Abstract
This study was designed to obtain data on the farmer’s approach to tick control and to determine whether Rhipicephalus appendiculatus Neuman, Amblyomma variegatum (Fabricius), and Rhipicephalus (Boophilus) microplus (Canestrini) were resistant to amitraz and cypermethrin acaricides, in Isoka District, Zambia. Prevailing tick control practices were documented by administering a semi-structured questionnaire to 80 randomly selected smallholder livestock farmers from four agricultural camps (Longwe, Kantenshya, Kapililonga, and Ndeke) in Isoka District. Modified larval packet test (LPT) bioassay experiments were used to determine the resistance status of the common tick species against amitraz and cypermethrin acaricides. Fifty percent of respondents practiced chemical tick control with amitraz (27 %) and cypermethrin (23 %) being the acaricides in use, and were applied with knapsack sprayers. Less than 3 l of spray wash per animal was used which was considerably lower than the recommended delivery rate of 10 l of spray wash per animal. No significant susceptibility change to amitraz at 95 % confidence level was observed in R. appendiculatus and A. variegatum against amitraz. However, a significant change in the susceptibility of R. (Bo.) microplus tested with amitraz was detected at 95 % confidence. The test population had a lower susceptibility (LD50 0.014 %; LD90 0.023 %) than the reference population (LD50 0.013 %; LD90 0.020 %). The results indicated that resistance to amitraz was developing in R. (Bo.) microplus. For cypermethrin, no significant susceptibility change at 95 % confidence was observed in any of the three species and thus resistance to this chemical was not observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks (Acari: Ixodidae) are by far the most important external parasites that attack livestock. They have been implicated as voracious blood suckers that cause heavy blood losses resulting in low-quality hides (Rajput et al. 2006), secondary bacterial infections (Muchenje et al. 2008), lowered productivity in terms of weight gain (Estrada-Pena and Salman 2013) and milk yield (Sajid et al. 2007), and increased mortality due to tick-borne diseases (TBD) (Esemu et al. 2013). Luguru et al. (1987) identified Rhipicephalus appendiculatus Neuman, Amblyomma variegatum (Fabricius), and Rhipicephalus (Boophilus) microplus (Canestrini) to be the most important tick species infesting cattle in Isoka District.
Although a number of techniques such as grooming (Mooring et al. 1996), genetic manipulation through increase of Bos indicus content in progeny (Ayres et al. 2013), biological control through the use of entomopathogenic fungi (Pirali-Kheirabadi et al. 2007), immunological control using anti-tick vaccines (Freeman et al. 2010), and the use of ethno-veterinary practices (Zaman et al. 2012), the application of acaricides in dips and sprays to control cattle ticks still remains the cornerstone of tick control in the developing world. Chemotherapeutic control has provided a rapid and efficient method of controlling livestock ticks, and has consequently influenced livestock productivity through the significant reduction in the prevalence of tick infestations and tick-borne diseases (Abbas et al. 2014). However, the progressive evolution of resistance of ticks to almost every available class of acaricide continues to frustrate the efforts of cattle farmers (George et al. 2004).
Acaricide resistance is an inherited phenomenon that stems from a directional selection pressure caused by the toxic effects of the chemical (Roush and McKenzie 1987). This results in the selection of a strain of individuals that posses specific genetically determined biochemical mechanisms that enable them to survive treatment (Meyer et al. 2012 and Corley et al. 2013). Resistance leads to the failure of control programs undertaken at recommended procedures (Kunz and Kemp 1994), and its development has been exacerbated by the misuse of drugs and the use of incorrect doses (Bianchi et al. 2003). In fact, the injudicious use of acaricides may represent the greatest threat to the livestock industry in many countries.
Periodic monitoring of the effectiveness of drugs and the identification of resistant strains is essential for an effective chemical control strategy. Therefore, this study was designed in order to obtain data on the farmer’s approach for tick control and to determine whether R. appendiculatus, A. variegatum, and R. (Bo.) microplus tick species in Isoka District were resistant to amitraz and cypermethrin.
Materials and methods
The study area
The study was conducted in Isoka District (10° 24’ S, 32° 61’ E), which is located in the North Eastern part of Zambia. This district is bordered by Nakonde District in the north, Chinsali District in the west, and Chama District in the south. Malawi borders its eastern boundary. A tropical climate prevails which is characterized by high rain fall (1000 to 1500 mm annually) and is restricted to the period of November to April with temperatures ranging from 17 to 28 °C. May to July is cool and dry with temperatures ranging from 11 to 25 °C. August to October is hot and dry and temperatures range from 13 to 30 °C.
Farming is dominated by smallholder farmers who practice a mixed crop livestock production system, with cattle, goats, and poultry being the major livestock. The main breed of cattle is the Angoni, a short-horned Zebu animal originating from the Eastern Province of Zambia (Yambayamba et al. 2003). Cattle are kraaled overnight for protection and are released in the morning for communal grazing.
Acaricide survey
A preliminary survey of smallholder cattle farmers was conducted using a semi-structured questionnaire that was administered to a sample of 80 respondents. This sample size was determined using the formula adopted from Campbell (2005); \( n={\left(\frac{Z}{\mathrm{SE}}\right)}^2(P)\left(1-P\right) \) where n is the sample size; Z is the confidence level which is 1.96 for 95 % confidence; SE is the standard error, taken as 10 %; P is the expected proportion of farmers applying acaricides, taken to be 30 %; and 1-P is the expected proportion of farmers not applying acaricides.
Interviews with 80 farmers (20 randomly selected from four agricultural camps; Longwe, Kantenshya, Kapililonga, and Ndeke) were conducted using semi-structured questionnaires in November 2011 to January 2012 as described by Moyo and Masika (2009). Data collected was mainly on the importance of tick-borne diseases, the major acaricides in use, and dosage and methods of acaricide application to cattle.
Experimental cattle
A total of 80 Angoni cattle from four agricultural camps in Isoka District (Longwe, Kantenshya, Kapililonga, and Ndeke) were randomly selected for the purpose of tick collection. No consideration was given to the sex and age of cattle as treatments with acaricides was done indiscriminately. A similar sampling procedure was used by Yilima et al. (2001) who detected organophosphate resistance in R. (Boophilus) decoloratus (Koch) in Central Ethiopia.
Test and reference ticks
Fully engorged female tick species were randomly collected from the four agricultural camps, during the peak occurrence period of adult ticks (January to March, 2012), from which R. (Bo.) microplus, R. appendiculatus, and A. variegatum were identified using the pictorial guide provided by Walker et al. (2007). To facilitated oviposition and harvest of sufficient larvae, 10 clean ticks of each species were placed in separate 150-mm glass rearing tubes which were closed firmly with a ventilated stopper. These rearing tubes were then incubated at a temperature of 27 ± 1 °C and a relative humidity of 85–95 %, as per standard procedures recommended by FAO (1984).
Reference tick strains (susceptible populations) of R. (Bo.) microplus, R. appendiculatus, and A. variegatum were collected from Sansamwenge agricultural camp where acaricide usage was confirmed to be minimal or nil. Acaricide usage in this area has been absent since the breakdown of subsidized government dipping services in 1996, a fact that was confirmed by key informant interviews with lead livestock farmers and agricultural extension officers. The two kraals selected for sample collection were located 45 Km from the nearest cattle herds to the north and east while the Kalungu River separates them from other camps to the east and south.
Chemicals
The acaricides used in the study were formulated amitraz 12.5 % m/v (Milbitraz - Bayer Animal Heath Pty Ltd), registration number G2084 and formulated cypermethrin 15 % m/v (Cydip-United Phosphorous Ltd), registration number G505. Both acaricides were registered according to Act 36/1947, South Africa.
Bioassay techniques
All tick strains were assayed using modifications of the larval packet test (LPT) (FAO 1984). For amitraz, the standard LPT does not produce dose-mortality relationships that can be used to discriminate between susceptible and resistant individuals (Ducornez et al. 2005). The cause for this lack of a dose-mortality relationship has been attributed to an inadequate exposure time, possible interaction of technical amitraz and the paper substrate, and the instability of technical amitraz which maybe degrading during the bioassay (Miller et al. 2002). Therefore, the bioassays used for amitraz involved increasing the exposure time from 24 to 48 h and replacing technical amitraz with formulated amitraz. Therefore, in amitraz assays, a top dose was prepared by adding a volume of formulated amitraz to a 2:1 ratio mixture of chloroform and olive oil diluent. Serial dilutions using the 2:1 chloroform and olive oil diluent were made in order to produce eight dose levels including the control (diluent only), with each dose level having three replicates. A volume of 0.67 ml of each dilution was applied to a 5 cm × 10 cm piece of Whatman 541 filter paper. Treated papers were hung on a rack in a fume hood for 2 h to allow the chloroform to evaporate. The filter papers were then folded in half and sealed with metal spring clips on both sides. Fourteen to 21-day-old larvae were used in the bioassays. Approximately 100 larvae of each tick species were introduced into the packets with a fine brush, and the top was sealed with another metal spring clip. The packets were then incubated at 27 ± 1 °C and a relative humidity of 85–95 % for 48 h. A similar procedure was used in cypermethrin assays except packets were incubated for 24 h.
Determination of mortality
Dead larvae were easily detected after exposure to cypermethrin as many were desiccated and obviously dead. For amitraz, mortality was more difficult to determine as many larvae did not desiccate after exposure and appeared to be alive. Therefore, we considered larvae that could walk across treated papers after incubation to be alive, but larvae that did not move or could only move legs without walking as dead (Miller et al. 2002).
Statistical analysis
Probit analysis was used to analyze the bioassay results with the aid of Polo Plus Probit and Logit Analysis 2.0 software (Le Ora Software 2002). This analysis included probit transformations of percentage mortality and natural logarithm transformations of dose. Assessment of goodness of fit was done using the chi-square goodness of fit test and the heterogeneity factor (chi-square divided by the degrees of freedom) of each bioassay. When the heterogeneity factor was greater than 1.0, the data was assumed not to follow the probit model used in the analysis. Lethal dose ratios at LD50 and LD90 were used to estimate resistance ratios relative to reference tick strains. Significance of each comparison was determined when the number 1 was not contained in the 95 % confidence interval of the lethal dose ratio (Robertson et al. 2007).
Results
Acaricide survey
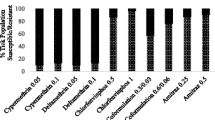
All respondents acknowledged that ticks were the major problem in their farming systems causing diseases such as East Coast fever (100 %), babesiosis (23 %), and anaplasmosis (17 %). Acaricide application was the main tick control method (50 %), with amitraz (27 %) and cypermethrin (23 %) being the most commonly used acaricides. All respondents applied acaricides to cattle using knapsack sprayers at weekly (3.5 %), biweekly (35 %), monthly (5 %), bimonthly (2 %), biannual (1.5 %), and annual intervals (1.5 %). Application was done during the peak tick season, November to April. Farmers were able to follow the recommended dosage of 20 ml of acaricide to 10 l of water for amitraz and 10 ml of acaricide to 10 l of water for cypermethrin. An average of 2.5 l of spray wash per animal was used.
Susceptibility to amitraz
The results of the bioassays for each tick species tested for amitraz susceptibility are summarized in Table 1. Data for all assays except for the test population of R. (Bo.) microplus fitted the probit model used in the analysis as the heterogeneity factors calculated were all less than 1.0 (Table 1). For R. (Bo.) microplus, the 95 % confidence interval of the lethal dose ratios at LD50 and LD90 did not include the number 1 (Table 1). This indicated that the susceptibility of the reference and test populations was significantly different at P = 0.05, with the test population exhibiting a lower susceptibility. Therefore, the log-dose probit-mortality line of the test population showed a shift to the right of that of the reference population (Fig. 1) as a result of higher values of LD50 and LD90 estimates, and an increase in the slope of the test population. Resistance ratios at LD50 and LD90 at 95 % confidence level were estimated at 1.10 (1.04–1.16) and 1.14 (1.08–1.22) respectively.
For R. appendiculatus and A. variegatum, the 95 % confidence interval of their lethal dose ratios at LD50 and LD90 both included the number 1 (Table 1). This indicated that for the two species, the susceptibility of the reference and test population were not significantly different at P = 0.05. Therefore, the LD50 and LD90 estimates and slopes of the reference and test populations were not significantly different.
Susceptibility to cypermethrin
Bioassay results for each tick species tested for cypermethrin susceptibility are summarized in Table 2. Data for all assays fitted the probit model used in the analysis as the heterogeneity factor for each assay was equal to or less than 1.0. The 95 % confidence interval of the lethal dose ratios at LD50 and LD90 for all three species included the number 1 (Table 2). This indicated that for all the three species, the susceptibility of the reference and test population of each species was not significantly different at P = 0.05. Therefore, for each species, the LD50 and LD90 estimates and slopes of the reference and test populations were not significantly different.
Discussion
Measurement of amitraz susceptibility in ticks using the traditional FAO LPT technique is not suitable because it produces dose-mortality lines with extremely high slopes (Li et al. 2004 and Miller et al. 2007). It is for this reason that Miller et al. (2002) developed a modified LPT for amitraz where formulated instead of technical amitraz was used on nylon substrate instead of filter paper. In the same study, it was also shown that assays using formulated amitraz on filter paper incubated for 48 h instead of 24 h produced reliable lethal dose estimates at 50 and 90 % mortality. The latter two modifications seem appropriate for rural laboratories with limited capacity and were therefore used in this study. In cypermethrin assays, formulated instead of technical cypermethrin was used in order to evaluate the commercial product that was being used by farmers.
Amitraz bioassay results indicated that the reference and test populations of R. appendiculatus and A. variegatum produced data that fitted the probit model. For R. (Bo.) microplus, only the reference strain indicated good fit. However, the chi-square test for goodness of fit for the test strain suggested that the departure from linearity was not sufficient enough to exclude the data from analysis (Heong et al. 2010). In cypermethrin assays, the data for all reference and test strains for the three tick species fitted the probit model. Therefore, since these data fitted the probit model used in the analysis, we can conclude that the modified bioassays used in the study produced valid results (Robertson et al. 2007 and Heong et al. 2010).
The susceptibility of the reference and test strains of R. (Bo.) microplus to amitraz was found to be significantly different, with the test strain having a lower susceptibility. This was indicative of resistance development, as a higher dose of amitraz was required in the test strain in order to illicit a similar level of response as in the reference strain (Yilima et al. 2001; George et al. 2004 and Abbas et al. 2014). However, the resistance ratio estimates at LD50 and LD90 were low. Ducornez et al. (2005) reported amitraz resistance ratio in R. (Bo.) microplus to be as high at 29.4 (23.1–37.3) while Cutullé et al. (2012) reported the resistance ratios of two field isolates of the same tick from Argentina to be 32.5 and 57.0, respectively. The detection of low order resistance indicates that amitraz resistance in R. (Bo.) microplus populations in Isoka District was in the initial or emerging phase of development. The detection of emerging amitraz resistance in R. (Bo.) microplus in the present study is similar to the findings of Ntondini et al. (2008) who detected emerging amitraz resistance in this tick on communally grazed cattle, in the eastern region of the Eastern Cape Province of South Africa. The detection of fully susceptible strains of R. appendiculatus also agreed with the results of Ntondini et al. (2008) who reported fully susceptible strains of this tick to amitraz.
All three tick species were also shown to be fully susceptible to cypermethrin. The lack of detected resistance to cypermethrin resistance in R. (Bo.) microplus was in contrast to the results reported by Caracostantogolo et al. (1996) who reported high levels of cypermethrin resistance in R. (Bo.) microplus in the Eastern parts of Argentina. Recently, cypermethrin resistance in R. (Bo.) microplus has been reported in Brazil (Mendes et al. 2007) and Iran (Enayati et al. 2010).
The results of the present study support the general notion that resistance development in ticks is not a universal phenomenon but is more common in the one-host tick species (Abbas et al. 2014). This is because multi-host ticks develop resistance more slowly as they have longer generation times, less acaricidal exposure of immature stages, and an availability of alternative hosts that reduces their overall exposure to acaricides (Jongejan and Uilenberg 2004). On the other hand, one-host ticks are subjected to considerably high selection pressure at all parasitic stages, even in poorly implemented acaricide treatment regimes (Abdullah et al. 2012).
The results of the acaricide survey suggested that the development of amitraz resistance in R (Bo.) microplus in the study area could be attributed to inadequate delivery of dip wash to cattle (less than 3 l instead of the recommended 10 l of dip wash per animal) and erratic treatments. Studies by Mekonnen (2002) support this assertion as they reported the highest percentage of confirmed resistance among farmers that delivered inadequate amounts of acaricide spray washes in South Africa. Brito et al. (2011) also attributed the reduction in efficacy of several synthetic pyrethroid and amidine acaricides to inadequate spraying and underdosage. Use of higher doses of amitraz at recommended delivery rates may serve to eliminate heterozygous resistant individuals (Thullner et al. 2007 and Adakal et al. 2013) but will further increase the cost of cattle tick control. Alternatively, another chemical, such as ivermectin or coumaphos, both of which belong to different chemical classes than the amidine and pyrethroid tested, can be used (George et al. 2004 and Abbas et al. 2014).
References
Abbas, R.Z., Zaman, M.A., Colwell, D.D., Gillard, J. and Iqbal, Z., 2014. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Veterinary Parasitology, 203(1–2), 6–20.
Abdullah, S., Yadav, C.L. and Vatsva, S., 2012. Esterase profile of Rhipicephalus (Boophilus) microplus population collected from northern India exhibiting varied susceptibility to deltamethrin. Experimental and Applied Acarology, 58, 315–325.
Adakal, H., Stachurski, F. and Chevillon, C., 2013. Tick control practices in Burkina Faso and acaricide resistance survey in Rhipicephalus (Boophilus) geigyi (Acari: Ixodidae). Experimental and Applied Acarology, 59, 483–491.
Ayres, D.R., Pereira, R.J., Boligon, A.A., Silva, F.F., Schenkel, F.S., Roso, V.M. and Albuquergue, L.G., 2013. Linear and poisson models for genetic variation of tick resistance in cross-bred Hereford × Nellore cattle. Journal of Animal and Breeder Genetics, 130, 417–424.
Bianchi, M.W., Barre, N. and Messad, S., 2003. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Veterinary Parasitology, 112, 75–89.
Brito, G.L., Barbieri, F.S., Rocha, R.B., Oliveira, M.C.S. and Ribeiro, E.S., 2011. Evaluation of the efficacy of acaricides used to control the cattle tick, Rhipicephalus microplus, in dairy herds raised in the Brazilian South Western Amazon. Veterinary Medicine International, 806093.
Campbell, M.J., 2005. Sample size determination in clinical trials. In Encyclopaedic Companion to Medical Statistics Eds: Everitt BS and Palmer CR: Hodder Arnold, 302–306.
Caracostantogolo, J., Munoz Cobenas, M.E., Eddi, C., Ambrustolo, R.R., Bulman, G.M. and Marangunich, L., 1996. Primera deteminacion en la Republica Argentina de una poblacion de Boophilus microplus (Can.) resistente al piretroide sintetico alfacipermetrina caraterizada mediante pruebas preliminares., Veterinary Argentina, 13, 575–582.
Corley, S.W., Jonsson, N.N., Piper, E.K., Cutullé, C., Stear, M.J. and Seddon, J.M., 2013. Mutation in RmBAOR gene is associated with amitraz resistance in the cattle tick Rhipicephalus microplus. Proceedings of the National Academy of Sciences USA, 110 (42), 16772–16777.
Cutullé, C., Lovis, L., D’Agostinoc, B.I., Balbiani, G.G., Morici, G., Citroni, D., Reggi, J. and Caracostantogolo, J.L., 2012. In vitro diagnosis of the first case of amitraz resistance in Rhipicephalus microplus in Santo Tomé (Corrientes), Argentina. Veterinary Parasitology, 192, 296–300.
Ducornez, S., Barré, N., Miller, R.J. and de Garine-Wichatitsky, M., 2005. Diagnosis of amitraz resistance in Boophilus microplus in New Caledonia with the modified Larval Packet Test. Veterinary Parasitology, 130, 285–292
Enayati, A.A., Asgarian, F., Amouei, A., Sharif, M., Mortazavi, H., Boujhmehrani, H. and Hemingway, J., 2010. Pyrethroid insecticide resistance in Rhipicephalus bursa (Acari: Ixodidae). Pesticide Biochemistry Physiology, 97, 243–248.
Esemu, S.N., Besong, W.O., Ndip, R.N. and Ndip, L.M., 2013. Prevalence of Ehrlichia ruminatium in adult Amblyomma variegatum from cattle in Cameroon. Experimental and Applied Acarology, 59, 377–387.
Estrada-Pena, A. and Salman, M., 2013. Current limitations in the control and spread of ticks that affect livestock: A review. Agriculture., 3, 221–235.
FAO. 1984. Acaricide resistance, pp. 246–299. In Ticks and tick-borne disease control. A practical field manual, vol. 1. Tick control. FAO, Rome.
Freeman, J.M., Davey, R.R., Kappmeyer, L.S., Kammlah, D.M. and Olafson, P.V., 2010. Bm 86 midgut protein sequence variation in south Texas cattle fever ticks. Parasites and Vectors, 3, 101.
George, J.E., Davey, R.B. and Pound, J.M., 2004. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology, 129, S353-S366.
Heong, K.L., Tan, K.H., Fabellar, L.T. and Garcia, C.P., 2010. Research methods in toxicology and insecticide resistance monitoring of rice plant hoppers. Technical report.
Jongejan, F. and Uilenberg, G., 2004. The global importance of ticks. Parasitology, 129, S3–S14.
Kunz, S.E. and Kemp, D.H., 1994. Insecticides and acaricides: Resistance and environmental impact. Revue scientifique et technique Office International desEpizooties., 13, 1249–1286.
Li, A.Y., Davey, R.B., Miller, R.J. and George, J.E., 2004. Detection of amitraz resistance in the southern cattle tick, Boophilus microplus (Acari: Ixodidae). Journal of Medical Entomology, 41, 193–200.
Luguru, S.M., Chizyuka, G.B. and Musisi, F.L. 1987. A survey for resistance to acaricides in cattle ticks (Acari: Ixodidae) in three major traditional cattle areas in Zambia. Bulletin of Entomological Research, 77, 569–574.
Mendes, M.C., Pereira, J.R. and Prado, A.P., 2007. Sensitivity of Boophilus microplus (Acari: Ixodidae) to pyrethroid and organophosphate in farms in the Vale Do Paraiba regions of SAO Paulo. Brazil and Argentina Institute of Biology, 74, 84–85.
Mekonnen, S., 2002. Acaricide resistance profiles of single and multi-host ticks in commercial and communal farming areas in the Eastern Cape and Northwestern Provinces of South Africa. M.V.Sc. Dissertation, University of Pretoria.
Meyer, J.M., Ejendal, K.F., Avramora, L.V., Garland-Kuntz, E.E., Giraldo-Calderon, G.I., Brust, T.F., Watts, V.J. and Hill, C.A., 2012. A genome to lead approach for insecticide discovery: pharmacological characterization and screening of Ades aegypti D (1) like dopamine receptors. PLoS Neglected Tropical Diseases, 6, 478.
Miller, R.J., Davey, R.B. and George, J.E., 2002. Modification of the food and agriculture organization larval packet test to measure amitraz-susceptibility against Ixodidae. Journal of Medical Entomology, 39, 645–651.
Miller, R.J., Davey, R.B., Hunter White, W. and George, J.E., 2007. A comparison of three bioassay techniques to determine susceptibility in Boophilus microplus (Acari: Ixodidae). Journal of Medical Entomology, 44, 283–294
Mooring MS, McKenzie, A.A. and Hart, B.L., 1996. Grooming in impala: role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiology and Behaviour 59:965–971
Moyo, B. and Masika, P.J., 2009. Tick control methods used by resource-limited farmers and the effect of ticks on cattle in rural areas of the Eastern Cape Province, South Africa. Tropical Animal Health and Production, 41, 517–523.
Muchenje, V., Dzama, K., Chimoyo, M., Raats, J.G. and Strydom, P.E., 2008. Tick susceptibility and its effects on growth performance and carcass characteristics of Nguni, Bonsmura and Angus steers raised on natural pasture. Animal., 2, 298–304.
Ntondini, Z., van Dalen, E.M.S.P. and Horak, I.G., 2008. The extent of acaricide resistance in 1-, 2- and 3-host ticks on communally grazed cattle in the eastern region of the Eastern Cape Province, South Africa. Journal of the South African Veterinary Association., 79(3), 130–135.
Pirali-Kheirabadi, K., Haddadzadeh, H., Razzaghi-Abyaneh, M., Bokaie, S., Zare, R., Ghazavi, M. and Shams-Ghahfarokhi, M., 2007. Biological control of Rhipicephalus (Boophilus) annulatus by different strains of Metarhizium anisopliae, Beauveria bassiana and Lecanicillium psalliotae fungi. Parasitological Research, 100, 1297–1302
Rajput, Z.I., Hu, S., Chen, W., Arijo, A.G. and Xiao, C., 2006. Importance of ticks and their chemical and immunological control in livestock. Journal of Zhejiany University Science B., 7, 912–921.
Robertson, J. L., Russell, R. M., Preisler, H.K. and Savin, N.E., 2007. Bioassays with arthropods. 2nd ed. CRC Press, Tayor and Francis Group, New York.
Roush, T.R. and McKenzie, J.A., 1987. Ecological genetics of insecticide and acaricide resistance. Annual Review of Entomology, 32, 361–80.
Sajid, M.S., Iqbal, Z., Khan, M.N., Muhammad, G. and Iqbal, M.U., 2007. Effect of Hyalomma ticks (Acari: Ixodidae) on milk production of dairy buffaloes (Bos Bubalus Bubalis) of Punjab (Pakistan). Italian Journal of Animal Science 6:939–941
Thullner, F., Willadsen, P. and Kemp, D., 2007. Acaricide rotation strategy for managing resistance in the tick Rhipicephalus (Boophilus) microplus (Acarina: Ixodidae): laboratory experiment with a field strain from Costa Rica. Journal of Medical Entomology, 44, 817–821.
Walker, A.R., Bouattour, A., Camicas, J.L., Estrada-Peña, A., Horak, I.G., Latif, A.A., Pegram, R.G. and Preston, P.M., 2007. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh.
Yilima, J., Adamu, G. and Zerbini, E., 2001. Biossay of acaricide resistance on three tick species at Holotta, Central Ethiopia. Revue Médecine Vétérinaire, 152(5), 385–390.
Yambayamba, K.E., Mukumbuta, W.M., Chileshe, E.C., Simainga, S. and Chonde, D., 2003. Assessment of the existing livestock status and production systems in the ASP operational areas. Technical report.
Zaman, M.A., Iqbal, Z., Abbas, R.Z., Khan, M.N., Muhammad, G., Yonnas M. and Ahmed, S., 2012. In vitro and in vivo acaricidal activity of a herbal extract. Veterinary Parasitology, 18, 431–436.
Acknowledgments
The authors wish to acknowledge financial support from the Ministry of Agriculture and livestock, Zambia. They also extend their thanks to Tedwell Mulutula, Boniface Mulima, Alfred Siame and Gilbert Munongo (Veterinary Assistants, Isoka District) whose kind assistance made this work possible. Similar thanks also go to Dr Hachamba (Veterinary Officer, Isoka District) who allowed use of his staff during the course of this work. Lastly, we thank all members of the farming community in Isoka District for their willingness to participate in the survey and allow their animals to be sampled for the study.
Statement of human and animal rights
Informed consent was obtained from all individual participants included in the study. All applicable international and national guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muyobela, J., Nkunika, P.O.Y. & Mwase, E.T. Resistance status of ticks (Acari; Ixodidae) to amitraz and cypermethrin acaricides in Isoka District, Zambia. Trop Anim Health Prod 47, 1599–1605 (2015). https://doi.org/10.1007/s11250-015-0906-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-015-0906-4