Abstract
This study investigated the comparative efficacy of ivermectin and cypermethrin pour-on, for the treatment of Hyalomma anatolicum (a.) anatolicum infestations in bovines. For examining acaricidal efficacy, 480 ticks were exposed in vitro to graded doses of both the acaricides and in vivo efficacy was examined in 360 tick-infested bovines treated at the recommended doses of ivermectin (IVM) and cypermethrin (CYM) pour-on. The comparative quantitative assessment of tick burden was done on days 0, 5, 10, 15, and 20 after treatment using “finger counting.” The results of the tick survival assay indicated both compounds were effective in vitro against H. a. anatolicum. The arc transformed mean surviving ticks, 24 h post immersion, was 2.66 and zero in groups treated with the highest dilutions of IVM and CYM, respectively. At 15 days post-treatment, the CYM pour-on showed a higher in vivo efficacy (no surviving ticks) compared to IVM (mean of 20 surviving ticks). A single dose of CYM and IVM was found effective for 20 and 15 days post-treatment, respectively. Additionally, a questionnaire was used to gather information from 30 small holder dairy farms on the farmer's approach toward the control of ticks. The majority (90%) of respondents were using acaricides incorrectly along with poor husbandry practices on their farms. Overuse of IVM in the tested area of Pakistan may be the reason the IVM is not as effective as expected. These results provide useful tools for the decision making in tick control, as well as providing the basis for testing the findings on provincial and national levels in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks have been recognized as important ectoparasites of livestock. They have been incriminated as voracious bloodsuckers, causing heavy blood losses resulting in low-quality hides (Jongejan and Uilenberg 2004), secondary bacterial infections (Ambrose et al. 1999), lowered productivity in terms of weight gain (Pegram and Oosterwijk 1990) and milk yield (Sajid et al. 2007), and increased mortality (Niyonzema and Kiltz 1986). In some countries, half of the earning from milk is spent on acaricidal treatment (Swai 2002). Hence, ticks and tick-borne diseases pose a serious impact on the individual and national economics of developing countries. Therefore, it has been suggested that developing countries like Pakistan should make tick control a priority (Bansal 2005). Although grooming (Mooring et al. 1996) and domestic poultry (Hassan et al. 1992) have been found to be effective for tick control, chemical control remains the cornerstone in developing countries (De Castro et al. 1997). The major constraint of chemical treatment is the selection for chemical-resistant strains of ticks (Ghosh et al. 2006). The predominant contributing factors in development of resistance may include misuse of drugs (Bianchi et al. 2003) and use of the wrong concentration of acaricide (Dolan 1999) leading to failure of the tick control program (Pegram et al. 2000). For an effective chemical control strategy, periodic monitoring of the effectiveness of drugs and identification of resistant strains is essential. In Pakistan, the over-the-counter availability of various brands of Ivermectin has led to the misuse of the drug by dairy farmers for the control of ecto- and endo-parasites (Sajid, personal observation), and this may be a predisposing factor in the development of ivermectin-resistant strains of ticks (Ghosh et al. 2006). The present study was designed in order to obtain data on the farmer's approach for the control of ticks and to determine the in vivo comparative efficacy of an avermectin (injectable ivermectin, IVM) and a pyrethroid (pour-on cypermethrin, CYM), against Hyalomma a. anatolicum, the most common cattle tick in lower Punjab, Pakistan (Sajid et al. 2008). The results of this study also provide a rough estimate of the efficacy of both the drugs in vivo.
Materials and methods
Survey
A preliminary survey of the small holder dairy farms of the selected areas was conducted using a pre-designed questionnaire (Thrusfield 1995), in order to record detailed relevant information about the current use of acaricides and/or other alternative therapies for the control of tick infestation in animals. The points emphasized were: (1) housing, management, and on-farm hygiene practices; (2) priority of treatment strategies for tick infestation; and (3) information about concentration, dosage, and administration of drugs for various species of animals.
Chemotherapeutic trials
The in vitro and in vivo trials were planned according to the recommendations of the World Association for the Advancement of Veterinary Parasitology (Holdsworth et al. 2006).
In vitro acaricidal efficacy
In vitro acaricidal efficacy was determined using live engorged female ticks collected from the study area. Ticks were exposed to IVM (Ivomec, Merial, France) or 5% CYM pour-on (Cipermetriven, Ivan) at various concentrations to estimate the acaricidal efficacy of these drugs. Ticks sham-treated with propylene glycol (Propandiol-(1, 2), Merck) acted as controls. The tick survival assay described by Mendes et al. (2001) was used. Briefly, 480 live engorged female Hyalomma a. anatolicum ticks were collected from the bovines of the selected area and separated into two equal groups of 240 ticks (1 and 2) for the efficacy trial of IVM and CYM, respectively. Each group of ticks was immersed in the different acaricides diluted in oil-based diluent for 5 min. Group 1 was further divided into four sub-groups (A through D) having 60 ticks in each (20 × 3 replicates). Sub-groups B, C, and D were immersed in 200, 400, and 600 µg of IVM concentrations, respectively, while sub-group A was treated with three concentrations of propylene glycol (sham treatment). Group 2 sub-groups B, C, and D were immersed in 1.0, 1.25, and 1.5 mg of CYM, while sub-group A was treated with corresponding doses of propylene glycol (sham treatment). After the immersion period, the engorged females were removed by passage through a plastic filter and dried on paper towels. These ticks were placed in Petri dishes and incubated at 27–28°C, 85–95% relative humidity, for 24 h. After this period, the number of live ticks in each group was counted in order to estimate the acaricidal efficacy of IVM and CYM. The live ticks (if any) were again provided the same conditions for 2 weeks in order to observe their reproductive potential.
In vivo acaricidal efficacy
Three hundred sixty bovines in the study area were selected for an in vivo acaricidal efficacy of the two compounds (IVM and CYM pour-on). The animals were selected based on the following criteria: (1) the animal had a tick burden of >100 ticks per animal, (2) the age of animal was more than 1 year, and (3) there was no history of application of acaricide to the animal.
The selected animals were divided into four equal groups (A through D) and treated with IVM or CYM, or with a sham treatment of propylene glycol (Propandiol-(1, 2), Merck) vehicle as a control. A layout of the treatment protocol is shown in Table 1.
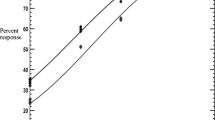
After a single treatment with either of the above-mentioned acaricides, the animals were examined quantitatively every 24 h through “finger counting” (Rugg and Hair 2007) of the ticks on one side of the body and multiplying the number by 2 to get the whole body count (Knopf et al. 2002). The number of ticks shed after the first 24 h and the duration for which the treatment remained effective was calculated from the data. The graphs show the post-treatment tick burden on days 0, 5, 10, 15, and 20.
Data analyses
The results of in vitro and in vivo acaricidal efficacy trials were analyzed using a general linear model with repeated measure analysis of variance and least square means of interaction (StatSoft 1999).
Results
Ninety percent (27/30) of the selected small holder dairy farm owners in the current trial had a history of inappropriate and blind treatment of animal tick infestation through self-administration of various brands of IVM (easily available at local markets) rather than consulting a veterinarian. Only 14% of these farmers were using correct doses and formulations of the drugs. All the selected farms farmed both small and large ruminants simultaneously. Only 10% (03/30) of farm owners were consulting veterinarians and had acceptable management and hygiene practices that included: (1) well-constructed sheds with cemented floors and an open housing system, (2) proper cleanliness and management of wastes, and (3) regular showering/bathing and deworming practices. IVM was the only drug used on all the farms, with various local and international brands being used. Good management and hygienic practices were found only in 10% of the farms surveyed.
The tick survival assay indicated the in vitro efficacy of 5% CYM pour-on and IVM against H. a. anatolicum. The arc transformed mean number of ticks surviving 24 h after treatment in IVM-treated groups was higher (P = 0.00) than that of the CYM-treated groups. However, a dose-dependent decrease in the mean number of ticks was observed in both of the treated groups (Figs. 1 and 2). All the live ticks found 24 h after treatment died during the 2-week incubation period except those in the sham-treated groups. The ticks of the control group not only survived, but they also laid eggs during incubation. The mean number of H. a. anatolicum surviving 24 h is given in Table 2. The results showed that H. a. anatolicum is susceptible to both of the test drugs. However, roughly, the death of the ticks treated with IVM was delayed as compared to those treated with CYM.
The in vivo post-treatment quantitative assessment of tick burden revealed that the sham-treated animals maintained a tick infestation throughout the study period. Both the IVM- and CYM-treated groups resulted in significantly lower (P < 0.05) tick counts relative to controls on all post-treatment counting days. The finger counts were significantly higher (F 12, 32 = 48.6; P = 0.00) in group A (IVM-treated group) than in Group C (5% CYM pour-on) as shown in Fig. 3. From day 0 (pre-treatment) to day 5 (post-treatment), the reduction in the mean number of ticks was not significant (P > 0.05) in the IVM-treated group. The maximum reduction in mean number of ticks in the IVM-treated group was found from day 5 to day 10, followed by day 10 to day 15. IVM was not found to be effective in controlling the tick burden after 15 days post-treatment. In the CYM-treated group, reduction in the mean number of ticks was significant (P < 0.05) even in the day 0 (pre-treatment) to day 5 (post-treatment) period. CYM 5% pour-on was found to be effective even after 15 days post-treatment; however, the rate of reduction in mean tick numbers was a little lower than in earlier periods. The lowest tick burden in the IVM-treated group was significantly higher (P < 0.05) than that of the CYM-treated group, the latter being close to zero. Hence, the in vivo efficacy trials of injectable IVM and CYM pour-on revealed better results for the latter.
Discussion
A number of tick control strategies have so far been used by the livestock farmers and animal practitioners. These include grooming (Mooring et al. 1996), genetic manipulation through increasing Bos indicus content in the progeny (Sutherst and Utech 1981; Frisch et al. 2000), biological control through domestic poultry (Hassan et al. 1992), entomopathogenic fungi (Bittencourt et al. 1994; Samish and Rehacek 1999; Gindin et al. 2001), immunological control through production of vaccines against some of the tick species (Willadsen 1987; Rodriguez et al. 1995; Brossard 1998), and ethnoveterinary practices (Sutherst et al. 1982; Carol et al. 1989; Regassa 2000); however, chemotherapeutic control remains the foundation of tick control programs for eradication of livestock infestations in the developing world (De Castro et al. 1997; Bianchi et al. 2003). However, a progressive decrease in efficiency of acaricidal drugs through the development of resistance (Beugnet et al. 1994) would undermine this method. Epidemiological investigations have suggested that a reduction in acaricide-treatment frequency that permits high tick-attachment rates allows the development of endemic stability (Norval et al. 1992). To this end, a regular screening of compounds is required for the determination of their efficacy. So far, various groups of insecticides and acaricides have been found to have significant efficacy for tick control, including pyrethroides (Miller 1987; Zerba 1988), avermectins (Miller 1987), organophosphates (Fiedler 1958; Miller 1988), organochlorines, carbamates, and insect growth regulators (Miller 1987).
The present research indicated that the vehicles of IVM and CYM do not have any acaricidal activity (Rugg and Hair 2007). Previous reports of comparative chemotherapeutic trials of IVM showed better efficacy (Khan et al. 1997; 1998; George et al. 1998). Our results are surprisingly different from the above-mentioned previous comparative studies indicating better in vivo efficacy of CYM (a pyrethroid) than IVM (an avermectin). The probable reason may be less exposure of domestic animals to pyrethroids for the control of ecto- and endo-parasites in this region of Pakistan. In a recent study, the impact of CYM on the vittelongenesis-inducing factor was determined by Friesen and Kaufman (2003), who showed that, instead of stimulating vitellogenesis, it has an inhibiting effect on egg development. The results of the tick survival assay do not allow the comparison of the efficacy of the two drugs because the doses used in the experiment may not be having physiological equivalence with each other. However, these results allow us to estimate the dose needed to kill all the ticks for each of the drugs and provide some tools to help manage the tick problem in the testing area.
According to reported speculation (Ghosh et al. 2006), the principal use of a limited number of chemicals (e.g., IVM) for tick control leads to the selection of chemical-resistant strains of ticks, along with environmental contamination. We suspect that over-the-counter availability and misuse of IVM in the selected farms may be the cause of the apparent decrease in its acaricidal efficacy and development of resistance as compared to 5% CYM pour-on, as previously reported in other countries by Bianchi et al. (2003), Beugnet et al. (1994), Dolan (1999), and Ogden et al. (2005). However, standardization of the permethrin hydrolytic assay (Jamroz et al. 2000) and larval packet test (LPT) (FAO 1984) still needs to be done in Pakistan for confirmatory screening of acaricide-resistant strains of ticks. Various studies have been conducted on the mechanism of acaricidal resistance. Organophosphate resistance mechanisms include the following: (1) Acetylcholine esterase affinity is changed in resistant tick populations (Pruett 2002), and (2) a link to cytochrome P450 monooxygenase activity (Foil et al. 2004). Pyrethroid resistance was found to be due to two mechanisms: (1) a mutation of Na+ channels (He et al. 1999), confirmed by Guerrero et al. (2001) through PCR, and (2) involvement of some metabolic activity leading to a much higher CzEst9 esterase activity in resistant populations (Jamroz et al. 2000). Avermectin resistance has also been suggested to be due to this mechanism by Jamroz et al. (2000), but this needs to be scientifically proved.
The application of integrated pest management (IPM), e.g., the use of seasonal treatment at the peak of tick activity, accompanied by good management and sanitary conditions, may be a prophylactic approach for tick control in small holder dairy farming systems of Pakistan. Hence, the future plans of research should not only be directed towards the development of modern drugs and searching of new drug targets with different modes of application, but also towards finding some feasible alternative strategies using ethnoveterinary medicine, immunotherapy, and genetic manipulation in order to get some useful tools in future tick control programs.
References
Ambrose N, Lloyd D, Maillard JC (1999) Immune response to Dermatophilus congolensis infections. Parasitol Today 15:295–300
Bansal GC (2005) Bovine theileriosis in India: an overview. Proc Nat Acad Sci India 75:134–143
Beugnet F, Costa R, Chardonnet L (1994) Adaptations des methods de lutte contre les tiques a l extension du phenomene de chimioresistance: example de Boophilus microplus on Nouvelle-Caledonie Revue. Med Vet 145:931–940
Bianchi MW, Barre N, Messad S (2003) Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Vet Parasitol 112:75–89
Bittencourt VREP, Massard CL, de Lima AF (1994) Acao do fungo Metarhizium anisopliae em ovos e larvas do carrapato Boophilus microplus. Rev Univ Rural Ser Cienc da Vida 16:41–47
Brossard M (1998) The use of vaccines and genetically resistant animals in tick control. Rev Sci Tech Off Intl Epizoo 17:188–199
Carol JF, Maradufu A, Warthen JD Jr (1989) An extract of Commiphora erythraea, a repellent and toxicant against ticks. Entomol Exp Appl 53:111–116
De Castro ABA, Bittencourt VREP, Daemon E, Viegas EDC (1997) Eficacia do fungo Metarhizium anisopliae sobrre o carrapato Boophilus microplus em teste de estabulo. Rev Univ Rural Ser Cienc da Vida 19:73–82
Dolan TT (1999) Dogma and misunderstanding in East Coast Fever. Trop Med Int Hlth 4:A3–A11
FAO (1984) Acaricidal resistance. Ticks and tick-borne disease control. A practical field manual, vol. 1. Tick control. FAO, Rome, pp 246–299
Fiedler OGH (1958) Control of cattle ticks in South Africa with special reference to the use of ‘Asuntol’ (Bayer 21/199). Vet Med Nach 3:133–146
Foil LD, Coleman P, Eisler M, Fragoso-Sanches H, Garcia-Vasquez Z, Guerrero FD, Jonsson NN, Langstaff IC, Li AY, Machila N, Miller RJ, Morton J, Pruett JH, Torr S (2004) Factors that influence the prevalence of acaricidal resistance and tick-borne diseases. Vet Parasitol 125:163–181
Friesen KJ, Kaufman WR (2003) Cypermethrin inhibits egg development in the ixodid tick, Amblyomma hebraeum. Pest Biochem Physiol 76:25–35
Frisch JE, O'Neill CJ, Kelly MJ (2000) Using genetics to control cattle parasites—the Rockhampton experience. Intl J Parasitol 30:253–264
George JE, Davey RB, Ahrens EH, Pound JM, Drummond RO (1998) Efficacy of amitraz (Taktic® 12.5% EC) as a dip for the control of Boophilus microplus (Canestrini) (Acari: Ixodidae) on cattle. Prev Vet Med 37:55–67
Ghosh S, Azhahianambi P, Furente JDL (2006) Control of ticks of ruminants, with special emphasis on livestock farming systems in India: present and future possibilities for integrated control—a review. Exp Appl Acarol 46:49–66
Gindin G, Samish M, Alekseev E, Glazer I (2001) The susceptibility of Boophilus annulatus (Ixodidae) ticks to entomopathogenic fungi. Biocont Sci Tech 11:111–118
Guerrero FD, Davey RB, Miller RJ (2001) Use of an allele-specific polymerase chain reaction assay to genotype pyrethroid resistant strains of Boophilus microplus (Acari: Ixodidae). J Med Entomol 38:44–50
Hassan SM, Dipeolu OO, Munyinyi DM (1992) Influence of exposure period and management methods on the effectiveness of chickens as predators of ticks infesting cattle. Vet Parasitol 43:301–309
He H, Chen AC, Davey RB, Ivie GW, George JE (1999) Identification of a point mutation in the para-type sodium channel gene from a pyrethroid-resistant cattle tick. Biochem Biophys Res Com 261:558–561
Holdsworth PA, Kemp D, Green P, Peter RJ, De Bruin C, Jonsson NN, Letonja T, Rehbein S, Vercruysse J (2006) World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of acaricides against ticks (Ixodidae) on ruminants. Vet Parasitol 136:29–43
Jamroz RC, Guerrero FD, Pruett JH, Oehler DD, Miller RJ (2000) Molecular and biochemical survey of acaricide resistance mechanisms in larvae from Mexican strains of the sourthern cattle tick, Boophilus microplus. J Insect Physiol 46:685–695
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitol 129:S3–S14
Khan MN, Hayat CS, Iqbal Z (1997) Evaluation of acaricidal efficacy of ivermectin, diazinon, permethrin and coumaphos in cattle and buffaloes. Pak Entomol 19:58
Khan MN, Iqbal Z, Hayat CS (1998) Evaluation of acaricidal efficacy of ivermectin, diazinon, permethrin and coumaphos in sheep and goats. Pak J Biol Sci 1:63–65
Knopf L, Komin-Oka C, Betschart B, Jongejan F, Gottstein B, Zinsstag J (2002) Seasonal epidemiology of ticks and aspects of cowardiosis in N'Dama village cattle in Central Guinea savannah of Cote d' Ivoire. Prev Vet Med 53:21–30
Mendes MC, Veríssimo CJ, Kaneto CN, Pereria JR (2001) Bioassays for measuring the acaricides susceptibility of cattle tick Boophilus microplus (canestrini, 1887) in São Paulo state, Brazil. Arq Inst Biol 68:23–27
Miller JA (1987) New approaches to the chemical control of arthropode pests of livestock. Intl J Parasitol 17:689–693
Miller TA (1988) Mechanims of resistance to pyrethroid insecticides. Parasitol Today 4:S10–S11
Mooring MS, McKenzie AA, Hart BL (1996) Grooming in impala: role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiol Behav 59:965–971
Niyonzema A, Kiltz HH (1986) Control of ticks and tick-borne diseases in Burundi. ACIAR Proc 17:16–17
Norval RAI, Barret JC, Perry BD, Mukhebi AW (1992) Economics, epidemiology and ecology: a multidisciplinary approach to the planning and appraisal of tick and tick-bonre disease control in Southern Africa. In: Fivaz B, Petney T, Horak I (eds) Tick vector biology: The medical and veterinary significance of ticks. Springer, Berlin, pp 35–54
Ogden NH, Swai E, Beauchamp G, Karimuribo E, Fitzpatrick JL, Bryant MJ, Kambarage D, French NP (2005) Risk factors for tick attachment to smallholder dairy cattle in Tanzania. Prev Vet Med 67:157–170
Pegram RG, Oosterwijk GPM (1990) The effect of Amblyomma variegatum on liveweight gain of cattle in Zambia. Med Vet Entomol 4:327–330
Pegram RG, Wilson DD, Hansen JW (2000) Past and present national tick control programs. Why they succeed or fail? Ann NY Acad Sci 916:546–554
Pruett JH (2002) Comparative inhibition kinetics for acetylcholinesterases extracted from organophophate resistant and susceptible strains of Boophilus microplus (Acari: Ixodidae). J Econ Entomol 95:1239–1244
Regassa A (2000) The use of herbal preparations for tick control in western Ethiopia. J S Afr Vet Assoc 71:240–243
Rodriguez M, Penichet ML, Mouris AE, Labarta V, Lorenzo LL, Rubiera R, Cordoves C, Sanchez PA, Ramos E, Soto A, Canales M, Palenzuela D, Triguero A, Lleonart R, Herrera L, De la Fuente J (1995) Control of Boophilus microplus populations in grazing cattle vacccinated with a recobinant Bm86 antigen preparation. Vet Parasitol 57:339–349
Rugg D, Hair JA (2007) Dose determination of a novel formulation of metaflumizone plus amitraz for control of cat fleas (Ctenocephalides felis felis) and brown dog ticks (Rhipicephalus sanguineus) on dogs. Vet Parasitol 150:203–208
Sajid MS, Iqbal Z, Khan MN, Muhammad G, Iqbal MU (2007) Effect of Hyalomma ticks (Acari: Ixodidae) on milk production of dairy buffaloes (Bos Bubalus Bubalis) of Punjab (Pakistan). Italian J Anim Sci 6:939–941
Sajid MS, Iqbal Z, Khan MN, Muhammad G (2008) Point prevalence of hard ticks (Ixodids) infesting domestic ruminants of lower Punjab, Pakistan. Intl J Agric Biol 10:349–351
Samish M, Rehacek J (1999) Pathogens and Predators of ticks and their potential in biological control. Ann Rev Entomol 44:159–182
StatSoft (1999) Statistica for Wndows (Computer program manual). StatSoft, Tulsa
Sutherst RW, Utech KBW (1981) Controlling livestock parasites with host resistnace. In: Pimentel D (ed) CRC handbook of pest management in agriculture, vol 2. CRC, Boca Raton
Sutherst RW, Jones RJ, Schnitzerling HJ (1982) Tropical legumes of the genus Stylosanthes immobilize and kill cattle ticks. Nature 295:320–321
Swai E (2002) Epidemiological studies of tick-borne diseases in small scale dairy farming systems in Tanzania. Ph.D. Thesis, University of Reading
Thrusfield M (1995) Veterinary epidemiology. Blackwell Science, Cambridge, pp 180–181
Willadsen P (1987) Immunological approaches to the control of ticks. Intl J Parasitol 17:671–677
Zerba E (1988) Insecticidal activity of pyrethroides on insects of medical importance. Parasitol Today 4:S8–S9
Acknowledgments
The study is a part of research conducted in an indigenous Ph.D. fellowship scheme funded by the Higher Education Commission of Pakistan. The authors would like to thank Dr. Thomas Nolan, Laboratory of Parasitology, University of Pennsylvania, Philadelphia, for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajid, M.S., Iqbal, Z., Khan, M.N. et al. In vitro and in vivo efficacies of ivermectin and cypermethrin against the cattle tick Hyalomma anatolicum anatolicum (Acari: Ixodidae). Parasitol Res 105, 1133–1138 (2009). https://doi.org/10.1007/s00436-009-1538-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1538-2