Abstract
The systematic position of the Orthonectida remains enigmatic. According to a classical point of view, they are placed together with Dicyemida in the phylum Mesozoa. Traditionally, orthonectids are regarded as rather primitive organisms, lacking digestive, muscular, and nervous systems. Here, using confocal laser scanning microscopy (CLSM) and immunohistochemical methods, we describe the musculature and serotoninergic nervous system of female adults of Intoshia linei (Orthonectida). The whole muscular system consists of 4 longitudinal and 9–11 circular muscle cells. The general muscular topography corresponds to the typical pattern for small-sized annelids or flatworms. Immunohistochemistry reveals six serotonin-like cells, which form part of a small nervous system comprising only 10–12 total cells based on nuclear counts. This is the first finding of a serotoninergic nervous system in orthonectids. Our analysis of muscular and neural organization in Orthonectida reveals significant differences from Diciemyda and aligns it more closely with the Lophotrochozoa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Orthonectida is probably one of the most enigmatic groups of so-called lower Metazoa, whose phylogenetic relationships remain uncertain (Giard 1877; Hatschek 1888; Hartmann 1925; Stunkard 1954; Caullery 1961; Hamlet et al. 1996; Pawlowski et al. 1996). Representatives of this group are parasites of a wide range of marine invertebrates. Their life cycle is rather simple and consists of two alternating generations: a free-living sexual stage and a parasitic stage, commonly referred to as plasmodium. The main mode of locomotion of orthonectid free living stages—males, females, and larvae—is ciliary swimming (Giard 1877; Giard 1879; Metschnikoff 1879; Metschnikoff 1881; Caullery and Mesnil 1901), which is generally considered to be a primitive feature. Most authors working with this group have either pointed to the absence of muscular and nervous systems in orthonectids or avoided mentioning their presence. In his final survey on orthonectids, Caullery (1961) stated that the female Rhopalura ophiocomae lacks any muscular system. Nevertheless, Kozloff (1969, 1971) described supposedly contractile cells in free-swimming stages of R. ophiocomae and Ciliocincta sabellariae using TEM and Protargol impregnated preparations. Later, morphologically similar cells were described in the female of Intoshia variabili (Slyusarev 1994, 2003). Moreover, female I. variabili have been observed to curve the anterior part of the body, which indirectly suggests the presence of a contractile system. Slyusarev and Manylov (2001) using whole mount rhodamine phalloidin staining of I. variabili demonstrated that the contractile system of Orthonectida is composed of true muscle cells. The presence of muscle cells requires neural integration, as found in all other animals; thus, both systems should be expected to occur in orthonectids.
Here, we describe the muscular and nervous systems of female Intoshia linei based on the combination of phalloidin staining, immunohistochemistry (anti-5HT), and confocal laser scanning microscopy (CLSM).
Materials and methods
The orthonectid I. linei (Giard, 1877) is a parasite of the nemertine Lineus ruber (Müller, 1774) (Nemertina: Enopla: Heteronemertea). The nemertines were collected in August, 2014, in the Barents Sea at the marine biological station Dalnie Zelentsi (69° 07′ N, 36° 05′ E). The hosts were collected at the low tide under the stones. The infeсted nemertines were maintained in Petri dishes with filtered sea water. The emission of the free-living stages was carried out by rising the temperature of the water up to 10–15 °C.
Specimens of I. linei were fixed for 8–12 h in 4 % paraformaldehyde (PFA) with 0.01 M phosphate-buffered saline (PBS). After fixation, specimens were washed three times in PBS, containing 0.1 % Triton X-100 (PBT), and then stored in PBS with 0.1 % NaN3 at +4 °С. Prior to immunostaining, specimens were blocked in PBT containing 1 % BSA for 2 h and incubated in primary antibodies against acetylated α-tubulin (Sigma, T-6793; dilution 1:1000–1:2000) and serotonin (Immunostar, 20080, diluted 1:2000) overnight at 21 °C. Afterward, specimens were washed three times in PBT for 15 min and incubated with secondary antibodies diluted 1:800–1:1000 overnight. We used the following secondary antibodies: Alexa Fluor 488 Donkey Anti-Rabbit (Molecular probes, A-21206) and Alexa Fluor 647 Donkey Anti-Mouse (Molecular probes, A-31571). To visualize muscular elements, after immunolabeling, the specimens were stained with TRITC-conjugated phalloidin in dilution 1:100 for 2 h. Cell nuclei were stained with Hoechst 33258 or DAPI. All the incubations were performed at 4 °C. Specimens were immersed in glycerol or Mowiol and mounted between two coverslips. Observations were made with Leica TCS SP5 or Leica TCS SPE laser confocal microscopes. Resulting stacks of images were processed using FIJI package (Schindelin et al. 2012). A total of more than 200 specimens were examined and documented.
Results
Muscular system of the female I. linei
The muscular system of the female I. linei consists of longitudinal and circular muscles only (Fig. 1). All muscle cells are located immediately under the ciliated epidermis. The longitudinal muscles lie outside the circular muscles (Figs. 1d and 3a, b). The longitudinal musculature consists of muscle bands that extend from anterior to posterior in dorsal, ventral, and lateral positions (Figs. 1 and 3a, b).
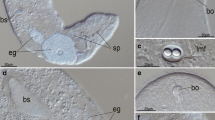
Muscle system of female I. linei. TRITC-phalloidin staining, CLSM. a General view, depth-coded image. b Lateral view. c Ventral view. d Longitudinal section at the level of longitudinal muscles. cm circular muscles, dm dorsal muscles, gp genital pore, lm lateral muscles, vm ventral muscle. Scale bar 10 μm
The dorsal and ventral muscle bands are of similar structure and parallel to each other. In lateral view, the dorsal muscle band curves inward in the anterior third of the body (Figs. 1a, c and 3a). In the middle of the body, the ventral muscle splits to form an oval opening, which is the site of the genital pore. The muscle then reunites into a singular unit posterior of the pore (Figs. 1b and 3b).
At the anterior end, both the dorsal and ventral muscle bands bifurcate. Each branch extends anterolaterally and connects with its ipsilateral (dorsal or ventral) counterpart at the anterior border, thereby forming an apparently continuous muscle band (Fig. 1b). Posteriorly, the dorsal and ventral muscles bifurcate again but do not connect as above. Instead, each dorsal branch curves ventrally and connects with a dorsal branch of the ipsilateral lateral longitudinal muscle, while the ventral branches curve dorsally and connect with the ventral branch of the ipsilateral lateral longitudinal muscles (Fig. 1b, c). These bifurcations and reconnections make the longitudinal muscle system appear as a continuous network of bands as opposed to a series of independent muscles.
The structure of the right and the left lateral muscles is alike, and similar to the dorsal and ventral muscles. At the anterior end, both bands bifurcate (Figs. 1c and 3a, b). Thin termini of the lateral muscles insert in the ciliated epithelium. At one angle, the terminus appears as a band extension, while at another angle, it appears tapering and pointed at the tip, suggesting that the band terminus tapers only in one plane. The muscle band termini are located in between the ciliated epithelial cells. Lateral longitudinal muscles are attached in two spots at the anterior end of the body. In cross section, the dorsal, the ventral, and the lateral muscle bands are flattened; the same is true for the circular ones. All longitudinal muscles are 6–7 μm wide throughout.
Female I. linei has approximately 9–11 circular muscles (Figs. 1а–c and 3a, b). The range reflects variability of the body length in different specimens, longer animals having more circular muscles. The circular muscles are nearly perpendicular to the longitudinal muscles, parallel to each other throughout the body, and located at about the same distance from one another. The only exception is the second anterior circular muscle, inclined to the longitudinal axis of the body (Figs. 1c and 3a). The width of the circular muscles is 1.5–2.5 μm, which is much thinner than the longitudinal muscles, and still narrower in the sites where they pass under the longitudinal muscle fibers.
The serotoninergic nervous system
The entire serotoninergic nervous system of female I. linei is composed of three pairs of cells (Figs. 2c, d and 3c, d). All serotonin-positive cells lie dorsally. They are multipolar and are compactly located in between the ciliated cells and the muscles (Fig. 2b). Two cells (the frontal ones) are shifted anteriorly. Each cell sends a process to the anterior end of the body; this process curves downward and projects along the lateral side of the body (Figs. 2b–d and 3c, d).
Nervous system of female I. linei. a Acetylated α-tubulin immunoreactivity (magenta) showing nerve plexus and unpaired anterior nerve (np) in the anterior part of the body. b A sagittal section at the level of dorsal (dm) and ventral (vm) muscles, showing serotonin-positive immunoreactivity (green) in the incurvation of the dorsal muscle. c, d Serotoninergic nervous system. View from the dorsal (c) and lateral (d) side. e Cell nuclei staining with DAPI. The ganglion region is outlined. an antherior nerve, cc ciliated cells, dm dorsal muscle, gc ganglion cells, np ganglionic nerve plexus, on oocyte nuclei, pn posterior nerves, vm ventral muscle. Scale bar 10 μm
Schematic drawings of musculature (a, b) and serotoninergic nervous system (c, d) in female I. linei. a, c lateral view. b, d dorsal view. an antherior nerve, cm circular muscles, cn circular nerve, dm dorsal muscle, gc ganglion cells, gp genital pore, lm lateral muscles, pn posterior nerves, vm ventral muscle
The two largest cells are located centrally (the central cells). Each cell has a long process extending backward along the longitudinal muscle band and nearly reaching the very end of the body (Figs. 2d and 3c, d). Also, each of the central cells appears to have several processes running forward and connecting these cells with the others to form a plexus.
Two cells are more lateral (the lateral cells; Figs. 2b, c and 3с, d). Each of them sends a process backward, which extends along the longitudinal muscles up to the middle of the body. Two processes of these cells bend ventrally and converge to form a ring perpendicular to the longitudinal axis of the body (Figs. 2d and 3c). These cells also have several processes, which run forward and connect them with the others forming the plexus. Thus, the plexus is formed by short processes of all nerve cells and is located between them (Figs. 2a, c, d and 3d). The plexus sends a thin unpaired process toward the anterior end of the body, projecting dorsally. Although immunohistochemistry demonstrated that this process was serotonin-positive (Fig. 3c, d), it is difficult to determine the origin of the apparent neurite. At the same time, antibodies against tubulin revealed microtubules both in the place of the plexus and the unpaired process extending forward (Fig. 2a).
Cell number counts
DAPI staining was used to quantify nuclei (hence, the cells; Fig. 3e). The total number of nuclei in the female I. linei is 369 ± 2–3. The body of the worm comprises a single-row epithelium, oocytes, muscle, and nerve cells. The number of the epithelial cells is 280. The number of oocytes was counted in living females with DIC and in DAPI-stained specimens, as their nuclei differ significantly in size from all other cells. The oocyte number is 35. Furthermore, the female genital pore comprises nine cells. Thus, the total number of epithelial cells, oocytes, and genital pore cells is 280 + 35 + 9 = 324. The rest of the cells (369 − 324 = 45) account for the cells of muscular and nervous systems described here. The muscle cells lie under the epithelial cells. The number of muscle cells is 35; hence, there should be only 10 nerve cells. Although the number of all cell types can vary from specimen to specimen, the variation is within a very small range (±2-3) and it does not affect the order of magnitude. Thus, the maximal number of nerve cells should not exceed 10–12.
Discussion
The general pattern of the muscular system in female I. variabili was first described by Slyusarev and Manylov (2001). Three main features of the orthonectid muscular system were revealed: the bilateral symmetry, the presence of only longitudinal and circular muscles, and the interior position of longitudinal muscles relative to the circular muscles. However, since we performed this study by means of epifluorescence microscopy, we failed to obtain images, permitting unequivocal interpretation of relative localization of longitudinal and circular muscle bands. In this study, performed by means of CLSM in the females of another species, I. linei, which is similar in general organization and larger in size, we reconsider the general pattern of the muscular system in Orthonectida. We confirmed the first two characteristic features of the muscular system in orthonectids—the bilateral symmetry and the presence of only two types of muscle bands. As for the third feature, by using CLSM, we managed to show that the longitudinal muscles lie outside the circular muscles, which presumably should be true for other species as well. Such muscular pattern (lacking additional muscles of reproductive and digestive systems) is typical for orthonectids. Of course, similar patterns can be found in larvae of different invertebrates (Wanninger 2005; Rawlinson 2010; Helm et al. 2013; Hindinger et al. 2013), but we do not consider males and females of orthonectids as neotenic larvae. On the other hand, both males and female orthonectids might be dwarfs. Dwarf males are known in some invertebrates and are characterized by reduction of different systems (Neves et al. 2009). A thorough further investigation would be necessary to prove or to reject any speculation of this kind. Overall, muscular pattern of orthonectids corresponds to the general pattern characteristic of small-sized animals, such as flatworms (Tyler and Hyra 1998; Hooge 2001; Kotikova et al. 2002; Tyler and Hooge 2004; Tekle et al. 2005), several small annelids (Müller and Worsaae 2006), cycliophorans (Neves et al. 2012), and many others. However, in annelids circular muscles are present mostly in burrowing forms and may not be characteristic of the annelid ground plan (Tzetlin et al. 2002; Tzetlin and Filippova 2005; Purschke and Müller 2006). Moreover, when present, circular muscles in annelids are situated on the outside of the longitudinal muscles.
In addition to our novel findings on the muscular system, we provide the first description of the serotoninergic nervous system in orthonectids. The simplicity of its structure makes the comparison with other groups highly speculative. Nevertheless, similarly structured nervous systems are present in Acoela (Reuter et al. 2001, Achatz and Martinez 2012), Plathelmintes (Reuter et al. 1998; Kotikova et al. 2002; Rawlinson 2010), Gnathostomulida (Müller and Sterrer 2004), Gastrotricha (Rothe et al. 2011), and very small Annelida (Müller and Westheide 2002; Orrhage and Müller 2005; Müller 2006). At the same time, analysis of the fine structure of orthonectids (the presence of the true cuticle and fibrous bands in the epithelial cells consisting of ciliated and non-ciliated cells) supported our hypothesis that orthonectids are closer to Annelida, or, at least, Trochozoa (Slyusarev 2000; Slyusarev and Kristensen 2003). To avoid unjustified speculations, so far we can conclude that the bilateral pattern of the serotoninergic nervous system of Ortonectida is reminiscent of different groups of lophotrochozoans (Reuter et al. 2001; Brinkmann and Wanninger 2008; Rothe and Schmidt-Rhaesa 2009; Hindinger et al. 2013), but the pattern itself does not provide further insights.
The key questions concerning orthonectids are as follows. What are their phylogenetic relationships? Are orthonectids related to dicyemids, and is it possible to join them in one group—Mesozoa?
Traditionally, orthonectids have been regarded as very primitive animals. Molecular data (Hamlet et al. 1996; Pawlowski et al. 1996) argue for placing them in Bilateria. Petrov’s data (Petrov et al 2010) permit to refer orthonectids to Spiralia, and among these, possibly, to annelids. Our data are in good agreement with this concept and allow placing them in Lophotrochozoa, specifically, in Trochozoa.
Joining Orthonectida and Diciemyda in one phylum has long been questioned. According to molecular data (Petrov et al. 2010), Diciemyda and Orthonectida are related. This seems rather doubtful, as dyciemids lack both nervous and muscle systems and, basically, have no real tissues at all. So, joining these two groups into one phylum, Mesozoa, seems controversial.
References
Achatz, J. G., & Martinez, P. (2012). The nervous system of Isodiametra pulchra (Acoela) with a discussion on the neuroanatomy of the Xenacoelomorpha and its evolutionary implications. Frontiers in Zoology, 9, 27.
Brinkmann, N., & Wanninger, A. (2008). Larval neurogenesis in Sabellaria alveolata reveals plasticity in polychaete neural patterning. Evolution and Developement, 10(5), 606–618.
Caullery, M. (1961). Classe des Orthonectides (Orthonectida Giard 1877). In: Grassé PP (ed) Traité de Zoologie. pp 695–706.
Caullery, M., & Mesnil, F. (1901). Recherches sur les Orthonectides. Archives d'anatomie microscopique et de morphologie expérimentale, 4, 381–470.
Giard, A. (1877). Sur les Orthonectida, classe nouvelle d’animaux parasites des Echinodermes et des Turbellariés. Comptes Rendus des Séances l’Académie des Sciences, 85, 812–814.
Giard, A. (1879). On the organization and classification of the Orthonectida. Annals and Magazine of Natural History, 5 Series, 4, 471–473.
Hamlet, B., Van Schyndel, D., Adema, C. M., Lewis, L. A., & Locker, E. S. (1996). The phylogenetic position of Rhopalura ophiocomae (Orthonectida) based on 18S ribosomal DNA sequence analysis. Molecular biology and evolution, 13, 1187–1191.
Hartmann, D.W. (1925). Mesozoa. In: Handbuch der Zoologie, Bd 1. pp 996–1014.
Hatschek, B. (1888). Lehrbuch der Zoologie. 1. Lieferung. Gustav Fischer, Jena.
Helm, C., Schemel, S., & Blendorn, C. (2013). Temporal plasticity in annelid development—ontogeny of Phyllodoce groenlandica (Phyllodocidae, Annelida) reveals heterochronous patterns. Journal of Experemental Zoology, 320B, 166–178.
Hindinger, S., Schwaha, T., & Wanninger, A. (2013). Immunocytochemical studies reveal novel neural structures in nemertean pilidium larvae and provide evidence for incorporation of larval components into the juvenile nervous system. Frontiers in Zoology, 10, 31.
Hooge, M. D. (2001). Evolution of body-wall musculature in the Platyhelminthes (Acoelomorpha, Catenulida, Rhabditophora). Journal of Morphology, 249, 171–194.
Kotikova, E. A., Raikova, O. I., Reuter, M., & Gustafsson, M. K. S. (2002). The nervous and muscular systems in the free-living flatworm Castrella truncata (Rhabdocoela): an immunocytochemical and phalloidin fluorescence study. Tissue & Cell, 34, 365–374.
Kozloff, E. N. (1971). Morphology of the orthonectid Ciliocincta sabellariae. Journal of Parasitoogy, 57, 377–406.
Kozloff, E. N. (1969). Morphology of the orthonectid Rhopalura ophiocomae. Journal of Parasitoogy, 55, 171–195.
Metschnikoff, E. (1881). Untersuchungen uber Orthonectiden. Zeitschrift für wissenschaftliche Zoologie, 35, 282–303.
Metschnikoff, E. (1879). Nachtragliche Bemerkungen uber Orthonectiden. Zoologischer Anzeiger, 2, 618–620.
Müller, M. C. M. (2006). Polychaete nervous systems: ground pattern and variations—cLS microscopy and the importance of novel characteristics in phylogenetic analysis. Integrative and Comparative Biology, 46, 125–133. doi:10.1093/icb/icj017.
Müller, M. C. M., & Westheide, W. (2002). Comparative analysis of the nervous systems in presumptive progenetic dinophilid and dorvilleid polychaetes (Annelida) by immunohistochemistry and cLSM. Acta Zoologica, 83, 33–48. doi:10.1046/j.1463-6395.2002.00096.x.
Müller, M. C. M., & Worsaae, K. (2006). CLSM analysis of the phalloidin-stained muscle system in Nerilla antennata, Nerillidium sp. and Trochonerilla mobilis (Polychaeta; Nerillidae). Journal of Morphology, 267, 885–896. doi:10.1002/jmor.10292.
Müller, M. C. M., & Sterrer, W. (2004). Musculature and nervous system of Gnathostomula peregrina (Gnathostomulida) shown by phalloidin labeling, immunohistochemistry, and cLSM, and their phylogenetic significance. Zoomorphology, 123, 169–177. doi:10.1007/s00435-004-0099-2.
Neves, R. C., Sørensen, K. J. K., Kristensen, R. M., & Wanninger. (2009). Cycliophoran dwarf males break the rule: high complexity with low cell numbers. The Biological Bulletin, 217(1), 2–5.
Neves, R. C., Kristensen, R. M., & Funch, P. (2012). Ultrastructure and morphology of the cycliophoran female. Journal of Morphology, 273, 850–869.
Orrhage, L., & Müller, M. C. M. (2005). Morphology of the nervous system of Polychaeta (Annelida). Hydrobiologia, 535–536, 79–111. doi:10.1007/s10750-004-4375-4.
Pawlowski, J., Montoya-Burgos, J.-I., Fahrni, J. F., Wuest, J., & Zaninetti, L. (1996). Origin of the Mesozoa inferred from 18S rRNA gene sequences. Molecular biology and evolution, 13, 1128–1132.
Petrov, N. B., Aleshin, V. V., Pegova, A. N., Ofitserov, M. V., & Slyusarev, G. S. (2010). New insight into the phylogeny of Mesozoa: evidence from the 18s and 28S RRNA Genes. Moscow University Biological Sciences Bulletin, 65, 168–170.
Purschke, G., & Müller, M. C. M. (2006). Evolution of body wall musculature. Integrative and Comparative Biology, 46, 497–507. doi:10.1093/icb/icj053.
Rawlinson, K. A. (2010). Embryonic and post-embryonic development of the polyclad flatworm Maritigrella crozieri; implications for the evolution of spiralian life history traits. Frontiers in Zoology. doi:10.1186/1742-9994-7-12.
Reuter, M., Mäntylä, K., & Gustafsson, M. K. S. (1998). Organization of the orthogon—main and minor nerve cords. Hydrobiologia, 383, 175–182.
Reuter, M., Raikova, O. I., Jondelius, U., Gustafsson, M. K. S., Maule, A. G., & Halton, D. W. (2001). Organisation of the nervous system in the Acoela: an immunocytolochemical study. Tissue Cell, 33(2), 119–128.
Rothe, B. H., & Schmidt-Rhaesa, A. (2009). Architecture of the nervous system in two Dactylopodola species (Gastrotricha, Macrodasyida). Zoomorphology, 128, 227–246.
Rothe, B. H., Schmidt-Rhaesa, A., & Kieneke, A. (2011). The nervous system of Neodasys chaetonotoideus (Gastrotricha: Neodasys) revealed by combining confocal laserscanning and transmission electron microscopy: evolutionary comparison of neuroanatomy within the Gastrotricha and basal Protostomia. Zoomorphology, 130, 51–84.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9, 676–682. doi:10.1038/nmeth.2019.
Slyusarev, G. S. (1994). The fine structure of the female Intoshia variabili (Alexandrov & Sljusarev) (Mesozoa: Orthonectida). Acta Zoologica, 75, 311–321.
Slyusarev, G. S. (2003). The fine structure the muscle system in the female of the orthonectid Intoshia variabili (Orthonectida). Acta Zoologica, 84, 107–111.
Slyusarev, G. S. (2000). Fine structure and development of the cuticle of Intoshia variabili (Orthonectida). Acta Zoologica, 81, 1–8.
Slyusarev, G. S., & Manylov, O. G. (2001). General morphology of muscle system in the female orthonectid, Intoshia variabili (Orthonectida). Cahiers de Biologie Marine, 42, 239–242.
Slyusarev, G. S., & Kristensen, R. M. (2003). Fine structure of the ciliated cells and ciliary rootlets of Intoshia variabili (Orthonectida). Zoomorphology, 122, 33–39.
Stunkard, H. W. (1954). The life-history and systematic relations of the Mesozoa. Quarterly Review of Biology, 29, 230–244.
Tekle, Y. I., Raikova, O. I., Ahmadzadeh, A., & Jondelius, U. (2005). Revision of the Childiidae (Acoela), a total evidence approach in reconstructing the phylogeny of acoels with reversed muscle layers. Journal of Zoological Systematics and Evolutionary Research, 43, 72–90.
Tyler, S., & Hooge, M. (2004). Comparative morphology of the body wall in flatworms (Platyhelminthes). Canadian Journal of Zoology, 82, 194–210.
Tyler, S., & Hyra, G. S. (1998). Patterns of musculature as taxonomic characters for the Turbellaria Acoela. Hydrobiologia, 383, 51–59.
Tzetlin, A. B., & Filippova, A. V. (2005). Muscular system in polychaetes (Annelida). Hydrobiologia, 535–536, 113–126. doi:10.1007/s10750-004-1409-x.
Tzetlin, A. B., Zhadan, A., Ivanov, I., Müller, M. C. M., & Purschke, G. (2002). On the absence of circular muscle elements in the body wall of Dysponetus pygmaeus (Chrysopetalidae, “Polychaeta”, Annelida). Acta Zoologica, 83, 81–85. doi:10.1046/j.1463-6395.2002.00104.x.
Wanninger, A. (2005). Immunocytochemistry of the nervous system and the musculature of the chordoid larva of Symbion pandora (Cycliophora). Journal of Morphology, 265, 237–243.
Acknowledgments
The study was performed at the Core Facility Centers for Microscopy and Microanalysis, Center for molecular and cell technologies, center “CHROMAS” and center “Culture Collection of Microorganisms” of St-Petersburg State University. The main financial support for this study was provided by grant of Russian Foundation for Basic Research (RFBR) №13-04-00725. The work was partially supported by the Saint-Petersburg State University research grant 1.50.1619.2013 to Viktor Starunov.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slyusarev, G.S., Starunov, V.V. The structure of the muscular and nervous systems of the female Intoshia linei (Orthonectida). Org Divers Evol 16, 65–71 (2016). https://doi.org/10.1007/s13127-015-0246-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0246-2