Abstract
An experimental infection of rabbits with a wild isolate of the gastric nematode Graphidium strigosum was carried out. Animals (3.5 months age) were infected with 1,000 L3 administered by bucoesophagic catheter (five rabbits) or kept as uninfected control group (five animals). The infection was maintained for 3 months. Along the experimental period, some parasitological, hematological and immunological parameters were determined. Prepatent period of the infection ranged from 30 to 38 days and, at necropsy, average adult helminth counts were 430.75 ± 126.12. No significant variations were found in packed cell volume, leukocyte, and eosinophil counts along the experimental period. Infection elicited a clear serum-specific IgG response, estimated by ELISA, during patency. Pooled sera from the patent period of the infection recognized some soluble antigens, particularly, a 67-kDa protein. Experimentally infected animals did not show cross recognition between G. strigosum, Haemonchus contortus, and Teladorsagia circumcincta. However, Western blot analysis with hyperimmune sera against H. contortus raised in rabbits and lambs showed cross reactivity between this helminth species and G. strigosum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasitic gastroenteritis (PGE) of sheep and goats are diseases of paramount economic importance worldwide. These infections are extremely prevalent and provoke, depending on the specific composition of the infections and parasite burdens, diminished production or deaths when acute cases are present (Bowman et al. 2004). Therefore, the studies addressed to reduce their presence or to minimize risks of infected animals still continue being a research priority in veterinary parasitology. Among PGE, one of the most significant processes is haemonchosis, caused by the abomasal nematode Haemonchus contortus. This blood-sucking helminth, affecting mainly young animals, has enough pathological and economic impact to be considered as the limiting factor of sheep and goat farming in some parts of the world (Krecek and Waller 2006).
Control of haemonchosis has relied almost entirely on the use of anthelmintics. However, the drug-dependent control is close to be socially and environmentally intolerable (pharmacologically active residues in meat, presence of metabolic residues in the environment, increase of anthelmintic resistances; Wolstenholme et al. 2004). In this scenario, the exploration of alternative control methods is a must; among them, immunoprophylaxis (Vercruysse et al. 2007). Different attempts have been carried out with variable results (Newton and Meeusen 2003). In general, notable protections have been obtained with a variety of purified antigens; however, these results could not be reproduced with antigens expressed by DNA recombinant technology (i.e., Knox et al. 1999; Domínguez-Toraño et al. 2000). In these conditions, availability of antigenic sources more accessible and comparable quality would be an advantage. A number of studies have been carried out to establish an experimental model of H. contortus in gerbils (Conder et al. 1992; Königová et al. 2008). Insofar, the life cycle has not been completed and in no case adults were obtained, thus, rendering the model inadequate for many purposes, in particular, the potentially protective immune response since in haemonchosis, it is stage-related.
Our approach involved the use of a parallel host–parasite model. When selecting the experimental model, several aspects were considered such as localization of adult helminths, close phylogenetic relationship between Trichostrongylidae affecting both host species (Durette-Desset et al. 1999; Audebert and Durette-Desset 2007); easy maintenance and affordable cost, and adequate quantity of helminths obtained at necropsy. On these grounds, we have selected the gastric trichostrongylid of the rabbit, Graphidium strigosum. Infections by this nematode are frequent in leporids (Massone et al. 2003; Lello et al. 2004; Wibbelt and Frölich 2005). However, the information on the endogenous life cycle of this helminth and the possible pathology involved is scarce and inconsistent (Enigk 1938; Cabaret 1981; Nickel and Haupt 1986) and no data are available on the immune response elicited by the infections.
Our research showed that experimental infections did not provoke notable alterations of the hematological parameters determined (packed cell volume, eosinophil counts) in the rabbits although a significant serum-specific IgG response was appreciated. More importantly, notable cross antigenicity between G. strigosum and H. contortus, but not Teladorsagia circumcincta, was found. These findings make this host–parasite model a good candidate to explore both the events taking place in a nematode–stomach environment and the potential use of heterologous antigens in the immunoprophylaxis of haemonchosis.
Materials and methods
Experimental animals
New Zealand × California Giant rabbits (ten) were purchased from Granja San Bernardo (Navarra, Spain) and maintained under conditions precluding undesired parasitic infections in a temperature-controlled room (Department of Animal Health, Faculty of Veterinary Medicine, Madrid). When they reached 3.5 months age they were separated onto two comparable groups: five animals (#18–22) were infected with 1,000 third-stage larvae (L3) of G. strigosum whereas the remaining rabbits (#13–17) were kept as uninfected control group. During the experimental period, animals were fed with commercial pelleted food (Global Diet 2030, Harlan Interfauna Iberica, Spain) and water ad libitum.
Parasites and experimental infection
G. strigosum L3 employed for the experimental infection were originally obtained from a recent isolate from wild rabbits in Central Spain obtained from local hunters. Briefly, wild-caught leporids (40) were necropsied and the adult helminths present in the stomach separated in phosphate buffer saline (PBS). Female G. strigosum were crushed and the eggs obtained cultured (26°C, 10 days) in a mixture of PBS and autoclaved rabbit feces. Infective L3 were isolated by Baermannization and were used to infect a donor rabbit. Eggs obtained from this first experimental infection were collected and treated as above and the L3 obtained used for our experiment. Infective doses (1,000 L3/animal) were administered by bucoesophagic catheter on a single dose. Infections were kept for 3 months (90 days).
Parasitological and hematological follow-up
Fecal egg counts were done before infection and once a week along patency, with a modified McMaster technique (MAFF 1971). After euthanasia (90 days post-infection) stomachs were immediately opened, macroscopic lesions observed and documented and helminths recovered and counted. In addition, gastric mucosa samples were preserved for further studies. Four blood samples were taken along the experiment (day −1 = pre-infection, day 16 = prepatency, day 32= early patency and 65 days post-infection). Using standard laboratory procedures, packed cell volume (PCV), leukocyte and eosinophil numbers were determined on the same days.
Antigens and sera
Soluble extracts of G. strigosum (ASE-Gs), T. circumcincta (ASE-Tc) and H. contortus (ASE-Hc) were obtained by freezing-and-thawing cycles of adult helminths after necropsy, and centrifugation (30,000×g; Klesius et al. 1984).
Anti T. circumcincta sera came from 8 donor lambs infected with doses ranging from 4200 to 25000 L3. Anti-F4, anti-F8 and, anti-F14 were pooled sera from a lamb immunization experiment carried out earlier with isolated protein fractions of adult H. contortus soluble extracts (Alunda et al. 2003). Anti-p26/23 lamb serum was obtained from one of the animals employed in a vaccination experiment with p26/23 of H. contortus (Domínguez-Toraño et al. 2000).
ELISA and Western blotting
Anti-G. strigosum serum IgG levels were determined by ELISA. Microtiter 96-well plates were coated with 5 μg/ml ASE-Gs (4°C, 16 h). Rabbit sera were diluted 1:100 and the secondary antibody was a goat anti-rabbit IgG alkaline phosphatase-labeled (1:1,000; Sigma). All incubations were carried out at 37°C, 1 h. Substrate was 1,4 p-nitrophenil phosphate (1 mg/ml; Boehringer Mannheim). Optical density (OD) at 405 nm was measured with Opsys MR reader (Dynex Technologies).
ASE-Gs, ASE-Tc and ASE-Hc were analyzed by electrophoresis under denaturing and reducing conditions (SDS-PAGE; 15%) following the method described (Cuquerella et al. 1994). After electrotransfer to Immobilon P membranes (Millipore), extracts were probed with anti-H. contortus (1:100), anti-T. circumcincta (1:50) and anti-G. strigosum sera (1:100) for 3 h at 37°C. The secondary antibodies were 1:1,000 diluted anti-rabbit IgG (Sigma) and anti-sheep IgG (Sigma) peroxidase-labeled. Incubations were carried out at 37°C for 1 h. The reaction was developed with 4-chloro-1-naphthol (0.5 mg/ml; BioRad). Low molecular weight (MW) markers employed were from Pharmacia.
Statistical analysis
The average values ± standard deviations of the hematological parameters and ELISA values determined from each group were compared by Student’s t test. A p value ≤0.05 was considered significant.
Results
The average prepatent period of the infection was 32 days (30 to 38). Fecal egg output showed a sustained rise until a peak value (1,175 ± 794.12 eggs per gram of feces, epg) was reached on day 76 post-infection (pi) to continue onwards with plateau values up to euthanasia of animals (90 days pi; Fig. 1). When the animals were necropsied, average adult helminth counts were 430.75 ± 126.12, this, representing an establishment rate of 43 ± 12.61%.
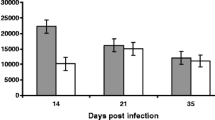
A slight increase in PCV values was found along the experimental infection (Fig. 2a), possibly related to the age increase of the animals, because it was observed in both infected and uninfected rabbits. Leukocyte counts were similar for both groups along the experimental infection (Fig. 2b) and no significant differences were seen in eosinophil counts (Fig. 2c). However, infection with G. strigosum elicited a significant serum-specific IgG response during patency (p < 0.01), reaching an average value of 1.4 OD in the last sera samples. OD values for specific IgG in the control animals remained unchanged for the entire duration of experiment (Fig. 3).
a–c Average values of hematological parameters in G. strigosum infected rabbits (solid bars) and uninfected control animals (open bars) in the four periods sampled: 1: pre-infection sample (Day −1), 2: prepatency period of the infection (Day 16 post-infection), 3: early patency (Day 32 post-infection), 4: late patency (Day 65 post-infection). PCV Packed cell volume. Values are means + standard deviation
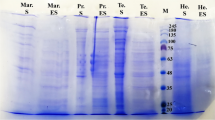
Since no information was available on the protein profile of G. strigosum, a SDS-PAGE analysis of ASE-Gs was carried out. The electrophoresis showed a variety of proteins in the MW range examined (between 94 and 10 kDa), although Coomassie-stained gel evidenced the presence of a very abundant low-MW peptide of ca. 17 kDa (Fig. 4A).
A Electrophoretic analysis under denaturing and reducing conditions (SDS-PAGE; 15%) of adult soluble extracts of G. strigosum (ASE-Gs) and H. contortus (ASE-Hc). MW Molecular weight markers in kDa. B Western blot analysis of adult soluble extract of G. strigosum (ASE-Gs) probed with pooled sera of uninfected rabbits (lane 1), early patent period of the infection (32 days post-infection; lane 2), late patency (65 days post-infection; lane 3). Molecular weight is shown in kDa
The follow-up of the infection by WB with pooled sera of infected rabbits produced parallel results to those obtained by ELISA; thus, although some reactivities (60, 48 kDa) were found in the pre-infection sample (Day −1; Fig. 4B, lane 1) an increase in the recognition of some ASE-Gs antigens was observed during early patency (day 32 post-infection, pi; Fig. 4B, lane 2) and late patency (day 65 pi; Fig. 4B, lane 3). Highest immune recognition was apparent ca. 67 kDa in both cases but, in addition, the late patency poled sera reacted with some antigens of 16, 30, 34, and >67 kDa.
Availability of rabbit and lambs’ sera allowed us to explore the possible cross reactivity between G. strigosum, T. circumcincta and H. contortus ASE. Pooled sera from rabbits infected with G. strigosum did not strongly react with ASE-Tc (Fig. 5A). There was a reactivity of over 50 kDa, already present in the pre-infection sample (Fig. 5A, lane 1), and a very faint recognition around 30 kDa with the sample from the patent period of the infection (Fig. 5A, lane 3). Pooled sera from lambs infected with T. circumcincta did not display any reactivity with G. strigosum antigens (Fig. 5B).
A Western blot analysis of adult soluble extracts of T. circumcincta (ASE-Tc) probed with pooled sera from rabbits infected with G. strigosum (lane 1 pre-infection sample; lane 2 16 days post-infection; lane 3 65 days post-infection). B Western blot of adult soluble extract of G. strigosum (ASE-Gs) probed with pooled sera from lambs monospecifically infected with T. circumcincta (lane 1 pre-infection sample; lane 2 prepatent period of the infection; lane 3 patent infection). MW Molecular weight markers in kDa
The extent of cross reactivity between G. strigosum and H. contortus was higher (Fig. 6). Recognition of bands over 50 kDa was observed in the pre-infection sample (Fig. 6A, lane 1). The reactivity over 50 kDa was maintained during patency of infection besides a doublet between 20–30 kDa (Fig. 6A, lanes 2 & 3). Similarly, H. contortus-infected-lambs’ sera did not react with ASE-Gs (Fig. 6B, lane 1). On the contrary, when sera from lambs immunized with different H. contortus fractions were used, cross reactivity in the MW range examined (14.4–94 kDa) was evident in the animals immunized with F4 (Fig. 6B, lane 2), F8 (Fig. 6B, lane 3), and F14 (Fig. 6B, lane 4), this suggesting the presence of common epitopes. More importantly, rabbit hyperimmune serum against H. contortus protein p26/23 did react not only with the ASE-Hc (Fig. 6A, lane 4) but also with G. strigosum soluble extracts (Fig. 6B, lane 5); other reactivities to molecules of 20, 43, and 60 kDa were also detected.
A Western blot analysis of adult soluble extract of H. contortus (ASE-Hc) probed with pooled sera from uninfected control rabbits (lane 1), rabbits infected with G. strigosum (lane 2 early patency; lane 3 late patency) and hyperimmunized animals against p26/23 of H. contortus (lane 4). B Western blot of adult soluble extract of G. strigosum probed with sera from lambs infected with H. contortus (lane 1) and immunized lambs with isolated fractions of H. contortus (lane 2 F4; lane 3 F8; lane 4 F14; lane 5 p26/23). MW Molecular weight markers in kDa
Discussion
The prepatent period of G. strigosum found under our experimental conditions, 32 days on average, was considerably longer than the observed in other close nematode species from both rabbit (9–13 for Trichostrongylus retortaeformis; Audebert et al. 2007); 16–22 days for Obeliscoides cuniculi and 21–22 days for Nematodiroides zembrae (Hadebert et al. 2002) or sheep (ca. 14–18 days). Results obtained by us were comparable to the 5 weeks reported by Cabaret (1981) or the 40–60 days range reported by Nickel and Haupt (1986) but not with those indicated by the exhaustive work of Enigk (1938; 12–35 days).
The unique nature of the endogenous life cycle of this Trichostrongylidae helminth is also evident by its long patency (21 weeks, according to Cabaret 1981). The pattern of fecal egg output by infected rabbits has been only partially studied but the increase in epg counts during the experimental period (12 weeks) supports the results obtained by Enigk (1938), who found a peak period between 5–15 weeks pi.
Parasite burdens were high, with an establishment rate over 40%, slightly lower than the value found by Nickel and Haupt (1986), but notably higher than the values reported in other trichostrongylids (see Gómez-Muñoz et al. 1999; Aumont et al. 2003). The pathology resulting from G. strigosum infections has been controversial. While some reports consider it a blood-sucking nematode (Enigk 1938; Massone et al. 2003; Wibbelt and Frölich 2005), other authors did not find notable alterations of hematological parameters (Nickel and Haupt 1986). In our studies, we also did not find any alteration in hematological parameters. It is possible, however, that higher parasite burdens could produce such effects.
We can not compare the results obtained on the serum IgG response against G. strigosum primary infections since, to our knowledge, no similar studies have been carried out. In spite of the preliminary nature of this study, the significant serum IgG response observed, that is not present in primary infections by gastric helminths (i.e., Haemonchus; Gómez-Muñoz et al. 1999; Kooyman et al. 2000), points towards an intimate host–parasite relationship. In fact, although this aspect is presently being addressed, in most cases, adult helminths did penetrate up to subserose level, only a few nematodes being actually on the gastric mucosa. Comparative studies could unravel the mechanisms involved in the response against gastric nematodes in other host–parasite combinations.
The lack of cross recognition (T. circumcincta, H. contortus, G. strigosum) in experimental infections was expected in spite of the close relationship between the Trichostrongylidae infecting both host species (rabbit and sheep; Durette-Desset et al. 1999; Audebert and Durette-Desset 2007) and the cross reactivity found among ovine Trichostrongylidae (Cuquerella et al. 1994). However, WB results showed that sera against ASE-Hc, some isolated fractions of H. contortus and, particularly, p26/23, strongly reacted with G. strigosum soluble extracts. This finding suggests the presence of similar antigenic determinants in both helminth species.
Immunization trials against sheep haemonchosis with recombinant proteins have been unsuccessful or behind expectations (Vercruysse et al. 2007), while native proteins did provoke significant protections. The presence of conserved epitopes, and in particular p26/23 which induced a notable protection in lambs younger than 4 months of age (Domínguez-Toraño et al. 2000), suggests that the use of proteins isolated from G. strigosum should be explored. Moreover, the differential immune responses observed in G. strigosum–rabbit and H. contortus–lamb host–parasite systems points towards the interest of this model to analyze the immune modulation mechanisms involved in stomach–helminth relationship.
References
Alunda JM, Angulo-Cubillán F, Cuquerella M (2003) Immunization against ovine haemonchosis with three low molecular weight somatic antigens of adult Haemonchus contortus. J Vet Med B 50:70–74
Audebert F, Durette-Desset MC (2007) Do lagomorphs play a relay role in the evolution of the Trichostrongylina nematodes. Parasite 14:183–197
Aumont G, Gruner L, Hostache G (2003) Comparison of the resistance to sympatric and allopatric isolates of Haemonchus contortus of black belly sheep in Guadeloupe (FW) and of INRA 401 sheep in France. Vet Parasitol 116:139–150
Bowman DD, Lynn RC, Eberhard ML (2004) Georgi’s Parasitología para veterinarios. Trad. esp. 8a edición. Elsevier, Madrid
Cabaret J (1981) Egg output of Graphidium strigosum (Nematoda) in low-level prime infection of rabbits. Folia Parasitologica 28:337–341
Conder GA, Jonson SS, May AD, Fleming MW, Millis MD, Guimond PM (1992) Growth and development of Haemonchus contortus in jirds, Meriones unguiculatus. J Parasitol 76:492–497
Cuquerella M, Gómez-Muñoz MT, Carrera L, de la Fuente C, Alunda JM (1994) Cross antigenicity among ovine trichostrongyloidea. Preliminary report. Vet Parasitol 53:243–251
Domínguez-Toraño IA, Cuquerella M, Gómez-Muñoz MT, Méndez S, Fernández-Pérez FJ, Alunda JM (2000) Vaccination of Manchego lambs against Haemonchus contortus with a somatic fraction (p26/23) of adult parasites. Parasite Immunol 22:131–138
Durette-Desset MC, Hugot JP, Darlu P, Chabaud AG (1999) A cladistic analysis of the Trichostrongyloidea (Nematoda). Int J Parasitol 29:1065–1086
Enigk K (1938) Ein Beitrag fur Physiologie und zum Wirt-Parasit-Verhaltnis von Graphidium strigosum (Trichostrongylidae, Nematoda). Zeitscrift fur Parasitenkunde 10:386–414
Gómez-Muñoz MT, Cuquerella M, Gómez-Iglesias LA, Méndez S, Fernández-Pérez FJ, de la Fuente C, Alunda JM (1999) Serum antibody response of Castellana sheep to Haemonchus contortus infection and challenge: relationship to abomasal worm burdens. Vet Parasitol 81:281–293
Hadebert F, Cassone J, Kerboeuf D, Durette-Desset MC (2002) The life cycle of Nematodiroides zembrae (Nematoda, Trichostrongylina) in the rabbit. J Parasitol 88:898–904
Klesius PH, Washburn SM, Ciordia H, Haynes TB, Snider TG (1984) Lymphocyte reactivity to Ostertagia ostertagi L3 antigen in type I ostertagiasis. Am J Vet Res 45:230–233
Knox DP, Smith SK, Smith WD (1999) Immunization with an affinity purified protein extract from the adult parasite protects lambs against infection with Haemonchus contortus. Parasite Immunol 21:201–210
Königová A, Hrčkova G, Velebný S, Čorba J, Várady M (2008) Experimental infections of Haemonchus contortus strains resistant and susceptible to benzimidazoles and the effect on mast cells distribution in the stomach of Mongolian gerbils (Meriones unguiculatus). Parasitol Res 102:587–595
Kooyman FM, Schallig HD, Van Leeuwen MA, Mackellar A, Huntley JF, Cornelissen AW, Vervelde L (2000) Protection in lambs vaccinated with Haemonchus contortus antigens is age related, and correlates with IgE rather than IgG1 antibody. Parasite Immunol 22:13–20
Krecek RC, Waller PJ (2006) Towards the implementation of the “basket of options” approach to helminth parasite control of livestock: emphasis on the tropics/subtropics. Vet Parasitol 139:270–282
Lello J, Boag B, Fenton A, Stevenson IR, Hudson PJ (2004) Competition and mutualism among the gut helminths of a mammalian host. Nature 428:840–844
Massone A, Capucci L, Macchi C, Giovannini S, Lavazza A (2003) Controllo sanitario di coniglio selvatici nel Parco del Serio (Provincia di Bergamo). J Mt Ecol 7(Suppl.):155–164
Ministry of Agriculture, Fisheries and Food (MAFF) (1971) Manual of Veterinary Parasitological laboratory techniques. HMSO London
Newton SE, Meeusen ENT (2003) Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol 25:283–296
Nickel EA, Haupt W (1986) Experimental studies on the course and consequences of infection with Graphidium strigosum (Nematoda, Trichostrongylidae) in Oryctolagus cuniculus (domestic rabbit). Angewandte Parasitologie 27:215–219
Vercruysse J, Schetters TPM, Knox DP, Willadsen P, Claerebout E (2007) Control of parasitic disease using vaccines: an answer to drug resistance. Rev Sci Tech Off Int Epiz 26:105–111
Wibbelt G, Frölich K (2005) Infectious diseases in European hare (Lepus europaeus). Wild Biol Pract June 1:89–93
Wolstenholme AJ, Fairweather I, Prochard R, von Samson-Himmelstjerna G, Sangster NC (2004) Drug resistance in veterinary helminths. Trends in Parasitology 20:469–476
Acknowledgements
Research was funded by CICYT project AGL2006-10589. Support from the UCM Special Action to JMA (AE3/08-16119) is acknowledged. Valuable comments by Dr. S. Méndez (J.A. Baker Institute, Cornell University) and an anonymous reviewer improved the manuscript. R. Paramio provided excellent technical assistance.
Experimental conditions comply with the Spanish laws on animal experimentation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cuquerella, M., Alunda, J.M. Immunobiological characterization of Graphidium strigosum experimental infection in rabbits (Oryctolagus cuniculus). Parasitol Res 104, 371–376 (2009). https://doi.org/10.1007/s00436-008-1206-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1206-y