Abstract
This study is the first detailed study of the organisation of the neuromuscular system of Cyathocephalus truncatus (Cestoda, Spathebothriidea). Five techniques have been used: (1) immunocytochemistry, (2) staining with TRITC-conjugated phalloidin, (3) NADPHdiaphorase histochemistry, (4) confocal scanning laser microscopy and (5) transmission electron microscopy. The patterns of nerves immunoreactive (IR) to antibodies towards serotonin (5-HT) and the invertebrate neuropeptide FMRFamide are described in relation to the musculature. The patterns of NADPHdiaphorase positive nerves and 5-HT-IR nerves are compared. The fine structure of the nervous system (NS) is described. The organisation of NS in the non-segmented, polyzoic C. truncatus differs clearly from that in the non-segmented, monozoic Caryophyllaeus laticeps and shows distinct similarities with the NS in pseudophyllidean cestodes. This supports the hypothesis that taxon Caryophyllidea and Spatheobothriidea form independent lineages within Eucestoda.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxon Spathebothriidea represents a group of polyzoic tapeworms with a polypleroid body type. This means that, unlike other polyzoic eucestodes that have a strobila body type with proglottisation and distinct segmentation, the spathebothriideans have a body with inner proglottisation, but they lack external segmentation. The taxonomic status, phylogeny and evolution of the spathebothriideans are widely discussed in the literature (see Olson and Tkach 2005). The general biology, taxonomy and morphology of this group has been dealt with by Nybelin (1922), Wardle and McLeod (1952), Gibson (1994), Protasova and Roitman (1995), Kearn (1998) and Okaka (2000). The fine structures of the male and female reproductive systems of two spathebotriidean species, the dixenous C. truncatus and the monoxenous Diplocotyle olriki, have recently been described by Poddubnaya et al. (2005a, b, c, d, 2006, 2007) and Brunanska et al. (2006). Emphasis was placed on those characteristics that might clarify the phylogenetic position of the spathebothriideans among the basal tapeworms and their relationships to the bothriocephalideans, the diphyllobothriideans (Kuchta et al. 2008) and the caryophyllideans (Mackiewicz 2003).
In the discussion about the phylogenetic position of flatworms, the organisation of the nervous system (NS) has been used as one of the discriminating criteria (Reuter et al. 2001; Raikova 2004). The presence of aminergic, cholinergic and peptidergic neuronal signal substances in flatworms has been verified with histochemical and immunocytochemical (ICC) methods (Gustafsson and Maule 2007). The neuronal signal substances have important roles in the regulation of the body musculature and the musculature in association with the attachment organs and the reproductive systems (see Terenina and Gustafsson 2003a; Halton and Maule 2004; Sebelová et al. 2004). Nitric oxide (NO) represents a new category of neuronal signalling substance: a transmitter gas. Information about the nitrergic NS in flatworms is limited. The NADPHdiaphorase (NADPH-d) reaction, i.e. the evidence for the presence of an active neuronal nitric oxide synthase (nNOS) enzyme, has been described in less than 20 flatworms (see Gustafsson et al. 2003a; Terenina et al. 2006). The pattern of acetycholinesterase in C. truncatus has been described by Kotikova and Kuperman (1978). Nothing is known about the aminergic, peptidergic and nitrergic neuronal signal substances in spathebothriidean tapeworms.
This study is the first detailed study of the organisation of the neuromuscular system of C. truncatus. Five techniques have been used: (1) immunocytochemistry, (2) staining with TRITC-conjugated phalloidin, (3) NADPH-d histochemistry, (4) confocal scanning laser microscopy and (5) transmission electron microscopy.
Firstly, the patterns of nerves immunoreactive (IR) to antibodies towards serotonin (5-hydroxytryptamine, 5-HT) and the invertebrate neuropeptide FMRFamide are described in relation to the musculature. Secondly, the patterns of NADPH-d-positive nerves and 5-HT-IR nerves are compared. Thirdly, the fine structure of the NS is described.
Material and methods
Specimens of adult C. truncatus (Pallas 1781) Kessler, 1868 were recovered from the pyloric caeca of whitefish (Coregonus lavaretus) from the White Sea, Russia. The material was fixed in 4% paraformaldehyde in 0.1 M phosphate buffer at 4°C. For storage, it was transferred to the same buffer with 10% sucrose. The material was embedded in Tissue-Tek, frozen and sectioned at 20 μm using a Bright cryostat. The sections were collected on chrom-alum-gelatin-coated glass slides, dried for about 2 h at room temperature and stained directly.
Immunocytochemistry
Cryostat sections of C. truncatus were stained with rabbit-anti-5-HT (Incstar, Stillwater, MN, USA) (1:500) or rabbit-anti-FMRFamide (Peninsula, Belmont, CA, USA) (1:500) according to the method described by Coons et al. (1955). The sections were incubated with the primary antibody for 2 days at 4°C and with the secondary antibody swine anti-rabbit FITC (DAKO) 1:50 for 3 h at 4°C. Controls included omission of the primary antibody and substitution of primary antibody with non-immune rabbit serum.
Phalloidin staining of the musculature
In order to study the relationship between the patterns of 5-HT-IR and FMRF-IR nervous elements and the musculature, staining with TRITC-labelled phalloidin (Sigma, St. Louis, MO, USA) (1:200) was performed for 20 min at 4°C (Wahlberg 1998). The phalloidin staining was performed after the ICC staining. The ICC and the phalloidin staining were performed at the Department of Biology, Åbo Akademi University, Finland.
NADPH-d histochemistry
The NADPH-d staining was performed on cryostat sections. The staining was performed according to Lindholm et al. (1998). In the incubation medium, the final concentration of nitroblue tetrazolium was 1 mg/ml, and that of β-NADPH was 2 mg/ml. For controls, β-NADPH was substituted with β-NAD, β-NADH or β-NADP in concentrations as above (2 mg/ml). The incubation time for sections was 2 h at 37°C. All the above-mentioned chemicals were from Sigma. To exclude coexistence, double staining with NADPH-d and anti-5-HT was performed. The NADPH-d staining was performed before the ICC staining. The staining was performed at the Centre of Parasitology of A. N. Severtsov Institute of Ecology and Evolution, RAS, Moscow. The ICC staining was performed at the Department of Biology, Åbo Akademi University, Finland.
Light microscopy and confocal scanning laser microscopy
The slides stained with NADPH-d were examined with Carl Zeiss microscope Axiostar plus at the Centre of Parasitology of A. N. Severtsov Institute of Ecology and Evolution, RAS, Moscow. The slides stained with anti-5HT, anti-FMRFamide and TRITC-labelled phalloidin were examined with a Leica TCS 4D confocal scanning laser microscope coupled to a Leitz Aristoplan fluorescence microscope at the Department of Biology, Ǻbo Akademi University, Finland.
Transmission electron microscopy
Adult C. truncatus were recovered from the pyloric caeca of whitefish (C. lavaretus) and grayling (Thymallus thymallus) from Lake Segozero, Karelia, Russia. The worms were processed as described by Poddubnaya et al. (2005a). They were examined in a JEM-1010 C transmission electron microscope at the Institute of Inland Waters, Russian Academy of Sciences, Borok, Russia.
Results
The confocal scanning laser microscopical method made it possible to follow the pattern of muscle fibres stained with TRITC-labelled phalloidin in relation to the pattern of 5-HT-IR and FMRFamide-IR nerve structures respectively.
The musculature
The musculature of C. truncatus consists of longitudinal, transverse, dorsoventral and subtegumental muscle fibres. In the funnel-shaped scolex and the neck region, the musculature is well developed (Fig. 1). The layer of longitudinal muscles is the strongest layer, and it divides the body into a medullary and a cortical parenchyma. The walls of the reproductive ducts are surrounded by circular and longitudinal muscle fibres (Figs. 4, 7 and 8).
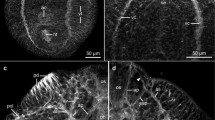
Frontal section of scolex and neck region showing the longitudinal muscles (lm), transverse muscles (tm), dorsoventral muscles (dvm), subtegumental muscles (stm) and tegument (t). Bar = 100 μm
Image pair of Fig. 1 showing the pattern of 5-HT-IR nerves. Brain ganglion (G), main nerve cords with cell bodies (MC), minor nerve cord (mc), transverse commissure (tc), subtegumental nerve terminals (snt) and tegument (t)
Section of neck region, showing the pattern of 5-HT-IR nerves. Main nerve cord (MC), longitudinal muscles (lm), 5-HT-IR nerve cell bodies (cb), minor nerve cord (mc), subtegumental nerve terminals (snt) and tegument (t). Bar = 100 μm
Pattern of 5-HT nerves around the reproductive ducts. Cirrus sac (cs), cirrus (c), uterovaginal duct (uva), longitudinal muscles (lm) and transverse nerve commissure (tc). Bar = 100 μm
The 5-HT IR NS
In C. truncatus, the central nervous system (CNS) consists of a bilobed brain (= two ganglia connected with a ring commissure) in the scolex and two main cords (MCs), extending in the medullary parenchyma from the brain ganglia to the posterior end of the body. The brain and the MCs consist of a densely interwoven fibrillar neuropile of axons and dendrites. The MCs measure approximately 30 μm in diameter in the neck region and become slightly thinner towards the posterior end of the body. The peripheral nervous system (PNS) consists of numerous thin minor cords, which run in the cortical parenchyma along the outside of the longitudinal muscles. Thin commissures connect the main and the minor cords. The minor cords gradually become thinner, forming nerve plexuses beneath the tegument and on the longitudinal muscles.
5-HT-IR nerve fibres occur in the brain, the MCs, the minor nerve cords and the commissures (Figs. 2 and 3). The 5-HT-IR nerve cell bodies are usually bipolar and measure approximately 20 × 8 μm. The 5-HT-IR cell bodies occur at the surface of the brain and along the MCs. Many 5-HT-IR cell bodies were also observed in the longitudinal muscle layer. These cell bodies send processes to the main and the minor cords, connecting them (Figs. 2 and 3). The 5-HT-IR nerve fibres end in terminals beneath the basal lamina of the tegument, forming a subtegumental nerve plexus in the whole worm (Figs. 2 and 3). Many 5-HT-IR transverse fibres connect the two MCs. A plexus of thin varicose 5-HT-IR nerve fibres occur in the muscle layers surrounding the reproductive ducts (Fig. 4).
The FMRFamide IR NS
The NS stains strongly with anti-FMRFamide. Staining was observed in the brain, the MCs, the minor nerve cords and in the commissures (Figs. 5 and 6). Thin FMRF-IR nerve fibres extend from the brain between muscle fibres, terminating beneath the basal lamina of the tegument covering the whole surface of the funnel-shaped scolex (Fig. 5). The density of FMRF-IR terminals is as high on the inside as on the outside of the scolex. In addition, FMRF-IR nerve fibres extend from the MCs through the longitudinal muscle layer to the cortical parenchyma, ending in terminals beneath the basal lamina of the tegument, forming a dense subtegumental nerve plexus (Figs. 5 and 6). A FMRFamide-IR nerve plexus surrounds the cirrus sac and the utero-vaginal atrium (Fig. 7). Furthermore, FMRF-IR fibres were observed on the surface of the ovary, the Mehlis glands and the uterine glands (Fig. 8).
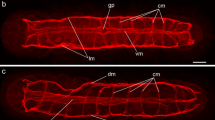
Frontal section of scolex and neck region showing the pattern of FMRF-IR nerves. Brain ganglion (G), main nerve cord (MC), transverse nerve commissure (tc), subtegumental nerve terminals (snt) and tegument (t). Bar = 100 μm
The pattern of FMRF-IR nerves in mid region. Main nerve cord (MC), minor nerve cord (mc), transverse commissure (tc) and longitudinal muscles (lm). Bar = 100 μm
A network of FMRF-IR nerves around the reproductive ducts. Cirrus sac (cs), cirrus (c), uterovaginal duct (uva) and egg (e). Bar = 100 μm
The pattern of FMRF-IR nerves close to Mehlis gland (MG), ovary (o), uterine gland (ug), uterus (u) and ovary duct (od). Bar = 100 μm
The patter of 5-HT-IR nerves in main cord (MC) and nerve cell bodies (cb) in nerves extending from the main cord towards the tegument. Bar = 100 μm
Image pair of Fig. 9 stained with NADPHd. Positive NADPHd reaction occurs in the main cord (MC) and close to muscle fibres. Testes (te)
The nitrergic NS
Deep blue NADPH-d staining was demonstrated in the CNS of C. truncatus. Double staining with NADPH-d and anti-5HT revealed that both occur in the MCs. However, the 5-HT-IR cell bodies that lie close to the MCs do not contain NADPH-d positive material (Figs. 9 and 10). Distinct NADPH-d staining was observed in very thin nerve fibres in close association with all types of muscle, i.e. the main longitudinal, transverse and dorsoventral muscles, as well as subtegumental musculature and the musculature of the reproductive organs.
The fine structure of the NS
The central part of the brain and the MC of C. truncatus are composed of densely packed small and large nerve fibres (Fig. 11). No extracellular stroma is present between the nerve fibres. Many of the nerve fibres are devoid of vesicles. However, some nerve fibres contain the following types of vesicles:
-
1.
Small, clear vesicles (sv), measuring about 30 nm in diameter
-
2.
Dense-core vesicles (dcv), measuring 70–120 nm in diameter
-
3.
Large, dense vesicles (ldv), measuring 130–560 nm in diameter
-
4.
Large, lucent vesicles (llv), measuring 90–100 nm in diameter
Small magnification of neuropile in main nerve cord showing the tightly packed nerve fibres. Dense core vesicles (dcv) occur in some nerve fibres. Mitochondrion (m), synapse (s). Bar = 1 μm
Synapses containing small clear vesicles (sv) and dense core vesicles (dcv). Presynaptic density (black arrow), postsynaptic density (white arrow). Bar = 0.5 μm
Synapses containing small clear vesicles (sv) and dense core vesicles (dcv). Presynaptic density (black arrow), postsynaptic density (white arrow). Bar = 0.5 μm
Nerve fibres containing large dense vesicles (ldv) running between muscle fibres (M). The arrow points to an omega figure, which indicates neuronal release. Bar = 1 μm
Nerve fibre containing large dense vesicles (ldv) close to basal lamina (BL) of tegument (T). Muscle fibres (M). Bar = 1 μm
Large magnification of nerve fibre containing large dense vesicles (ldv). The arrow points to an omega figure, which indicates neuronal release. Extra cellular filaments (ecf). Muscle fibre (M). Bar = 0.5 μm
Nerve cell body containing large dense vesicles (ldv). Nucleus (n), mitochondria with short cristae (m), rough endoplasmatic reticulum (rer) and muscle fibres (M). Bar = 1 μm
Nerve fibres close to Mehlis gland (MG). Dense core vesicles (dcv), small clear vesicles (sv) and neurotubules (nt). Bar = 1 μm
The vesicles occur in different combinations in the nerve fibres. Nerve fibres containing sv and dcv were observed in the CNS and the PNS. The most common type of synapse contains sv and dcv. The sv outnumber by far the dcv (Fig. 12). Both types of vesicle are tightly accumulated on the presynaptic side. Generally, the dcv are located farther from the synaptic site than the sv. The synaptic cleft is 20 nm wide and filled with material of moderate density. Both pre- and postsynaptic densities occur. The synapses measure 200–400 nm in length. Shared and single synapses were observed (Fig. 13).
Nerve fibres containing ldv occur in the CNS and the PNS. These nerve fibres run very close to the muscle fibres, and many terminate beneath the basal lamina of the tegument, forming a subsurface nerve plexus (Figs. 14 and 15). Generally, these nerve fibres are packed with ldv. A few omega figures indicating release from the ldv were observed (Figs. 14 and 16). Only seldom were a few llv observed in the same nerve fibres. The muscle fibres are composed of tightly packed thin and thick myofilaments (Figs. 14, 15, 16, 17). Extracellular filaments were observed between the muscle fibres (Fig. 16). Figure 17 shows a nerve cell body containing ldv. The mitochondria are round and contain few cristae. Many free ribosomes but very few RER membranes occur in the cell body.
Figure 18 shows nerve fibres close to the Mehlis gland. Some of the nerve fibres do not contain vesicles. Some of them contain sv and dcv. Neurotubules were observed in the nerve fibres.
Discussion
Gross anatomy
The plan for the flatworm NS is the so-called orthogon, a rectilinear, ladder-like configuration of longitudinal cords connected at intervals by transverse commissures (see Halton and Gustafsson 1996). Thirty years ago, Kotikova and Kuperman (1978) described the pattern of acetycholinesterase in C. truncatus. They found four ganglia connected by a nerve ring in the scolex, two main lateral nerve cords extending longitudinally in the medullary parenchyma, numerous small nerve cords running along the outside of the longitudinal muscles and nerve plexuses beneath the tegument. According to Protasova and Roitman (1995), the NS in C. truncatus consists of two brain ganglia and two MCs. In the scolex, four minor cords branch out from the ganglia and form nerve plexuses.
The serotoninergic, peptidergic and nitrergic NS of C. truncatus has never been described before. The ICC analysis of the patterns of 5-HT-IR and FMRFamide-IR nerves also gives, in addition to information about the occurrence of the above-mentioned neuronal signal substances, a picture of the general neuroanatomy of the worm. The ultrastructural part deepens the knowledge of the NS. The NS of C. truncatus follows the general plan for NS in flatworms with a bilobed brain, two MCs, many minor cords, connecting commissures and nerve plexuses.
The 5-HT IR NS
5-HT-IR nerves have been described from the NS in all flatworm taxons investigated so far (see Terenina and Gustafsson 2003a; Biserova 2004; Halton and Maule 2004; Raikova 2004). The pattern of 5-HT-IR nerves in C. truncatus conforms to that of other tapeworms, and the 5-HT-IR cell bodies are of the same size as in other parasitic flatworms (Gustafsson et al. 1985; Terenina et al. 2006). 5-HT is generally regarded as the main excitatory neurotransmitter of motor activity in flatworms (see Halton and Maule 2004).
The FMRFamide IR NS
This is the first demonstration of a neuropeptide in a spathebothriidean flatworm. FMRFamide has been localised with ICC methods in flatworms from all taxons studied so far (see Day and Maule 1999; Gustafsson et al. 2002; Halton and Maule 2004; Gustafsson and Maule 2007). The pattern of FMRFamide-IR nerves in C. truncatus conforms to that of other tapeworms. Generally, FMRFamide and 5-HT occupy separate sets of neurones and fibres in flatworms (see Gustafsson et al. 2002; Halton and Maule 2004). Unfortunately, double staining with anti-5-HT and anti-FMRF was not performed in this study. FMRFamide belongs to the FaRP neuropeptide family and has been shown to be myoexcitatory in a concentration-dependent manner when applied exogenously to isolated muscle cells or muscle strips from free-living and parasitic flatworms (Day and Maule 1999). The authors point out that the potent, specific, and immediate action of FaRPs on flatworm muscles suggest that they are acting as fast transmitters rather than as modulators.
The NADPH-d positive NS
To date, the pattern of NADPH-d has been studied in less than 20 flatworms (see Gustafsson et al. 2003a; Terenina et al. 2006). Cellular signalling mediated by NO involves the highly regulated synthesis of NO by nNOS, the diffusion of NO into adjacent target cells and the synthesis of the second messenger cGMP (Garthwaite and Boulton 1995). An nNOS-like enzyme has been identified by radiometric analysis in Hymenolepis diminuta and Fasciola hepatica (Terenina et al. 2000, 2003). The patterns of cGMP-IR nerves have been described in adult H. diminuta and F. hepatica, plerocercoids of Diphyllobothrium dendriticum and cercaria of Diplostomum chromatophorum (Gustafsson et al. 2003a, b; Terenina and Gustafsson 2003b). The effect of a NO donor on the synthesis of cGMP in H. diminuta has been followed by radiometric analysis (Onufriev et al. 2005). When studying the pattern of the NADPH-d reaction in flatworms, a close association to the muscle fibres has consistently been observed. A myoinhibitory role of NO in flatworms has been suggested (Gustafsson et al. 2001). For the first time, the presence of NADPH-d staining in nerve fibres in a spathebothriidean flatworm has been demonstrated. The pattern of NADPH-d in C. truncatus conforms to that in other flatworms (Gustafsson et al. 1996, 2001; Lindholm et al. 1998). Further studies are needed.
The fine structure of the NS
In flatworms, which lack a coelom and a circulatory system, integration takes place through versatile and highly secretory neurones engaged in the synthesis and export of material by axonal transport in vesicles (Halton and Gustafsson 1996). They release the neuronal mediators to the intercellular space close to target cells or organs, in a synaptical or non-synaptical paracrine way (Gustafsson 1992). A diversity in both size and structure of vesicles has been recognised in the NS of flatworms, and they have been used as markers for the different neuronal cell types. The small clear vesicles (sv) of the synaptic type are regarded as cholinergic or as vesicles for recapturing membranes or for retrieval of Ca2+. The dense-cored vesicles (dcv) are regarded as aminergic and the large dense vesicles (ldv) as peptidergic. However, the results of immunogold-labelling experiments at the electron microscopical level have shown these broad categories to be unreliable, with immunoreactivities for neuropeptides most often observed in dcv. In all probability, vesicle ultrastructure likely depends on the developmental stage observed, the processing state of the neuroactive substances involved and the co-existence of neuroactive substances (Halton and Gustafsson 1996). This is the first study of the fine structure of the NS in C. truncatus. Four kinds of vesicles were observed in the nerve fibres. The vesicles and the synapses are of the same type as those found in the NS of D. dendriticum, Triaenophorus nodulosus and Amphilina foliacea (Gustafsson 1984; Biserova et al. 1996, 2000). The ldv-filled nerve terminals beneath the basal lamina of the tegument in C. truncatus correspond to the nerve terminals identified by the ICC analysis. The same pattern has been observed in many tapeworms. The tegument is the nutrient-absorbing surface of tapeworms, and the need for innervation of this surface is obvious (Gustafsson 1992).
Phylogenetic aspects
Taxon Spathebothriidea is regarded to be related to both the monozoic taxon Сaryophyllidea and the polyzoic taxon Pseudophyllidea (see Olson and Tkach 2005). The NS of the non-segmented, monozoic Caryophyllaeus laticeps (Caryophyllidea) has recently been described by Biserova (2004). The level of centralisation in C. laticeps is very low. No brain ganglia, no neuropile and no brain commissures were observed. The organisation of NS in the non-segmented, polyzoic C. truncatus thus differs clearly from the organisation of the NS in the non-segmented, monozoic C. laticeps and shows distinct similarities with the NS in pseudophyllidean cestodes (see Halton and Maule 2004). According to Poddubnaya et al. (2006) and Brunanska et al. (2006), taxons Caryophyllidea and Spatheobothriidea form independent lineages within Eucestoda.
This investigation was supported by the Russian Foundation for Fundamental Research grant no.08–04–00271a and the Research Institute of the Foundation for Åbo Akademi University. We wish to thank Mr. Jari Korhonen and Mr. Esa Nummelin for technical assistance.
References
Biserova NM (2004) The nervous system in Cestoda and Amphilinidea. Doctoral thesis, Moscow University (in Russian)
Biserova NM, Gustafsson MKS, Reuter M, Terenina NB (1996) The nervous system of the pike-tapeworm Triaenophorus nodulosus (Cestoda: Pseudophyllidea)—ultrastructural and immunocytochemical mapping of aminergic and peptidergic elements. Invert Biol 115:273–285
Biserova NM, Dudicheva VA, Terenina NB, Reuter M, Halton DW, Maule AG, Gustafsson MKS (2000) The nervous system of Amphilina foliacea (Platyhelminthes, Amphilinidea). An immunocytochemical, ultrastructural and spectrofluorometrical study. Parasitology 121:441–453
Brunanska M, Scholz T, Dezfuli BS, Poddubnaya LG (2006) Spermiogenesis and sperm ultrastructure of Cyathocephalus truncatus (Pallas 1781) (Cestoda: Spathebothriidea). J Parasitol 92:176–184
Coons AH, Leduc EH, Connelly JM (1955) Studies on antibody production. I. A method for histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med 102:42–60
Day TA, Maule AG (1999) Parasitic peptides! The structure and function of neuropeptides in parasitic worms. Peptides 20:999–1019
Garthwaite J, Boulton BL (1995) Nitric oxide signalling in the central nervous system. Annu Rev Physiol 57:683–706
Gibson DI (1994) Order Spathebothriidea Wardle & McLeod, 1952. In: Khalil LF, Jones A, Bray RA (eds) Keys to the cestode parasites of vertebrates. CAB, Wallingford, pp 15–19
Gustafsson MKS (1984) Synapses in Diphyllobothrium dendriticum (Cestoda). An electronmicroscopical study. Ann Zool Fennici 21:167–175
Gustafsson MKS (1992) The neuroanatomy of parasitic flatworms. Adv Neuroimmunol 2:267–286
Gustafsson MKS, Maule MG (2007) The nervous system of helminths. In: Mehlhorn H (ed) Encyclopedia of parasitology. Springer, Berlin Heidelberg New York
Gustafsson MKS, Wikgren MC, Karhi TJ, Schot LPC (1985) Immunocytochemical demonstration of neuropeptides and serotonin in the tapeworm Diphyllobothrium dendriticum. Cell Tissue Res 240:255–260
Gustafsson MKS, Lindholm AM, Terenina NB, Reuter M (1996) NO nerves in a tapeworm. NADPHdiaphorase histochemistry in adult Hymenolepis diminuta. Parasitology 113:559–565
Gustafsson MKS, Terenina NB, Kreshchenko ND, Reuter M, Maule AG, Halton DW (2001) Comparative study of the spatial relationship between nicotinaminde adenine dinucleotide phosphate-diaphorase activity, serotonin immunoreactivity, and GYIRF-amide immunoreactivity and the musculature of the adult liver fluke, Fasciola hepatica (Digenea, Fasciolidae). J Comp Neurol 429:71–79
Gustafsson MKS, Halton DW, Kreshchenko ND, Movsessian SO, Raikova OI, Reuter M, Terenina NB (2002) Neuropeptides in flatworms. Peptides 23:2053–2061
Gustafsson MKS, Gaivoronskaja TV, Terenina NB, Tolstenkov OO (2003a) The nitrergic nervous system in flatworms. Helminthologia 40:79–85
Gustafsson MKS, Terenina NB, Reuter M, Movsessian SO (2003b) NO nerves and their targets in a tapeworm: an immunocytochemical study of cGMP in Hymenolepis diminuta. Parasitol Res 90:48–152
Halton DW, Gustafsson MKS (1996) Functional morphology of the platyhelminth nervous system. Parasitology 113:S47–S72
Halton DW, Maule AG (2004) Flatworm nerve-muscle: structural and functional analysis. Can J Zool 82:316–333
Kearn GC (1998) Parasitism and the platyhelminths. Chapman & Hall, London, p 221
Kotikova EA, Kuperman BI (1978) New data on the structure of the nervous system in cestodes of the order Pseudophyllidea. Biologiya Morya 6:41–46 (in Russian)
Kuchta R, Scholz T, Brabec J, Bray RA (2008) Suppression of the tapeworm order Pseudophyllidea (Platyhelminthes: Eucestoda) and the proposal of two new orders, Bothriocephalidea and Diphyllobothriidea. Int J Parasitol 38:49–55
Lindholm AM, Reuter M, Gustafsson MKS (1998) The NADPH-diaphorase staining reaction in relation to the aminergic and peptidergic nervous system and the musculature of adult Diphyllobothrium dendriticum. Parasitology 117:283–292
Mackiewicz JS (2003) Caryophyllidea (Cestoidea): molecules, morphology and evolution. Acta Paraitol 48:143–154
Nybelin O (1922) Anatomisch-systematische studien über pseudophyllideen. Göteborgs Kungl. Vetenkaps- och Vitterhets-Samhälles Handlingar, Fjärde Följden, 26, 228 pp
Okaka CE (2000) Maturity of the procercoid of Cyathocephalus truncatus (Eucestoda: Spathebothriidea in Gammarus pulex (Crustacea: Amphipoda) and tapeworm life cycle using the amphipod as the sole host. Helminthologia 37:153–157
Olson PD, Tkach VV (2005) Advances and trends in the molecular systematics of the parasitic Platyhelminthes. Adv Parasitol 60:165–243
Onufriev MV, Gulyaeva NV, Terenina NB, Tolstenkov OO, Gustafsson MKS (2005) The effect of a nitric oxide donor on the synthesis of cGMP in Hymenolepis diminuta: a radiometric study. Parasitol Res 95:22–24
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Dezfuli BS (2005a) Fine structure of the male reproductive ducts, vagina and seminal receptacle of Cyathocephalus truncatus (Cestoda: Spathebothriidea). Folia Parasitologica 52:241–250
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Scholz T (2005b) Fine structure of the female reproductive ducts of Cyathocephalus truncatus (Cestoda: Spathebothriidea), from salmonid fish. Folia Parasitologica 52:323–338
Poddubnaya LG, Mackiewicz JS, Bruňanská M, Scholz T (2005c) Ultrastructural studies on the reproductive system of progenetic Diplocotyle olrikii (Cestoda: Spathebothriidea): ovarian tissue. Acta Parasitologica 50:199–207
Poddubnaya LG, Mackiewicz JS, Świderski Z, Bruňanská M, Scholz T (2005d) Fine structure of egg-forming complex ducts, eggshell formation and supporting neuronal plexus in progenetic Diplocotyle olrikii (Cestoda: Spathebothriidea). Acta Parasitologica 50:292–304
Poddubnaya LG, Gibson DI, Olson P (2006) Vitellocyte ultrastructure in the cestode Didymobothrium rudolphii Monticelli, 1890: possible evidence for the recognition of divergent taxa within the Spathebothriidea. Acta Parasitologica 51:255–263
Poddubnaya LG, Gibson DI, Olson PD (2007) Ultrastructure of the ovary, ovicapt and oviduct of the spathebothriidean tapeworm Didymobothrium rudolphii (Monticelli, 1890). Acta Parasitologica 52:127–134
Protasova EN, Roitman VA (1995) Cyathocephalates, tapeworm helminths of marine and freshwater fish (Cestoda: Pseudophyllidea: Cyathocephalata). Essentials of cestodology. Institute of Parasitology RAN, Moskva, p 12,134 (in Russian)
Raikova OI (2004) Neuroanatomy of basal bilaterians (Xenoturbellida, Nemertodermatida, Acoela) and it phylogenetic implications. Doctoral thesis, Department of Biology, Åbo Akademi University, Åbo, Finland
Reuter M, Raikova OI, Jondelius U, Gustafsson MKS, Maule AG, Halton DW (2001) Organisation of the nervous system in the Acoela: an immunocytochemical study. Tissue Cell 33:119–128
Sebelová Ś, Stewart MT, Mousley A, Fried B, Marks NJ, Halton DW (2004) The musculature and associated innervation of adult and intramolluscan stages of Echino-stoma caproni (Trematoda) visualised by confocal microscopy. Parasitol Res 89:199–206
Terenina NB, Gustafsson MKS (2003a) Neurotransmitters in helminthes (biogenic amines, nitric oxide) In: Movsessian SO (ed) Moscow NAUKA, 178 p. (in Russian)
Terenina NB, Gustafsson MKS (2003b) Nitric oxide and its target cells in cercaria of Diplostomum chromatophorum: a histochemical and immunocytochemical study. Parasitol Res 89:199–206
Terenina NB, Onufriev MV, Gulyaeva NV, Lindholm AM, Gustafsson MKS (2000) A radiometric analysis of nitric oxide synthase activity in Hymenolepis diminuta. Parasitology 120:91–95
Terenina NB, Onufriev MV, Gulyaeva NV, Moiseeva YV, Gustafsson MKS (2003) Nitric oxide synthase activity in Fasciola hepatica: a radiometric study. Parasitology 126:585–590
Terenina NB, Tolstenkov OO, Fagerholm H-P, Serbina EA, Vodjanitskaja SN, Gustafsson MKS (2006) The spatial relationship between the musculature and the NADPH-diaphorase asctivity, 5-HT and FMRFamide immunoreactivity in redia, cercaria and adult Echinoparyphium aconiatum (Digenea). Tissue Cell 38:151–157
Wahlberg MH (1998) The distribution of F-actin during the development of Diphyllobothrium dendriticum. Cell Tiss Res 291:561–570
Warde, McLeod JA (1952) The zoology of tapeworms. University of Minnesota Press, Minneapolis, p 780
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terenina, N.B., Poddubnaya, L.G., Tolstenkov, O.O. et al. An immunocytochemical, histochemical and ultrastructural study of the nervous system of the tapeworm Cyathocephalus truncatus (Cestoda, Spathebothriidea). Parasitol Res 104, 267–275 (2009). https://doi.org/10.1007/s00436-008-1187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1187-x