somatic extracts of the parasite as an antigen source. Excretory-secretory (E/S) products from Strongyloides infective larvae can be used to improve the serodiagnosis. The combined use of western blot and proteomics became an interesting strategy to identify immunological markers for the serodiagnosis of strongyloidiasis. The present study describes the proteomic analysis of the antigenic components from E/S products of S. venezuelensis infective larvae that were recognized by IgG antibodies from patients with strongyloidiasis. Our results showed that IgG antibodies from patients with strongyloidiasis recognized between 15 and 16 antigenic bands in the E/S products from S. venezuelensis that were incubated in PBS or in RPMI culture medium, respectively. Overall, antigenic bands of low and high molecular weight were more specific than those of intermediate molecular weight, which were cross-reactive. A 36-kDa antigenic band was 93% sensitive and 100% specific (a probably arginine kinase of 37 kDa), while other antigenic bands were highly sensitive but low specific. Proteomic analysis revealed differences between the protein profiles from E/S-RPMI and E/S-PBS since only one-third of all proteins identified were common in both types of E/S products. Bioinformatic analysis showed that more than 50% of the proteins from E/S products are secreted within extracellular vesicles and only a small percentage of them are actually released by the classical secretory pathway. Several components from the E/S products were identified as plasminogen-binding proteins, probably used as an immune evasion mechanism. The data provided here provide valuable information to increase understanding of E/S products from S. venezuelensis infective larvae. This may help us to find new targets for the immunodiagnosis of human strongyloidiasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human strongyloidiasis is a parasitic disease caused predominantly by the nematode Strongyloides stercoralis with a worldwide distribution, especially in tropical and subtropical regions including Africa, Southeast Asia, and Latin America. Human strongyloidiasis is still considered a neglected tropical disease, and it is estimated that approximately 600 million individuals are infected worldwide (Nutman 2017; Buonfrate et al. 2020).
S. stercoralis infection begins when the infective third-stage filariform larvae (iL3) penetrate the host skin and migrate to reach the lungs and then the digestive tract, where they become parasitic female adults in the small intestine, producing eggs by parthenogenesis; these eggs develop into rhabditiform larvae that are excreted along with the feces into the environment where they develop either into iL3 forms (homogonic route) or into free-living adult stages (heterogonic route). These adult forms will mate to produce eggs which then develop into rhabditoid larvae and then into iL3. The possibility of host autoinfection exists when rhabditoid larvae are retained in the intestine and become iL3 while still in the human host. Therefore, massive re-penetration and larval migration may result in disseminated infection or hyperinfection syndrome (Nutman 2017).
Human Strongyloides infection is usually chronic, asymptomatic, and longstanding. However, the use of corticosteroids as part of immunosuppressive therapies after solid organ transplantation or previous history of HTLV-I infection can lead to hyperinfection syndrome with a mortality rate from 15 to 87% (Vasquez-Rios et al. 2019).
Definitive diagnosis of Strongyloides infection using conventional parasitological methods to detect larvae in feces may be difficult due to low or irregular parasitic load in the feces, resulting in high rates of false-negative results (Requena-Méndez et al. 2013; Vasquez-Rios et al. 2019). For these reasons, detection of antibodies by immunoserological tests was developed and used as complementary alternatives to parasitological diagnosis, and these include indirect immunofluorescence assay, enzyme-linked immunosorbent assay (ELISA), and western blot (WB) (Requena-Méndez et al. 2013).
Among them, ELISA is the most used and preferred test due to its high sensitivity and usefulness in seroepidemiological studies (Casado et al. 2019; Salvador et al. 2020). However, a critical limitation is the occurrence of cross-reactivity with other helminth infections, probably due to the use of somatic extracts of the parasite (Conway et al. 1993a; Requena-Méndez et al. 2013). On the other hand, WB has been used for the recognition of Strongyloides antigens by serum antibodies from patients with strongyloidiasis with high sensitivity and specificity (Sato et al. 1990; Conway et al. 1993b; Sudré et al. 2007). When the WB was used together with proteomic analysis became an important tool to identify potential immunological markers for the serodiagnosis of human strongyloidiasis (Rodpai et al. 2016, 2017; Corral et al. 2017).
Strongyloides iL3 release a wide range of excretory-secretory (E/S) products that play a fundamental role in the host-parasite interaction, including recognition, invasion, and immune evasion, making them main targets for activation of host immune response. Due to limitations in obtaining a continuous source of S. stercoralis iL3, other species such as S. venezuelensis offer an alternative source of E/S products to be applied to the serodiagnosis of human strongyloidiasis (Cunha et al. 2017; Roldán Gonzáles et al. 2021).
Recently, we have tested the usefulness of E/S products from S. venezuelensis for the detection of IgG antibodies in patients with strongyloidiasis by ELISA, obtaining high values of sensitivity and specificity, when compared with different somatic extracts of the parasite (Roldán Gonzáles et al. 2021). On the other hand, secretomes of S. ratti and S. venezuelensis were characterized from E/S products obtained in serum-free culture media, showing a wide variety of molecules involved in facilitating host invasion and migration, nutrient acquisition, antioxidant activity, and immune evasion or suppression, enabling their survival into the host (Soblik et al. 2011; Maeda et al. 2019). However, there are no studies related to the antigenic characterization of the secretome from S. venezuelensis to search for potential markers to improve the serodiagnosis of human strongyloidiasis.
The present study describes the proteomic analysis of the antigenic components of E/S products from S. venezuelensis iL3 that were recognized by serum IgG antibodies from patients with human strongyloidiasis.
Materials and methods
Patients and serum samples
A total of 260 serum samples were analyzed, including 71 patients with laboratory diagnosis of S. stercoralis infection (presence of larvae in stool samples) and 105 serum samples from apparently healthy individuals with negative results for parasites in stool samples and no history of Strongyloides infection. In order to determine the rate of cross-reactivity, 84 serum samples from individuals with other helminth infections were used (17 patients infected with Ascaris lumbricoides, 20 patients infected with Trichuris trichiura, 2 patients infected with Enterobius vermicularis, 7 patients infected with hookworms, 13 patients infected with Hymenolepis nana, one patient infected with adult Taenia solium, 11 patients with neurocysticercosis (larval stages of T. solium), 5 patients infected with Diphyllobothrium pacificum, 2 patients infected with Schistosoma mansoni, and 6 patients with Fasciola hepatica). Positive and negative control sera were made from a mixture of sera from a patient with intestinal strongyloidiasis and sera from healthy individuals, respectively.
Production of S. venezuelensis iL3
The strain of S. venezuelensis used in this study was maintained by serial passages in male Wistar rats (Rattus norvegicus) at the Instituto de Medicina Tropical de São Paulo, USP, São Paulo, Brazil (Comitê de ética no uso de animais da FMUSP, protocol N° 0356A). Male Wistar rats (30 days of age) were inoculated subcutaneously with approximately 30,000 iL3 in the abdominal region. Six days post-infection, the feces containing Strongyloides eggs were collected and moistened with tap water and then mixed with animal bone charcoal; the mixture was placed in wide glass jars and incubated at 28 ℃ for three days. After this procedure, iL3 were recovered according to the modified Baermann technique (Lok 2007), treated with sodium hypochlorite 0.25% for 5 min and then washed four times with sterile distilled water at 4000 × g for 1 min.
Preparation of E/S products
In our latest study, we have observed that S. venezuelensis iL3 is able to release antigenic proteins after incubation in 0.01 M phosphate buffer saline, pH 7.2 (PBS) (Roldán Gonzáles et al. 2021). Therefore, we decided to compare the antigenic profile of the E/S products from iL3 after incubation in RPMI 1640 medium and in PBS. Approximately 400,000 living iL3 larvae were placed in sterile polypropylene tubes containing either 5.0 mL of RPMI 1640 culture base medium (LGC Biotecnologia Ind., Cotia, SP, Brazil) or phosphate-buffered saline 0.01 M, pH 7·2 (PBS), both supplemented with a mixture antibiotics (Penicillin 100 U/mL, Streptomycin 100 U/mL, Gentamicin 50 µg/mL, and Amphotericin B 2.5 µg/mL), and incubated at 37 ℃ under 5% CO2 for 24 h to obtain E/S products. After incubation, the supernatants were collected and mixed with a cOmplete™ Protease Inhibitor Cocktail (Sigma-Aldrich Chemical Co., Saint Louis, MO, USA) and stored at − 20 ℃. All the supernatants were mixed and concentrated 100-fold using an Amicon YM10 membrane (EMD Millipore, Billerica, MA, USA) and then dialyzed against distilled water, centrifuged at 13,000 × g for 30 min at 4 ℃, filtered through a membrane of 0.22 µm (EMD Millipore, Billerica, MA, USA), and stored at − 20 ℃ until use. In those conditions, two types of antigenic preparations were obtained: E/S-RPMI and E/S-PBS, respectively. The protein content was determined by using the Bio-Rad DC protein assay (Bio-Rad Laboratories Inc., Hercules, CA, USA).
SDS–polyacrylamide gel electrophoresis and electroblotting of E/S products
E/S products (E/S-RPMI or E/S-PBS) were resolved according to their molecular weights by vertical SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Briefly, the E/S products were treated with a 5 × sample SDS-PAGE buffer (0.01 M Tris–HCl, pH 6.8, 1% SDS, 6% urea, 3.2% 2-mercaptoetanol, 10% glycerol, 0.005% bromophenol blue), heat-denatured at 75 ℃ for 15 min, and loaded on a 12% SDS–polyacrylamide gel at a ratio of 2.5 µg of protein per millimeter of gel. Electrophoresis was performed in a Mini-Protean II apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA) at 80 V for 10 min and then at 150 V for 140 min. Precision Plus Protein™ All Blue Standards (Bio-Rad Laboratories Inc., Hercules, CA, USA) were used as electrophoresis running control. The apparent molecular weight (MW) of each component from E/S products was calculated following the method described by Matsumoto et al. (2019).
SDS–polyacrylamide gel containing separated E/S products were stained with a silver staining protocol compatible with mass spectrometry described by Shevchenko et al. (1996) or electro-transferred to 0.45 μm pore size, 7.5 cm × 8 cm nitrocellulose (NC) sheets in a Mini Trans-blot apparatus II (Bio-Rad Laboratories Inc.) at constant 100 V for 120 min at 4 ℃. The NC sheets were washed for 30 min with PBS containing 0.1% Tween-20 (PBS-T) at room temperature (RT), cut into 3.5-mm-wide strips, and then stored at − 20 ℃ until use.
Detection of human IgG antibodies against Strongyloides E/S products by western blot
NC strips were blocked with 5% nonfat milk diluted in PBS-T for 1 h at RT and then incubated with serum samples diluted 1:100 in blocking solution overnight at 4 ℃ in constant agitation. NC strips were washed three times for 5 min with PBS-T and then incubated with peroxidase-conjugated goat anti-human IgG (Fc specific) antibodies (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) diluted 1:2000 in blocking solution for 2 h at RT. After three washes with PBS-T as described above, NC strips were incubated with a chromogenic-substrate solution of 3,3′-diaminobenzidine containing cobalt salts (SIGMAFAST™ DAB with Metal Enhancer, Sigma-Aldrich Co. St. Louis, MO, USA) for 2 min at RT, and the enzymatic reaction was stopped by rinsing four times with tap water. A positive reaction on the NC strips was determined by visualizing defined black brown bands. All stages of this procedure were carried out under constant agitation using a volume of 800 µL for all reagents, and positive and negative control sera were used as reaction controls in all assays.
Proteomic analysis of antigenic bands by mass spectrometry
Antigenic bands that showed strong reactivity with sera from infected patients were located on silver-stained polyacrylamide gels and sliced into small fragments with the aid of a disposable scalpel and then deposited in 1.5 mL plastic tubes. The gel fragments were washed for 15 min in ultrapure water and then incubated in a silver destaining solution (equal parts of 30 mM potassium ferricyanide and 100 mM sodium thiosulfate pentahydrate) until the stain disappears and then washed for 15 min in ultrapure water. The gel fragments were washed three times with 50 mM ammonium bicarbonate buffer containing 40% acetonitrile (ACN) for 30 min; the gel fragments were dehydrated with 100% ACN for 15 min. The gel fragments were reduced with 10 mM dithiothreitol at 37 ℃ for 30 min and then alkylated with 55 mM iodoacetamide for 30 min in the dark. The gel fragments were dehydrated with 100% acetonitrile, and the remaining ACN was evaporated by vacuum centrifugation. Dehydrated gel fragments were hydrated for 45 min in sequencing grade trypsin solution (300 ng per fraction; Sigma-Aldrich Co. St. Louis, MO, USA) on ice. The hydrated gel pieces were covered with additional 50 mM ammonium bicarbonate and incubated overnight at 37 ℃. Following incubation, the enzymatic digestion was stopped by adding 10% formic acid and all the supernatants containing the tryptic peptides were transferred into new 1.5 ml plastic tubes. The gel pieces were then extracted three times with 50 mM ammonium bicarbonate containing 40% acetonitrile and then pooled with their respective supernatant. The extracted solutions were evaporated by vacuum centrifugation and then dissolved in 2% ACN prior to analysis.
All the peptide suspensions were analyzed by liquid chromatography-mass spectrometry in an ESI-IT-TOF instrument coupled to a UPLC 20A Prominence (Shimadzu, Kyoto, Japan). Samples (15 µL aliquots) were loaded into a C18 column (Kinetex C18, 5 μm; 50 × 2.1 mm) and fractionated by a binary gradient employing as solvents 0.1% formic acid (A) and ACN containing 0.1% formic acid (B). An elution gradient of 0–45% B was applied for 120 min at a constant flow of 0.2 mL/min after initial isocratic elution for 5 min. The eluates were monitored by a Shimadzu SPD-M20A PDA detector before being injected into the mass spectrometer. Raw LCD LCMS solution Shimadzu data were converted into an mzXML file by the LCM Solution (PRIDE) tool and then loaded into Peaks Studio V 7.0 (BSI, Canada). The false discovery rate was adjusted to ≤ 0.5%, and only proteins with a peptide confidence score (− 10lgP) ≥ 20 and containing at least 1 unique peptide were considered in this study. For more details, see https://www.bioinfor.com/wp-content/uploads/2016/12/PEAKS7Manual.pdf.
Bioinformatic analysis of the identified proteins
Proteins were identified by Peaks Studio V 7.0 (BSI, Canada) using the UniProtKB database (Taxid: 75,913 – Strongyloides venezuelensis) and then were categorized according to their predicted molecular function, biological process, and cellular component using both the UniProtKB database (http://www.uniprot.org/) and QuickGO (http://www.ebi.ac.uk/QuickGO/).
SignalP 5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0) was used to predict the presence of an N-terminal signal peptide typical from the classical secretory pathway and TMHMM 2.0 (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0) to predict the presence of transmembrane helices in the first 60 amino acids.
OutCyte 1.0 (http://www.outcyte.com) was used to predict potential unconventional pathways of protein secretion (UPS) and check the presence of transmembrane helices. This tool was chosen because it showed improved performance when compared to the well-known SecretomeP 2.0 (Zhao et al. 2019).
ExoPred web server (http://imath.med.ucm.es/exopred/) was used to predict potential protein secretion within exosomes. Proteins that had neither signal peptide, UPS, nor evidence of being secreted within exosomes were considered intracellular or non-secreted proteins.
NetNGlyc 1.0 (https://services.healthtech.dtu.dk/service.php?NetNGlyc-1.0) and NetOGlyc 4.0 (https://services.healthtech.dtu.dk/service.php?NetOGlyc-4.0) were used for N-glycosylation and O-glycosylation site predictions, respectively.
The antigenic density of each identified protein was estimated by means of its abundance of antigenic regions (AAR) value using the Secret-AAR tool (http://microbiomics.ibt.unam.mx/tools/aar/toolaar.php). The AAR value is the ratio between the sequence lengths to the number of predicted antigenic regions (Cornejo-Granados et al. 2019). Proteins with an AAR value ≤ 50 were considered potentially antigenic.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 5.0 program. Sensitivity, specificity, and predictive values for WB were calculated using 2 × 2 tables with a confidence interval of 95%. To compare the degree of concordance between the two tests, the kappa index (k) was used. Differences were considered statistically significant at a level of 5%.
Results
Western blot analysis
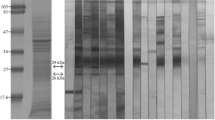
Sixteen antigenic bands were recognized in E/S-RPMI by the positive control serum and were then grouped into low-molecular-weight bands (LMW: 30, 32, 36, 39, 41, 45, 47), intermediate-molecular-weight bands (IMW: 55, 60, 66, 71, 89), and high-molecular-weight bands (HMW: 94 kDa, 105–115 kDa, 179 kDa, 224 kDa). With the exception of the 179-kDa antigenic band, all the same bands were recognized in ES-PBS, although there was a weaker reactivity in some antigenic bands from the LMW group (30 kDa, 41 kDa, 45 kDa, and 47 kDa) (Table 1 and Fig. 1a).
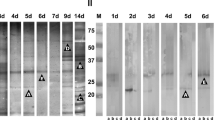
Antigenic components from S. venezuelensis iL3 E/S products (E/S-RPMI and E/S-PBS), obtained by Western blot. (a) Using a positive control serum (pool of sera from patients with strongyloidiasis) and as control of reactivity, negative control serum (pool of sera from healthy individuals). The high molecular weight fraction (HMW), intermediate molecular weight fraction (IMW), and low molecular weight fraction (LMW) were shown. (b) Western blot results showing the cross-reactivity of E/S products from S. venezuelensis iL3 obtained in RPMI medium (E/S-RPMI) with a representative panel of serum samples from individuals with other helminthiases, including infections caused by A. lumbricoides (Asl), T. trichiura (Trt), hookworms (Hkw), H. nana (Hym), neurocysticercosis (Ncy), D. pacificum (Dip), S. mansoni (Sch), and F. hepatica (Fas). Control positive ( +) and negative ( −) sera were included as a comparison
Sera from patients with strongyloidiasis recognized the 16 antigenic bands from E/S-RPMI and the 15 antigenic bands from E/S-PBS, showing a predominant reactivity with bands of 47 kDa (97.2%), 36 kDa (92.9%), 224 kDa (83.1%), 55 kDa (80.3%), 60 kDa and 179 kDa (76.1%), and 41 kDa and 105–115 kDa (73.2%). On the other hand, sera from healthy individuals recognized the bands of 60 kDa (72.4%) and 55 kDa (18.1%) (Table 1).
Sera from patients with other helminthiasis recognized bands of 60 kDa (42.9%), 55 kDa (33.3%), and 66 kDa (21.4%), followed by bands of 47 kDa and 105–115 kDa (11.9%), and the band of 224 kDa (10.7%). There was an unexpected recognition of two bands of 15 kDa (8.3%) and 136 kDa (4.8%) in both types of E/S products that were not recognized by the sera from strongyloidiasis patients. Bands of 36 kDa, 71 kDa, and 94 kDa were not recognized by this group of sera (Table 1).
Patients infected with A. lumbricoides recognized the bands of 55 kDa (76.5%), 60 kDa (64.7%), and 66 kDa (52.9%); patients infected with T. trichiura recognized the bands of 60 kDa (35%), but also with an unexpected band of 136 kDa (20%); patients infected with E. vermicularis recognized the band of 60 kDa (50%); patients infected with hookworms recognized the band of 60 kDa (28.6%), but also to a lesser extent with the bands of 55 kDa and 224 kDa (14.3%); patients infected with H. nana recognized the bands of 32 kDa, 39 kDa, 55 kDa, and 60 kDa (30.8%), 41 kDa and 45 kDa (15.4%), and 30 kDa (7.7%); patients with neurocysticercosis recognized the bands of 55 kDa and 66 kDa (54.5%), but also with the bands of 89 kDa, 105–115 kDa, and 179 kDa (45.5%). An unexpected band of 15 kDa was also recognized by 54.5% of these patients, as well as the only patient infected with T. solium. Patients infected with D. pacificum recognized predominantly the band of 60 kDa (80%), followed by the bands of 66 kDa and 89 kDa (60%). All the patients infected with S. mansoni recognized the bands of 60 kDa and 224 kDa, and half of them recognized the bands of 47 kDa and 55 kDa; all the patients infected with F. hepatica recognized the band of 224 kDa, and half of them recognized the bands of 32 kDa, 60 kDa, and the wide band of 105–115 kDa (Fig. 1b). A perfect concordance was observed in both types of E/S products for the detection of IgG antibodies in the three groups of sera (kappa index = 1).
Based on the WB results, three antigenic fractions named LMW (from 30 to 47 kDa), IMW (from 55 to 89 kDa), and HMW (from 94 to 224 kDa) from both E/S products were chosen to be analyzed by mass spectrometry (Fig. 1a). Tables 2 and 3 show all the proteins identified in the three selected fractions from E/S-RPMI and E/S-PBS, respectively.
Mass spectrometry and bioinformatic analysis from the selected antigenic fractions of E/S-RPMI
A total of 44 proteins were identified by mass spectrometry in the three selected fractions from E/S-RPMI: 8 proteins (18.2%) had a signal peptide to be released through the classical secretory pathway, 24 proteins (54.5%) had evidence of being secreted within exosomes, and 12 proteins (27.3%) were considered intracellular. Twenty-eight proteins (63.6%) were predicted to be O-glycosylated (12 proteins in fraction HMW, 6 proteins in fraction IMW, and 10 proteins in fraction LMW), and 6 proteins (13.6%) were predicted to be N-glycosylated (5 proteins in fraction HMW and 1 protein in fraction LMW). There were 4 proteins with both types of glycosylation (3 proteins in fraction HMW and 1 protein in fraction LMW). With regards to predicted antigenicity, a total of 28 proteins (63.6%) had AAR ≤ 50 and were considered potentially antigenic (Table 2).
Gene ontology analysis classified the identified proteins according to the cellular component, biological process, and molecular function in a total of 40 categories. In terms of cellular components, these proteins were mainly related to cytoplasm (38.6%), cell membrane (22.7%), extracellular region (13.6%), and nucleus (9.1%). In terms of biological processes, these proteins were involved mainly in protein transport (11.4%), larval development (9.1%), biosynthetic process (9.1%), oxidative stress (9.1%), and carbohydrate metabolic process (6.8%), among others. In terms of molecular function, these proteins were involved mainly in metal ion binding (22.7%), ATP binding (18.2%), protein binding (13.6%), nucleic acid binding (11.4%), transferase activity (11.4%), kinase activity (9.1%), and peptidase activity (9.1%), among others (Fig. 2, Supplementary Table 4).
Gene ontology analysis of the identified proteins from S. venezuelensis iL3 E/S products obtained following 24-h incubation in RPMI medium (E/S-RPMI) or in PBS (E/S-PBS). The proteins were grouped into cellular component (green bars), biological process (blue bars), and molecular function (red bars) in accordance with their GO signatures
In fraction HMW, a total of 16 proteins were identified by mass spectrometry (5 proteins had a signal peptide, 7 proteins were secreted within exosomes, and 4 proteins were considered intracellular) and 10 of these proteins were considered potentially antigenic (AAR ≤ 50). Among these proteins that can be highlighted because their potential antigenicity and their similar MW with the WB results are Degenerin unc-8 (94.56 kDa; pI = 6.05), two peroxidasin-like proteins of 107.66 kDa (pI = 7.04) and 114.6 kDa (pI = 9.64), calsyntenin-1 (111.15 kDa; pI = 4.99), and two uncharacterized proteins of 169.075 kDa (pI = 6.47) and 220.3 kDa (pI = 5.03).
In fraction IMW, a total of 9 proteins were identified (6 proteins were secreted within exosomes and 3 proteins were intracellular) and 6 of these proteins were considered potentially antigenic (AAR ≤ 50). Among these proteins that can be highlighted because their potential antigenicity and their similar MW with the WB results are actin, alpha cardiac muscle 1 (55.68 kDa; pI = 6), phosphoglycerate mutase (57.56 kDa; pI = 5.89), glucose-6-phosphate isomerase (62.19 kDa; pI = 6.52), phosphoenolpyruvate carboxykinase (73.94 kDa; pI = 6.03), and calpain-2 catalytic subunit (89.93 kDa; pI = 4.87).
In fraction LMW, a total of 19 proteins were identified (2 proteins had a signal peptide, 11 proteins were secreted within exosomes, and 6 were considered intracellular) and 12 of these proteins were considered potentially antigenic (AAR ≤ 50). Among these proteins that can be highlighted because their potential antigenicity and their similarity with the WB results are two CAP domain-containing proteins of 30.5 kDa (pI = 9.79) and 40.67 kDa (pI = 10.33), 14–3-3 protein zeta (33.74 kDa; pI = 4.81), arginine kinase (37.04 kDa; pI = 7.57), adenylate kinase isoenzyme 1 (38.7 kDa; pI = 7.51), fructose-bisphosphate aldolase (39.49 kDa; pI = 6.32), malate dehydrogenase (40.96; pI = 8.91), two isoforms of actin, alpha cardiac muscle 1 of 41.79 kDa and 41.8 kDa (pI = 5.3), uncharacterized protein of 44.3 kDa (pI of 7.43), isocitrate dehydrogenase (46.015 kDa; pI = 6.04), and 2-phospho-D-glycerate hydro-lyase (47.17 kDa; pI = 6.04).
Mass spectrometry and bioinformatic analysis from the selected antigenic fractions of E/S-PBS
A total of 44 proteins were also identified by mass spectrometry in the three selected fractions from the E/S-PBS: 7 proteins (15.9%) had a signal peptide to be released through the classical secretory pathway, 29 proteins (65.9%) had evidence of being secreted within exosomes, and 8 proteins (18.2%) were considered intracellular. Thirty proteins (68.2%) were predicted to be O-glycosylated (10 proteins in fraction HMW, 13 proteins in fraction IMW, and 7 proteins in fraction LMW), and 8 proteins (18.2%) were predicted to be N-glycosylated (2 proteins in fraction HMW, 3 proteins in fraction IMW, and 3 proteins in fraction LMW). There were 7 proteins with both types of glycosylation (1 protein in fraction HMW, 3 proteins in fraction IMW, and 3 proteins in fraction LMW). With regards to potential antigenicity, a total of 30 proteins (68.2%) had AAR ≤ 50 and were considered potentially antigenic (Table 3).
Gene ontology analysis classified the identified proteins according to the cellular component, biological process, and molecular function in a total of 31 categories. In terms of cellular components, these proteins were mainly related to cytoplasm (31.8%), nucleus (20.4%), cell membrane (13.6%), and cytoskeleton (13.6%). In terms of biological processes, the proteins were involved mainly in the catabolic process (15.9%), biosynthetic process (15.9%), phosphorylation (13.6%), carbohydrate metabolic process (6.8%), proteolysis (6.8%), apoptotic process (6.8%), and larval development (4.5%), among others. In terms of molecular function, the proteins were involved mainly in ATP binding (25%), kinase activity (22.8%), metal ion binding (20.5%), protein binding (18.2%), transferase activity (6.8%), peptidase activity (6.8%), and dehydrogenase activity (6.8%), among others (Fig. 2, Supplementary Table 5).
In fraction HMW, a total of 11 proteins were identified by mass spectrometry (2 proteins had a signal peptide, 4 proteins were secreted within exosomes, and 5 proteins were considered intracellular) and 7 of these proteins (AAR ≤ 50) were considered potentially antigenic. Among these proteins that can be highlighted because their potential antigenicity and their similar MW with the WB results are an uncharacterized protein of 102.4 kDa (pI = 6.04), calsyntenin-1 (111.15 kDa; pI = 4.99), protein transport protein sec16 (204.68 kDa; pI = 5.17), and non-specific serine/threonine protein kinase (208.13 kDa; pI = 8.83).
In fraction IMW, a total of 15 proteins were identified (2 proteins had a signal peptide, 12 proteins were secreted within exosomes, and 5 proteins were considered intracellular) and 12 of these proteins (AAR ≤ 50) were considered potentially antigenic. Among these proteins that can be highlighted because their potential antigenicity and their similar MW with the WB results are retinal dehydrogenase 2 (55.11 kDa; pI = 5.78), alanine aminotransferase 1 (55.57 kDa; pI = 8.35), actin, alpha cardiac muscle 1 (55.67 kDa; pI = 6), phosphoglycerate mutase (57.56 kDa; pI = 5.89), glucose-6-phosphate isomerase (62.19 kDa; pI = 6.52), uncharacterized proteins of 66.2 kDa (pI = 6.88), septin-2 (67.28 kDa; pI = 8.68), malic enzyme (71.99 kDa; pI = 8.91), phosphoenolpyruvate carboxykinase (73.94 kDa; pI = 6.03), and neuroglian (77.24 kDa; pI = 8.04).
In fraction LMW, a total of 18 proteins were identified (3 proteins had a signal peptide, 13 proteins were secreted within exosomes, and 2 proteins were considered intracellular) and 11 of these proteins (AAR ≤ 50) were considered potentially antigenic. Among these proteins that can be highlighted because their potential antigenicity and their similar MW with the WB results are peptidase S1 domain-containing protein (30.56 kDa; pI = 8.6), 14–3-3 protein zeta (33.74 kDa; pI = 4.81), an uncharacterized protein of 33.83 kDa (pI = 6.19), two isoforms of arginine kinase of 37.04 kDa (pI = 7.57) and 40.54 kDa (pI = 8.37), two isoforms of fructose-bisphosphate aldolase of 39.5 kDa (pI = 6.32 and 7.98), malate dehydrogenase (40.96 kDa; pI = 8.91), two isoforms of actin, alpha cardiac muscle 1 of 41.79 kDa and 41.81 kDa (pI = 5.3), and 2-phospho-D-glycerate hydro-lyase (47.17 kDa; pI = 6.04).
Fourteen proteins were in common in both types of E/S products (1 protein with signal peptide, 11 proteins secreted within exosomes, and 2 intracellular proteins), of which 12 proteins were considered potentially antigenic (AAR ≤ 50) (Tables 2 and 3).
Discussion
Our results showed that several components of E/S products from S. venezuelensis iL3 were recognized by serum IgG antibodies from patients with human strongyloidiasis, but also by sera from individuals with other helminth infections and healthy individuals. In general, the antigenic bands of the LMW and HMW fractions were more specific than those of the IMW fraction, which had cross-reactivity and were therefore unspecific.
Brindley et al. (1988) were the first to characterize the E/S products from S. stercoralis iL3 using SDS-PAGE and immunoprecipitation, showing antigenic bands of 240 kDa, 90 kDa, 66 kDa, 60 kDa, 54 kDa, 50 kDa, 40 kDa, 35 kDa, 33 kDa, 30 kDa, 25 kDa, and 12 kDa. It is interesting to note that most of the antigenic bands reported by them agreed with the MW of the antigenic bands from E/S products of S. venezuelensis iL3, in particular those of the LMW and IMW groups.
Among the different components of the LMW group, the antigenic band of apparent MW of 36 kDa was 93% sensitive and 100% specific and without evidence of cross-reactivity. Interestingly, this antigenic band matches with an arginine kinase of 37 kDa (Uniprot A0A0K0EZR7), which lacks glycosylation sites and possesses a high antigenic potential (AAR = 38.9), as determined following the bioinformatic analysis. Furthermore, there is no other identified protein that has a similar molecular weight. Therefore, it is very likely that the 36-kDa antigenic band is indeed an arginine kinase, as it was present in both types of E/S products, according to the mass spectrometry results.
Arginine kinase belongs to the family of phosphagen kinases that are involved in energy homeostasis in a wide range of invertebrate species. Among helminths, only nematodes possess arginine kinase whereas cestodes and trematodes do not have this enzyme, although other types of phosphotransferases may be present (Barrett 2009). In the present study, up to three arginine kinase isoforms were identified in the E/S products of S. venezuelensis iL3 (Tables 2 and 3).
Some authors have evaluated the diagnostic potential of a recombinant arginine kinase from Toxocara canis as a marker of human toxocariasis, showing 100% sensitivity and minimal cross-reactivity with Echinococcus granulosus, Toxoplasma gondii, and Entamoeba histolytica. (Varghese et al. 2017). However, there are no studies so far on the diagnostic potential of this enzyme for the serodiagnosis of human strongyloidiasis and this arginine kinase of 37 kDa could be a candidate antigen for future research.
The antigenic band of 47 kDa was 97% sensitive but cross-reactive with sera from patients infected with A. lumbricoides and H. nana. Indeed, around 30% of sera from patients infected with H. nana reacted with most components of the LMW group. This antigenic band matches with a 2-phospho-D-glycerate hydro-lyase or enolase (Uniprot A0A0K0FKU3; MW = 47.17 kDa) which lacks glycosylation sites, is potentially antigenic (AAR = 31.1), and is identified in both types of E/S products. Enolase is a metabolic enzyme that belongs to the pathway glycolysis and is involved in the synthesis of pyruvate. Despite long being known as a cytosolic protein, enolase has been found on the surface of a variety of pathogenic bacteria, fungi, and parasites acting as the main plasminogen-binding protein and possesses a high diagnostic potential (Gao et al. 2016; Ayón-Núñez et al. 2018; Ponce et al. 2018).
The antigenic band of 60 kDa (IMW group) was the most cross-reactive and unspecific of all antigenic bands of the E/S products from S. venezuelensis iL3, reacting with 43% of sera from individuals infected with other helminth infections, but also with 72% of sera from healthy individuals. This antigenic band matched with the Glucose-6-phosphate isomerase (Uniprot A0A0K0G292; MW = 62.2 kDa) which lacks glycosylation sites, is potentially antigenic (AAR = 39.57), and is identified in both types of E/S products. This metabolic enzyme has been found both on the surface and in the E/S products from different helminths and is involved in parasite development and/or in modulating the host-parasite relationship (Stadelmann et al. 2010; Diosdado et al. 2020).
Another cross-reactive and unspecific component from the IMW group was the antigenic band of 55 kDa, which matched with an Actin isoform of 55.7 kDa (Uniprot ID: A0A0K0F6C3) which lacks glycosylation sites, is potentially antigenic (AAR = 36.14), and is identified in both types of E/S products. Actin is a structural protein involved in many important cellular functions related to motility, division, shape, and signaling and also seems to be acting as a plasminogen-binding protein (González-Miguel et al. 2016).
Regarding the protein components of the HMW group, the antigenic band of 224 kDa was highly sensitive and did not react with sera from healthy individuals. However, there was cross-reactivity with sera from patients infected with trematodes. This antigenic band could be a non-specific serine/threonine protein kinase (Uniprot ID: A0A0K0EXN8; MW = 208 kDa) or an uncharacterized protein of 220 kDa (Uniprot ID: A0A0K0F3W6); both highly O-glycosylated and potentially antigenic (AAR of 39.2 and 47.4, respectively). Likewise, the antigenic bands of 105–115 kDa and of 179 kDa also were sensitive and without reactivity in sera from healthy individuals, but were cross-reactive mainly with sera from patients with neurocysticercosis. Among the identified proteins that may match the antigenic band of 105–115 kDa are two Peroxidasin-like proteins of 107 kDa (Uniprot ID: A0A0K0FL11) and 115 kDa (Uniprot ID: A0A0K0F465) and the Calsyntenin-1 (Uniprot ID: A0A0K0F0E3; MW = 111 kDa; AAR = 40.1) which was identified in both types of E/S products. With regards to the antigenic band of 179 kDa, an uncharacterized protein of 169 kDa (Uniprot ID: A0A0K0FZN1; AAR = 40.1), which are highly O-glycosylated, may match this antigenic band. It is important to mention that the predicted MW of several identified proteins may vary since 68.2% and 70.4% of the proteins from E/S-RPMI and E/S-PBS, respectively, are glycosylated.
The two types of E/S products, obtained after incubation of iL3 in RPMI medium or in PBS, gave apparently similar antigenic profiles according to the WB results. However, proteomic analysis revealed that there were considerable differences between the protein profiles of E/S-RPMI and E/S-PBS since only one-third of all proteins identified by mass spectrometry were common in both types of E/S products. A possible explanation for this phenomenon is that the RPMI medium (rich in amino acids, glucose, vitamins, and minerals) may mimic better the host environment at 37 ℃ compared to PBS, which is a simple buffer solution that just mimics the physiological host pH. Using similar incubation conditions at 37 ℃, Maeda et al. (2019) observed a high mortality rate of S. venezuelensis iL3 and low yield in the production of E/S products after 12 h of incubation in PBS, while Dulbecco’s modified Eagle’s medium (DMEM) was able to stimulate much more the production of E/S products. Brindley et al. (1988) observed that S. stercoralis iL3 was able to incorporate amino acids or glucose from DMEM at 33 ℃, demonstrating that the parasite could be metabolically active “in vitro.” Similarly, Tsuji et al. (1997) also observed that S. venezuelensis iL3 was able to incorporate [35S]-methionine from the medium and synthetize proteins as a result of the temperature change during the “in vitro” transformation from free-living to the parasitic stage. These environmental differences could alter the protein profile that S. venezuelensis iL3 secretes “in vitro.”
Host manipulation by helminth parasites is primarily due to the release of E/S products which are known to have immunomodulatory properties such as suppression of allergic responses, modulation of antigen-presenting cells, and induction of regulatory T-cell responses (Maizels et al. 2018). However, there is increasing evidence that helminths release a wide variety of proteins and microRNAs within extracellular vesicles, probably as a strategy to shape their immediate environment and modulate the host immunity (Drurey and Maizels 2021). Our results suggest that more than 50% of the proteins identified in the antigenic fractions of both types of E/S products from S. venezuelensis iL3 could be secreted within extracellular vesicles and only a small percentage of them are actually released by the classical secretory pathway. Marcilla et al. (2012) reported that more than 50% of the proteins from the secretomes of Echinostoma caproni and Fasciola hepatica are actually secreted within extracellular vesicles.
Proteins identified in the antigenic fractions of both types of E/S products from S. venezuelensis iL3 include metabolic and detoxifying enzymes, peptidases, proteins related to biosynthetic and signaling processes, cytoskeletal and nuclear proteins, calcium ion-binding proteins, and many of them were also identified in extracellular vesicles from other helminths such as Ascaris suum (Hansen et al. 2019), Trichuris muris (Tritten et al. 2017), Fasciola hepatica (Marcilla et al. 2012), and Taenia pisciformis (Wang et al. 2020), among others. The release of proteins to the host environment within extracellular vesicles by S. venezuelensis iL3 could explain the unusual presence of all these mentioned proteins.
Several studies have shown that metabolic enzymes from many species of parasitic protozoa and helminths can also be present extracellularly, being attached to the surface or being secreted within extracellular vesicles. At this extracellular level, these metabolic enzymes can also perform other additional functions, such as acting as ligands for a variety of components of the host. This binding would allow the parasites to participate in various important interactions such as adherence and invasion, modulation of host hemostatic and immune response, promoting angiogenesis, or acquiring molecules for nutrition (Gómez-Arreaza et al. 2014). In the present study, various metabolic enzymes including Phosphoenolpyruvate carboxykinase, Glucose-6-phosphate isomerase, Phosphoglycerate mutase, Enolase, Malate dehydrogenase, Fructose-bisphosphate aldolase, Adenylate kinase, and L-lactate dehydrogenase were found in both types of E/S products from S. venezuelensis iL3 and were previously reported as plasminogen-binding proteins (Gómez-Arreaza et al. 2014; González-Miguel et al. 2016; Ayón-Núñez et al. 2018; Diosdado et al. 2020). Likewise, other proteins such as actin, galectin, and 60S acidic ribosomal protein found in E/S products from S. venezuelensis iL3 have also been reported as plasminogen-binding proteins (Ayón-Núñez et al. 2018; Diosdado et al. 2020).
These findings show that S. venezuelensis iL3 could be manipulating the host fibrinolytic system through the recruitment of plasminogen (in a lysine-dependent manner) and its subsequent activation to plasmin by the host plasminogen activators as a survival mechanism, facilitating its migration through the different organs and host tissues.
An interesting group of proteins found in the LMW fraction of E/S-RPMI but not in E/S-PBS are two CAP domain (cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins) proteins, of which one of them (A0A0K0FXA8; MW = 30.5 kDa; pI = 9.79) possesses 50% identity with the recombinant protein antigen NIE derived from S. stercoralis iL3 which is being used as a potential marker for the serodiagnosis of human strongyloidiasis. Maeda et al. (2019) have reported the presence of seven CAP domain proteins in E/S products from S. venezuelensis iL3, which includes the abovementioned protein. However, another CAP domain protein of 40.67 kDa (A0A0K0G1T0) was not reported by them and therefore constitutes the first report. CAP domain proteins are also known as sperm-coating protein/Tpx/antigen 5/pathogenesis-related-1/Sc7 (SCP/TAPS) and share a common domain which adopts a unique α-β-α sandwich fold; these types of proteins have been reported in bacteria, plants, animals, and viruses and have been implicated in a variety of physiological contexts. CAP proteins were found in E/S products from hookworms and other helminths and are named as activation-associated secreted proteins (ASPs) or venom allergen-like proteins (VALs or VAPs). Although their molecular function is still somewhat enigmatic, recent studies have shown their participation as transporters of sterols or scavengers of leukotrienes. (Wilbers et al. 2018).
Another protein that deserves special attention is the 14–3-3 zeta protein (Uniprot ID: A0A0K0FYU3; MW = 33.7 kDa), which was identified in both types of E/S products from S. venezuelensis iL3. 14–3-3 proteins have been found within extracellular vesicles from several helminth parasites (Marcilla et al. 2012; Wang et al. 2020) and were able to bind the host mononuclear cell surface with modulatory effects on cytokine production, cell proliferation, and migration (Gadahi et al. 2016; Tian et al. 2018). A recombinant form of S. stercoralis 14–3-3 protein was produced and evaluated as a potential candidate for the serodiagnosis of human strongyloidiasis, showing 96% sensitivity and 93.8% specificity (Masoori et al. 2019).
Perhaps the main limitation of the present study was the lack of use of two-dimensional electrophoresis to separate the E/S products and then carry out the proteomic identification of those spots recognized by the IgG antibodies of patients with strongyloidiasis. However, the use of bioinformatics tools for the prediction of a signal peptide for protein secretion by the classical pathway, prediction of protein secretion by unconventional pathways including protein secretion within extracellular vesicles or exosomes, prediction of glycosylation sites, and estimation of the antigenic density helped to obtain more information on the nature and antigenic potential of E/S products from S. venezuelensis iL3.
All our results show that S. venezuelensis iL3 possesses proteins with immunomodulatory properties that can be released to the external environment using the classical secretory pathway or strategically secreted within extracellular vesicles or exosomes. Using mass spectrometry and bioinformatic analysis, we have been able to determine the biochemical characteristics of the antigenic components from the E/S products of S. venezuelensis iL3 identified by WB, showing that S. venezuelensis iL3 can release “in vitro” different protein profiles depending on the type of environment where they are exposed. Furthermore, our results also show that E/S products from S. venezuelensis iL3 have an interesting set of proteins with antigenic potential that could be explored as possible new markers for the immunodiagnosis of human strongyloidiasis, especially the arginine kinase of 37 kDa that matches with an antigenic band with high sensitivity and specificity. Future studies will be necessary to characterize and determine the specific functions of E/S products from S. venezuelensis iL3 and their role in the host-parasite interaction.
Data availability
Appendix A. Supplementary Data.
References
Ayón-Núñez DA, Fragoso G, Bobes RJ, Laclette JP (2018) Plasminogen-binding proteins as an evasion mechanism of the host’s innate immunity in infectious diseases. Biosci. Rep 38:BSR20180705. https://doi.org/10.1042/BSR20180705
Barrett J (2009) Forty years of helminth biochemistry. Parasitology 136:1633–1642. https://doi.org/10.1017/S003118200900568X
Brindley PJ, Gam AA, Pearce EJ, Poindexter RW, Neva FA (1988) Antigens from the surface and excretions/secretions of the filariform larva of Strongyloides stercoralis. Mol Biochem Parasitol 28:171–180. https://doi.org/10.1016/0166-6851(88)90001-1
Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, French M, Reithinger R, Gobbi F, Montresor A, Bisoffi Z (2020) The global prevalence of Strongyloides stercoralis infection. Pathogens 9:468. https://doi.org/10.3390/pathogens9060468
Casado L, Rodriguez-Guardado A, Boga JA, Fernández-Suarez J, Martínez-Camblor P, Rodríguez-Perez M, García-Pérez A, Vazquez F, Gascon J (2019) Use of serology in a systematic screening programme for strongyloidiasis in an immigrant population. Int J Infect Dis 88:60–64. https://doi.org/10.1016/j.ijid.2019.09.003
Conway DJ, Atkins NS, Lillywhite JE, Bailey JW, Robinson RD, Lindo JF, Bundy DA, Bianco AE (1993) Immunodiagnosis of Strongyloides stercoralis infection: a method for increasing the specificity of the indirect ELISA. Trans R Soc Trop Med Hyg 87:173–176. https://doi.org/10.1016/0035-9203(93)90477-8
Conway DJ, Bailey JW, Lindo JF, Robinson RD, Bundy DA, Bianco AE (1993) Serum IgG reactivity with 41-, 31-, and 28-kDa larval proteins of Strongyloides stercoralis in individuals with strongyloidiasis. J Infect Dis 168:784–787. https://doi.org/10.1093/infdis/168.3.784
Cornejo-Granados F, Hurtado-Ramírez JM, Hernández-Pando R, Ochoa-Leyva A (2019) Secret-AAR: a web server to assess the antigenic density of proteins and homology search against bacterial and parasite secretome proteins. Genomics 111:1514–1516. https://doi.org/10.1016/j.ygeno.2018.10.007
Corral MA, Paula FM, Meisel DM, Castilho VL, Gonçalves EM, Levy D, Bydlowski SP, Chieffi PP, Castro-Borges W, Gryschek RC (2017) Potential immunological markers for diagnosis of human strongyloidiasis using heterologous antigens. Parasitology 144:124–130. https://doi.org/10.1017/S0031182016001645
Cunha RA, de Carvalho EFG, de Sousa JEN, Costa-Cruz JM (2017) Excretory/secretory antigens of Strongyloides venezuelensis applied to IgG detection in human strongyloidosis. Parasitol Int 66:671–676. https://doi.org/10.1016/j.parint.2017.07.001
Diosdado A, Simón F, Morchón R, González-Miguel J (2020) Pro-fibrinolytic potential of the third larval stage of Ascaris suum as a possible mechanism facilitating its migration through the host tissues. Parasit Vectors 13:203. https://doi.org/10.1186/s13071-020-04067-5
Drurey C, Maizels RM (2021) Helminth extracellular vesicles: interactions with the host immune system. Mol Immunol 137:124–133. https://doi.org/10.1016/j.molimm.2021.06.017
Gadahi JA, Ehsan M, Wang S, Zhang Z, Wang Y, Yan R, Song X, Xu L, Li X (2016) Recombinant protein of Haemonchus contortus 14–3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs in vitro. Exp Parasitol 171:57–66. https://doi.org/10.1016/j.exppara.2016.10.014
Gao H, Xiao D, Song L, Zhang W, Shen S, Yin X, Wang J, Ke X, Yu C, Zhang J (2016) Assessment of the diagnostic efficacy of enolase as an indication of active infection of Schistosoma japonicum. Parasitol Res 115:151–164. https://doi.org/10.1007/s00436-015-4730-6
Gómez-Arreaza A, Acosta H, Quiñones W, Concepción JL, Michels PA, Avilán L (2014) Extracellular functions of glycolytic enzymes of parasites: unpredicted use of ancient proteins. Mol Biochem Parasitol 2014(193):75–81. https://doi.org/10.1016/j.molbiopara.2014.02.005
González-Miguel J, Siles-Lucas M, Kartashev V, Morchón R, Simón F (2016) Plasmin in parasitic chronic infections: friend or foe? Trends Parasitol 32:325–335. https://doi.org/10.1016/j.pt.2015.12.012
Hansen EP, Fromm B, Andersen SD, Marcilla A, Andersen KL, Borup A, Williams AR, Jex AR, Gasser RB, Young ND, Hall RS, Stensballe A, Ovchinnikov V, Yan Y, Fredholm M, Thamsborg SM, Nejsum P (2019) Exploration of extracellular vesicles from Ascaris suum provides evidence of parasite-host cross talk. J Extracell Vesicles 8:1578116. https://doi.org/10.1080/20013078.2019.1578116
Lok JB (2007) Strongyloides stercoralis: a model for translational research on parasitic nematode biology. WormBook, pp: 1–18. https://doi.org/10.1895/wormbook.1.134.1
Maeda Y, Palomares-Rius JE, Hino A, Afrin T, Mondal SI, Nakatake A, Maruyama H, Kikuchi T (2019) Secretome analysis of Strongyloides venezuelensis parasitic stages reveals that soluble and insoluble proteins are involved in its parasitism. Parasit Vectors 12:21. https://doi.org/10.1186/s13071-018-3266-x
Maizels RM, Smits HH, McSorley HJ (2018) Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity 49:801–818. https://doi.org/10.1016/j.immuni.2018.10.016
Marcilla A, Trelis M, Cortés A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sánchez del Pino MM, Muñoz-Antoli C, Toledo R, Bernal D (2012) Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 7:e45974. https://doi.org/10.1371/journal.pone.0045974
Masoori L, Falak R, Mokhtarian K, Bandehpour M, Razmjou E, Jalallou N, Jafarian F, Akhlaghi L, Meamar AR (2019) Production of recombinant 14–3-3 protein and determination of its immunogenicity for application in serodiagnosis of strongyloidiasis. Trans R Soc Trop Med Hyg 113:326–331. https://doi.org/10.1093/trstmh/trz006
Matsumoto H, Haniu H, Komori N (2019) Determination of protein molecular weights on SDS-PAGE. Methods Mol Biol 1855:101–105. https://doi.org/10.1007/978-1-4939-8793-1_10
Nutman TB (2017) Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144:263–273. https://doi.org/10.1017/S0031182016000834
Ponce R, León-Janampa N, Gilman RH, Liendo R, Roncal E, Luis S, Quiñones-Garcia S, Silverstein Z, García HH, Gonzales A, Sheen P, Zimic M, Pajuelo MJ, Cysticercosis Working Group in Peru (2018) A novel enolase from Taenia solium metacestodes and its evaluation as an immunodiagnostic antigen for porcine cysticercosis. Exp. Parasitol 191:44–54. https://doi.org/10.1016/j.exppara.2018.06.001
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J (2013) The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7:e2002. https://doi.org/10.1371/journal.pntd.0002002
Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, Wongkham C, Insawang T, Maleewong W (2016) Strongyloides stercoralis diagnostic polypeptides for human strongyloidiasis and their proteomic analysis. Parasitol Res 115:4007–4012. https://doi.org/10.1007/s00436-016-5170-7
Rodpai R, Intapan PM, Thanchomnang T, Sanpool O, Janwan P, Laummaunwai P, Wongkham C, Insawang T, Maleewong W (2017) Identification of antigenic proteins in Strongyloides stercoralis by proteomic analysis. Parasitol Res 116:1687–1693. https://doi.org/10.1007/s00436-017-5443-9
Roldán Gonzáles WH, Meisel DMCL, de Paula FM, Gryschek RCB (2021) Diagnostic accuracy of somatic and excretory−secretory antigens from Strongyloides venezuelensis infective larvae for the immunodiagnosis of human strongyloidiasis. Parasitology 148:1522–1527. https://doi.org/10.1017/S0031182021001207
Salvador F, Treviño B, Bosch-Nicolau P, Serre-Delcor N, Sánchez-Montalvá A, Oliveira I, Sulleiro E, Aznar ML, Pou D, Sao-Avilés A, Molina I (2020) Strongyloidiasis screening in migrants living in Spain: systematic review and meta-analysis. Trop Med Int Health 25:281–290. https://doi.org/10.1111/tmi.13352
Sato Y, Inoue F, Matsuyama R, Shiroma Y (1990) Immunoblot analysis of antibodies in human strongyloidiasis. Trans R Soc Trop Med Hyg 84:403–406. https://doi.org/10.1016/0035-9203(90)90337-e
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68:850–858. https://doi.org/10.1021/ac950914h
Soblik H, Younis AE, Mitreva M, Renard BY, Kirchner M, Geisinger F, Steen H, Brattig NW (2011) Life cycle stage-resolved proteomic analysis of the excretome/secretome from Strongyloides ratti-identification of stage-specific proteases. Mol Cell Proteomics 10(M111):010157. https://doi.org/10.1074/mcp.M111.010157
Stadelmann B, Spiliotis M, Müller J, Scholl S, Müller N, Gottstein B, Hemphill A (2010) Echinococcus multilocularis phosphoglucose isomerase (EmPGI): a glycolytic enzyme involved in metacestode growth and host-parasite cell interactions. Int J Parasitol 40:1563–1574. https://doi.org/10.1016/j.ijpara.2010.05.009
Sudré AP, Siqueira RC, Barreto MG, Peralta RH, Macedo HW, Peralta JM (2007) Identification of a 26-kDa protein fraction as an important antigen for application in the immunodiagnosis of strongyloidiasis. Parasitol Res 101:1117–1123. https://doi.org/10.1007/s00436-007-0596-6
Tian AL, Lu M, Calderón-Mantilla G, Petsalaki E, Dottorini T, Tian X, Wang Y, Huang SY, Hou JL, Li X, Elsheikha HM, Zhu XQ (2018) A recombinant Fasciola gigantica 14–3-3 epsilon protein (rFg14-3-3e) modulates various functions of goat peripheral blood mononuclear cells. Parasit Vectors 11:152. https://doi.org/10.1186/s13071-018-2745-4
Tritten L, Tam M, Vargas M, Jardim A, Stevenson MM, Keiser J, Geary TG (2017) Excretory/secretory products from the gastrointestinal nematode Trichuris muris. Exp Parasitol 178:30–36. https://doi.org/10.1016/j.exppara.2017.05.003
Tsuji N, Ohta M, Fujisaki K (1997) Expression of a 70-kDa heat-shock-related protein during transformation from free-living infective larvae to the parasitic stage in Strongyloides venezuelensis. Parasitol Res 83:99–102. https://doi.org/10.1007/s004360050218
Varghese A, Raina OK, Chandra D, Mirdha BR, Kelawala NH, Solanki JB, Kumar N, Ravindran R, Arun A, Rialch A, Lalrinkima H, Kelawala RN, Samanta S (2017) Sero-detection of Toxocara canis infection in human with T. canis recombinant arginine kinase, cathepsin L-1 and TES-26 antigens. Acta Parasitol 62:775–778. https://doi.org/10.1515/ap-2017-0093
Vasquez-Rios G, Pineda-Reyes R, Pineda-Reyes J, Marin R, Ruiz EF, Terashima A (2019) Strongyloides stercoralis hyperinfection syndrome: a deeper understanding of a neglected disease. J Parasit Dis 43:167–175. https://doi.org/10.1007/s12639-019-01090-x
Wang LQ, Liu TL, Liang PH, Zhang SH, Li TS, Li YP, Liu GX, Mao L, Luo XN (2020) Characterization of exosome-like vesicles derived from Taenia pisiformis cysticercus and their immunoregulatory role on macrophages. Parasit Vectors 13:318. https://doi.org/10.1186/s13071-020-04186-z
Wilbers RHP, Schneiter R, Holterman MHM, Drurey C, Smant G, Asojo OA, Maizels RM, Lozano-Torres JL (2018) Secreted venom allergen-like proteins of helminths: conserved modulators of host responses in animals and plants. PLoS Pathog 14:e1007300. https://doi.org/10.1371/journal.ppat.1007300
Zhao L, Poschmann G, Waldera-Lupa D, Rafiee N, Kollmann M, Stühler K (2019) OutCyte: a novel tool for predicting unconventional protein secretion. Sci Rep 9:19448. https://doi.org/10.1038/s41598-019-55351-z
Funding
WHR is receiving support from Edital MCT/CNPq-Brazil, Process N° 142056/2018–9. RCBG has received support from Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP, Grant N° 2013/04236–9).
Author information
Authors and Affiliations
Contributions
WHR designed the study, performed the experiments, wrote the manuscript, and prepared all figures. GRC helped to carry out the experiments and discuss the results. DCP helped to discuss proteomic results. FMP designed and supervised the study, discussed the results, and contributed to writing the manuscript. RCBG provided reagents, antigens, and human serum samples, supervised the study, and sought funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethical Committee from Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (Protocol N° 4.012.674). The strain of S. venezuelensis followed the animal ethics guidelines adopted by Ethical Committee on Animal of Instituto de Medicina Tropical da Faculdade de Medicina da Universidade de São Paulo (protocol N° 356A).
Consent to participate
Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Bruno Gottstein.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roldán Gonzáles, W.H., Coelho, G.R., Pimenta, D.C. et al. Proteomic analysis of the excretory-secretory products from Strongyloides venezuelensis infective larvae: new insights for the immunodiagnosis of human strongyloidiasis. Parasitol Res 121, 3155–3170 (2022). https://doi.org/10.1007/s00436-022-07636-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07636-y