Abstract
A potential vaccine candidate for visceral leishmaniasis should favour the development of CD4+ Th1 type of immune response which is further dependent on the dose of antigen and the route of inoculation. The present study was carried out to check the effective dose (low, medium and high) and route (subcutaneous, intradermal, intraperitoneal and intracardiac) of inoculation for the development of a CD4+ Th1 type of immune response in BALB/c mice. The parasite load was found to be the lowest in mice inoculated with low dose of promastigotes through the subcutaneous route, followed by intradermal, intraperitoneal and intracardiac routes. A reduced parasite load in mice inoculated through subcutaneous route was found to be associated with heightened DTH responses. The IgG2a levels were found to be the maximum in case of mice inoculated with the low dose of promastigotes through subcutaneous route followed by intradermal and intraperitoneal routes. In contrast, mice inoculated with high dose of promastigotes through the intracardiac route showed increased levels of IgG1. Low-dose inoculation with subcutaneous route elicited maximum IFN-γ levels, which points towards the generation of Th1 response. Maximum IL-4 and IL-10 levels were detected in high-dose inoculation through intracardiac route suggesting the development of Th2 response. In conclusion, inoculation through subcutaneous route with low dose of live whole parasite antigen evokes a strong Th1 response in BALB/c mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites belonging to the genus Leishmania are among the most diverse of human pathogens, both in terms of geographical distribution and in the variety of clinical syndromes caused by them (Melby 2002; Tripathi et al. 2007). The spectrum of signs and symptoms caused range from simple, self-healing skin ulcers caused by Leishmania major and other dermatropic species, more severe chronic mucocutaneous infections caused by Leishmania braziliensis to severe, life-threatening visceral disease caused by the Leishmania donovani complex (Alexander et al. 1999). Visceral leishmaniasis (VL) is the severest systemic disease among the three main categories of leishmaniasis (Graham 1987). Five hundred thousand new cases of VL occur each year (Ahmed et al. 2003) and 90% of cases of VL are concentrated in five countries—Bangladesh, India, Nepal, Sudan and Brazil (Saha et al. 2006). In India, almost 44 million people in 28 districts of Bihar and 55 million people in eight districts of West Bengal are at risk of VL (Mukhopadhyay et al. 2000).
Treatment of VL is associated with serious side effects. Also, the emergence of drug resistance is a major hurdle in the control of this disease. Therefore, much attention has been given to finding suitable prophylactic agents which can induce protective immunity against VL. The development of a safe and effective vaccine is besieged with problems. Peptide vaccines are poorly immunogenic and genetic vaccines are not 100% protective because they are restrictive and lack full repertoire of antigens involved in protective immune responses (Mukhopadhyay et al. 2000; Nossal 1988 and Van Regenmortel 1989). With the establishment of culture conditions to support the growth of Leishmania, whole parasites have been tried for immunization to cure leishmaniasis. The technique of using live organisms, also known as leishmanization, dates back to 1911 to 1980, during the Iran–Iraq war (Liew and O’Donnell 1993). Leishmanization as a prophylactic vaccine was used in Israel in 1970s and in Iran in 1980s (Nadim and Javadian 1988) against cutaneous leishmaniasis and then in a massive programme covering over two million people during the Iran–Iraq war of 1982–1986 (Nadim and Javadian 1988). Leishmanization has certain drawbacks like the danger of developing disease, immunosuppression as seen in reduced responsiveness to diphtheria, pertusis, tetanus (DPT) vaccine in children followed by leishmanization (Khamesipour et al. 2006 and Sukumaran and Madhubala 2004). Due to the hazards, the focus shifted to killed parasites but none could provide 100% protection till now. Recent reports of a low-dose infection generating a Th1 response and immunity to further infection has again revived the interest in this field (Bretscher et al. 1992). However, the reports on the effect of dose of antigen on the development of type of Th 1 response are controversial. Since, the Leishmania parasite being intracellular, escape from the humoral responses by hiding as amastigotes inside the phagolysosome of the host macrophages, the effective immunity against leishmaniasis is entirely cell mediated (Sukumaran and Madhubala 2004). The CD4+ subset of T cells is crucial for resistance (Awasthi et al. 2004) since they play a major role in generating specific and memory responses. Th1 cells secrete activators of cell-mediated immunity such as IFN-γ, while Th2 cells secrete cytokines such as IL-4, which promote antibody responses. Thus, the control of leishmanial infection is mediated by Th1 type of immune response (Tripathi et al. 2007). It is the lack of the Th1 response rather than the presence of a Th2 response that determines disease susceptibility in VL (Ahmed et al. 2003). Antigen dose has been found to affect T cell subset development; but as already mentioned, the reports are controversial. Some reports suggest the development of Th1 response after low-dose inoculation of parasite and Th2 response after high-dose inoculation of parasite whereas others suggest vice-versa (Uzonna et al. 2004). At present, the prevailing thought is that low doses of L. major promote a Th1 response, based on the finding that although BALB/c mice infected with high parasite doses develop an uncontrolled infection, a protective Th1 response is induced after low-dose infection (Bretscher et al. 1992; Menon and Bretscher 1998). An important role for CD8+ T cells as regulators of CD4+ Th1 cell development has been suggested and also that low-antigen doses may preferentially promote a CD4+ Th2 response in vivo, but in situations where CD8+ T cells are concomitantly activated this Th2 response might be masked (Uzonna et al. 2004; Belkaid et al. 2002).
Besides dose of antigen, a number of potentially important elements have been proposed which influence the initial activation of different cytokine responses, among these are the nature of the early antigen-presenting cell, the overall cytokine milieu and the presence of costimulatory molecules (Gajewski et al. 1991; O’Garra and Murphy 1994; Wenner et al. 1996). The type of APC involved in the early response is itself largely determined by the site at which antigen presentation occurs, the tropism of the infectious agent or antigen, the route by which antigen is acquired and many other factors (Doherty and Coffman 1996). Sandfly transmits promastigotes into the dermis of the mammalian host. Therefore, intradermal or subcutaneous routes of infection mimic the natural infection. Infection through both the routes has been achieved in the dog and hamster models (Killick-Kendrick et al. 1994; Wilson et al. 1987). But as far as the mouse model is concerned, controversial reports have been obtained by both the routes of infection (Melby et al. 1998; Nuwayri-Salti et al. 1998). The administration of the parasite by an intraperitoneal route resulted in a high homogeneity of infection (Rolao et al. 2004). Low-dose infection by intravenous route might lead to establishment of infection in susceptible strains of mice (Howard et al. 1987; Ulczak and Blackwell 1983). Higher levels of protection have been achieved using high dermal infection or low-dose intravenous challenge models (Ahmed et al. 2003).
Since the dose and route of administration of antigen influence the development of a protective Th response and controversial reports are available in the literature, the present study was carried out to evaluate different combinations of dose of antigen (low, medium or high) and route of inoculation (subcutaneous, intraperitoneal, intradermal and intracardiac) for the development of CD4+ Th1 or Th2 type of immune response against L. donovani in a murine model.
Materials and methods
Parasite strain
Leishmania donovani promastigotes of strain MHOM/IN/80/Dd8, originally obtained from London School of Tropical Medicine and Hygiene, London, were used for the present study and maintained in vitro at 22 ± 1°C in modified NNN medium (Rao et al. 1984) by serial subcultures after every 48–72 h.
Animals
Inbred BALB/c mice of either sex, weighing 20–25 g, were obtained from the Central Animal House of Panjab University, Chandigarh. They were fed with water and mouse feed ad libitum.
Infection of mice
BALB/c mice were inoculated with low (103), medium (105) and high (107) dose of promastigotes of L. donovani by subcutaneous, intradermal, intraperitoneal and intracardiac routes. Animals receiving only PBS served as controls.
Animals were sacrificed on 15, 30 and 45 days post inoculation. Impression smears of liver were made and the parasite load was assessed in terms of Leishman Donovan Units (LDU; Bradley and Kirkley 1977; Yadav et al. 2004).
DTH responses to leishmanin
All groups of mice were challenged in the right foot pad with a subcutaneous injection of leishmanin. After 48 h, the thickness of the right and left foot pads were measured using a pair of Vernier calipers. The percentage increase in the thickness of the right foot pad as compared to the left foot pad of mice was calculated.
Enzyme-linked immunosorbent assay (ELISA)
The specific serum immunoglobulin G (IgG) antibody response was measured by conventional enzyme-linked immunosorbant assay (ELISA). Ninety-six-well ELISA plates were coated with crude promastigote antigen at a concentration of 2 μg per well. Sera were added at a dilution of 1:100 followed by addition of HRP-conjugated specific secondary antibody (rabbit anti-mouse IgG). The substrate and the chromogen were added and absorbance was read on an ELISA plate reader at 450 nm.
IgG isotype ELISA
IgG isotype (IgG1 and IgG2a) ELISA was carried out with the serum samples following the same procedure as described above for ELISA.
Cytokine response profile
The lymphocytes from spleens of all groups of mice were cultured in 24-well plates in RPMI-1640 containing 20 mM NaHCO3, 10 mM HEPES, 10 U/ml of penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine and 10% FCS. Cells were stimulated with 50 μg/ml with the crude promastigote antigen. Cells were then incubated at 37 °C for 72 h and supernatant was collected and stored at −20°C. This was then assayed for IL-4, IL-10, and IFN-γ by ELISA kits (Bender MedSystems Inc, USA and Diaclone, USA) following manufacturer’s protocol.
Statistical analysis
All the experiments were performed three times independently. All data comparisons were tested for significance by using Student’s t-test; p-values below 0.05 were considered significant.
Results
Parasite load
Minimum parasite load was observed in mice inoculated with a low dose of parasite subcutaneously, followed by the parasite load in mice inoculated with medium and high doses, respectively. The least parasite burden occurred in mice inoculated with subcutaneous route followed by intradermal, intraperitoneal and then intracardiac route. Maximum parasite load was observed in mice inoculated intracardially with high dose of parasite (Fig. 1A). Parasite load in liver increased significantly in all groups of BALB/c mice on different post-inoculation days.
A Parasite load in terms of LDU in BALB/c mice upon inoculation with different doses of Leishmania donovani through different routes at various post-inoculation days. (a) 15 p.i.d., (b) 30 p.i.d., (c) 45-p.i.d. B DTH responses in BALB/c mice upon inoculation with different doses of Leishmania donovani through different routes at various post-inoculation days. (a) 15 p.i.d., (b)-30 p.i.d., (c) 45-p.i.d. [SC Subcutaneous, ID intradermal, IP intraperitoneal, IC intracardiac]
Delayed type hypersensitivity (DTH) responses
Inoculation of mice with low dose of parasite through the subcutaneous route induced the highest level of DTH response followed by the DTH responses in the inoculation through intradermal, intraperitoneal and intracardiac routes, respectively, suggesting the generation of cell-mediated immune response. The DTH responses in case of intradermal, intraperitoneal and intracardiac routes were lower than in the case of subcutaneous route and were the least in the intracardiac route; this points towards the generation of cell-mediated immune response. The DTH responses were the maximum with the inoculation of low dose followed by medium and high dose. The DTH responses in infected animals were significantly higher than the normal controls. DTH responses were the maximum on 15 days post inoculation and, thereafter, declined significantly till 45 post-inoculation days (Fig. 1B).
Specific antibody response
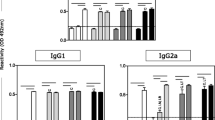
IgG1 and IgG2a antibody responses were evaluated by ELISA using specific anti-mouse isotype antibodies. Mice inoculated with low dose of parasite through the subcutaneous route showed the highest levels of Th1 cytokine regulated antibody, IgG2a, followed by the mice inoculated through intradermal, intraperitoneal and intracardiac routes, respectively. The level of Th2 cytokine regulated antibody, IgG1 was the maximum with intracardiac inoculation of high dose of parasite followed by intraperitoneal, intradermal and subcutaneous routes. The highest IgG2a titres were observed in mice inoculated with low dose of parasite as compared to medium and high dose whereas the maximum IgG1 titres were found in mice inoculated with high dose of the parasite as compared to medium and low dose. The antibody levels were found to increase with increase in post-inoculation days (Fig. 2A and B).
Specific antibody responses in BALB/c mice upon inoculation with different doses of Leishmania donovani through different routes at various post-inoculation days. A IgG1; B-IgG2a, (a)-15 p.i.d.; (b)-30 p.i.d.; (c) 45-p.i.d. (Straight line inside graphs represents the baseline value) [SC Subcutaneous, ID intradermal, IP intraperitoneal, IC intracardiac]
Cytokine response profile
The levels of IFN-γ, IL-4 and IL-10 were evaluated by ELISA. Mice inoculated with low dose of parasite through the subcutaneous route showed the highest levels of Th1-specific cytokine, IFN-γ. Mice inoculated through intradermal, intraperitoneal and intracardiac routes, respectively, showed a decrease in the IFN-γ levels in comparison to those inoculated through subcutaneous route. The level of Th2-specific cytokines IL-4 and IL-10 were the maximum with intracardiac inoculation of high dose of parasite followed by intraperitoneal, intradermal and subcutaneous routes, the levels of Th2-specific cytokines were seen to decrease as we move from intraperitoneal to subcutaneous route. The levels of IFN-γ were found to decrease with increase in the parasite dose, whereas the opposite was observed in case of IL-4 and IL-10. Maximum levels of IFN-γ, IL-4 and IL-10 were observed on 30 post-inoculation days (Fig. 3A,B and C).
Discussion
This study was carried out with a view to determining the consequences of the parasite inoculation in murine visceral leishmaniasis with respect to dose vis-à-vis route of inoculation. We have tested the outcome of different doses of Leishmania inoculation administered through subcutaneous, intradermal, intraperitoneal, and intracardiac routes in BALB/c mice. Our results suggest that the infective parasite inoculum is critical in determining the direction that the immune response of the infected animal takes. The administration of 103, 105 and 107 parasites in BALB/c mice established detectable infection which was reflected in the rising parasite load, presence of antileishmanial antibodies and heightened DTH responses. The parasite load as detected by enumeration of LDU can be resolved in two directions—low dose leads to minimum infection which increases with the change in dose from medium to high as the number of post-inoculation days increase. With regard to the route of inoculation, subcutaneous, intradermal and intraperitoneal routes showed decreased parasite load which increased with an increase in the infective inoculum. This can be attributed to the fact that in all of these three routes, the parasite travels from the initial site of infection to lymph nodes, as a result of the first line of defence of the host against an infection and is faced with the prospect of elimination by way of cell death en route to the establishment in target organs. Subcutaneous route is always considered as a slow release route in case of antigen presentation, allowing the body to generate enough resources to counter the invading microorganisms. Our findings correlate very well with these assumptions. We have found that the parasite inoculation through the intracardiac route leads to the maximum infection in mice, which is understandable since the parasite is effectively delivered to the visceral organs, namely the liver and spleen, in case of leishmaniasis. The DTH responses were in agreement with the parasite load levels and heightened foot-pad swellings were recorded 48 h after administration of leishmanin antigen and were the maximum in mice inoculated with low dose through the subcutaneous route. Though the DTH responses were elevated in all the cases, comparatively lower levels in other routes indicate that the subcutaneous route was the most efficient in priming the immune system against the leishmanin antigen. That Leishmania-specific antibodies were found to be elicited in the infective inoculum administered through all the routes points towards the fact that a good humoral immune response is activated by the host which is associated with the disease progression as evidenced by the levels of parasite burden. The estimation of cytokine profile for all the doses through three different routes indicates the generation of a mixed Th1/Th2 type of immune response. The levels of all three cytokines namely, IFN-γ, IL-4, IL-10 were observed to peak around day 30 post inoculation. In case of inoculation through subcutaneous route with low dose of parasite, the levels of IFN-γ were the maximum which indicates the potential of this route and dose for further vaccine studies. The levels of Th2-regulated cytokines IL-4 and IL-10 were the maximum in the intracardiac route.
Most in vitro studies indicate that lower antigen doses favour a Th2 response whereas higher doses favour a Th1 response. On the other hand, some in vivo studies indicate that low-antigen doses favour a Th2 response (Uzonna et al. 2004). It has been shown that BALB/c mice infected with a very low dose of L. major can contain the infection and develop a long-lasting immunity (Bretscher et al. 1992 and Doherty and Coffman 1996). An infective dose of 105 parasites in BALB/c mice embodies a model providing a greater number of representative markers (Carrion et al. 2006). At present the prevailing thought is that the low dose of parasite promotes a Th1 response. Inoculation of an excessive dose of parasite in an unnatural site may mask or subvert the factors responsible for the control of infection resulting from sandfly bite. The exogenous parasite antigen provided by high-dose inocula might elicit a level of CD4+ reactivity, adequate to control infection in the site, whereas intracellular infections evolving from low-dose challenge might generate a relatively poor source of antigen for class II presentation (Belkaid et al. 2002).Vaccine studies in case of visceral leishmaniasis need critical evaluation of the immunological parameters of the animal models currently in vogue. It is extremely important to optimize the conditions of the artificial infection to a point where it can be argued with confidence that the situation best or near-best represents the natural infection that the sandfly causes. Mouse model being the most widely used as the first choice owing to its relatively easier maintenance and handling, is naturally selected over other models for this purpose. Establishment of an infection model that is convenient to handle and is economical will be a step forward in testing the efficacy of various antigens, cocktails, DNA vaccines, etc.—protective or non-protective. In this regard, not only does the careful choice of dose have a say, but an equally important part is how effectively that dose is administered in the animal to mimic the onset and the course of the natural infection. In this investigation, we have attempted to address this very problem and have come to a conclusion that low dose of live parasite generates a protective Th1 response when inoculated through subcutaneous route.
References
Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D (2003) Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun 71(1):401–410
Alexander J, Satoskar AR, Russell DG (1999) Leishmania species: models of intracellular parasitism. J Cell Sci 112:2993–3002
Awasthi A, Mathur RK, Saha B (2004) Immune response to Leishmania infection. Indian J Med Res 119(6):238–258
Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, Udey MC, Sacks D (2002) CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol 168(8):3992–4000
Bradley DJ, Kirkley J (1977) Regulation of Leishmania populations within host I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol 30(1):119–129
Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H (1992) Establishment of stable, cell mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257(5069):539–542
Carrion J, Nieto A, Iborra S, Iniesta V, Soto M, Folgueira C, Abanades DR, Requena JM, Alonso C (2006) Immunohistological features of visceral leishmaniasis in BALB/c mice. Paraite Immunol 28(5):173–183
Doherty TM, Coffman RL (1996) Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol 84(2):124–135
Gajewski TF, Pinnas M, Wong T, Fitch FW (1991) Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol 146(6):1750–1758
Graham PCC (1987) Introduction. In: Peters W, Killick-Kendrick R (eds) The Leishmaniasis in biology and medicine. Academic Press, New York
Howard MK, Sayers G, Miles MA (1987) Leishmania donovani metacyclic promastigotes: transformation in vitro, lectin agglutination, complement resistance and infectivity. Exp Parasitol 64(2):147–156
Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F (2006) Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res 123(3):423–438
Killick-Kendrick R, Killick-Kendrick M, Pinelli E, Del Real G, Molina R, Vitutia MM, Canavate MC, Nieto J (1994) A laboratory model of canine leishmaniasis: the inoculation of dogs with Leishmania infantum promastigotes from midguts of experimentally infected phlebotomine sandflies. Parasite 1(4):311–318
Liew FY, O’Donnell CA (1993) Immunology of leishmaniasis. Adv Parasitol 32:161–259
Melby PC (2002) Recent developments in leishmaniasis. Curr Opin Infect Dis 15(5):485–490
Melby PC, Yang YZ, Cheng J, Zhao W (1998) Regional differences in the cellular immune responses to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun 66(1):18–27
Menon JN, Bretscher PA (1998) Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol 28(12):4020–4028
Mukhopadhyay S, Bhattacharyya S, Majhi R, De T, Naskar K, Majumdar S, Roy S (2000) Use of an attenuated leishmanial parasite as an immunoprophylactic and immunotherapeutic agent against murine visceral leishmaniasis. Clin Diagn Lab Immunol 7(2):233–240
Nadim A, Javadian E (1988) Leishmanization in the Islamic Republic of Iran. In: Walton BC, Wijayaratne PM, Modabber F (eds) Research on control strategies for control of leishmaniasis. International Development Research Center, Ottawa, pp 336–369
Nossal GJ (1988) Triumphs and trials of immunology in the 1980s. Immunol Today 9(10):286–291
Nuwayri-Salti N, Matta M, Shbaklo Z, Lakkis M, Kabbani ZE (1998) Behavior in a mouse model of isolates of Leishmania donovani sensu lato cultured from the blood of patients with chronic cutaneous lesions. Am J Trop Med Hyg 58(6):710–714
O’Garra A, Murphy K (1994) Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol 6(3):458–466
Rao RR, Mahajan RC, Ganguly NK (1984) Modified media for in vitro cultivation of Leishmania promastigotes. A Comparative study. Bull PGI 18:125–128
Rolao N, Melo C, Campino L (2004) Influence of the inoculation route in BALB/c mice infected by Leishmania infantum. Acta Trop 90(1):123–126
Saha S, Mondal S, Banerjee A, Ghose J, Bhowmick S, Ali N (2006) Immune responses in kala-azar. Indian J Med Res 123(3):245–266
Sukumaran B, Madhubala R (2004) Leishmaniasis: current status of vaccine development. Curr Mol Med 4(6):667–679
Tripathi P, Singh V, Naik S (2007) Immune response to Leishmania: paradox rather than paradigm. FEMS Immunol Med Microbiol 51(2):229–242
Ulczak OM, Blackwell JM (1983) Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: the effects of parasite dose, cyclophosphamide and sublethal irradiation. Parasite Immunol 5(5):449–463
Uzonna JE, Joyce KL, Scott P (2004) Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. J Exp Med 199(11):1559–1566
Van Regenmortel MH (1989) Structural and functional approaches to the study of protein antigenicity. Immunol Today 10(8):266–272
Wenner CA, Guler ML, Macatonia SE, O’Garra A, Murphy KM (1996) Roles of IFN-gamma and IFN-alpha in IL-12-induced T helper cell-1 development. J Immunol 156(4):1442–1447
Wilson ME, Innes DJ, Sousa AD, Pearson RD (1987) Early histopathology of experimental infection with Leishmania donovani in hamsters. J Parasitol 73(1):55–63
Yadav M, Nagill R, Kaur S (2004) Leishmania donovani: Effect of pH on the infectivity of axenic amastigotes. J Parasit Dis 28(2):90–95
Acknowledgement
The authors hereby declare that the experiments comply with the current laws in India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, S., Kaur, T., Garg, N. et al. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis. Parasitol Res 103, 1413–1419 (2008). https://doi.org/10.1007/s00436-008-1150-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1150-x