Abstract
Different molecular markers have been employed for typing Trypanosoma cruzi strains from endemic areas of Chagas disease. The low-stringency single specific primer–polymerase chain reaction (LSSP–PCR) has been a sensitive and informative technique that uses the variable region of kinetoplast DNA minicircles as a genetic marker, allowing detection of DNA sequence variation. In the present study, we analyzed the intra-lineage genetic variability of the T. cruzi strains obtained from triatomine feces collected on filter paper FTA card by LSSP–PCR. The hybridization of the PCR products with a probe for the subgenus Schizotrypanum and a clone-specific probe from Dm28c confirmed the subgenus as T. (S.) cruzi and respective lineages as T. cruzi I. Phenetic analysis showed the presence of three clusters that diverged by different coefficients of similarity. Thirteen T. cruzi I genotypes were observed circulating among Triatoma pseudomaculata and Rhodnius nasutus from peridomiciliary and natural environments in five peri-urban and urban localities of Jaguaruana, Ceará, Brazil. These data indicate the importance of the circulation of T. cruzi I genotypes among T. pseudomaculata and R. nasutus in different environments and the possible risk of Chagas disease domestic transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosoma cruzi, a causative agent of Chagas disease, is mainly transmitted by hematophagous triatomines found in different ecotopes in Latin America. The disease can also be transmitted by blood transfusion, organ transplantation, congenital transmission, contaminated food, and laboratory accidents. During the 1980s, Chagas disease affected approximately 16–18 million people, and 100 million were exposed to the risk of contracting the infection (WHO 2002). Since the successful control measures adopted by the Southern Cone Initiative for Triatoma infestans control, there has been a decline in the annual incidence of domiciliary vector transmission of T. cruzi in several areas. Current estimates suggest that less than 8 million people remain infected. However, the incidence has increased in other countries, such as Europe, Canada, and the USA due to the lack of effective control during blood transfusion and organ transplantation (Schmunis 2007; WHO 2007). Furthermore, sylvatic triatomines may occupy areas where T. infestans was prevalent as a consequence of environmental damage and the destruction of natural ecotopes (Silveira and Vinhaes 1998; Almeida et al. 2000; Oliveira and Silva 2007). New knowledge of the molecular genetics of T. cruzi and vectors can be used to identify areas where sylvatic and domestic transmission cycles occur, information that can subsequently be used to help measure the transmission risk.
Recently, the polymerase chain reaction (PCR) has also been applied to the detection of T. cruzi in feces of triatomines (Breniere et al. 1992; Russomando et al. 1996; Hamano et al. 2001; Brito et al. 2005). Due to the high sensitivity of the low-stringency single specific primer–PCR (LSSP–PCR) through amplification of the variable region of the T. cruzi kinetoplast DNA (kDNA) minicircles, it has been a useful tool for molecular typing of natural T. cruzi populations (Salazar et al. 2006).
In the present study, we investigated the presence of T. cruzi DNA in triatomine feces spotted in filter paper. Through LSSP–PCR, which allows detection of informative genetic signatures, we observed genetic variability in T. cruzi strains circulating in artificial and natural ecotopes found in peri-urban and urban areas of Jaguaruana, in the state of Ceará, Brazil.
Materials and methods
Origin of triatomines
Triatomines were captured in the following five localities of Jaguaruana municipality, in the state of Ceará, Brazil: Dió, Córrego das Melancias, Caatinguinha, Perímetro Irrigado, and Saquinho (Fig. 1). These localities were situated 1 km (Dió and Córrego das Melancias) to 5 km from downtown (Saquinho). The first three localities were situated within the urban perimeter, and the other two were in peri-urban areas. Except Dió and the biggest part of Córrego das Melancias, the localities presented characteristics that tended to be part urban and part rural. The captures were carried out in artificial ecotopes, such as domestic animal shelters and piles of bricks, tiles, woods, and straws found in the peridomiciles, as well as in the top of the palm tree Copernicia prunifera growing up to 200 m from the dwellings of these localities.
Two species, identified as T. pseudomaculata and Rhodnius nasutus, were captured. T. pseudomaculata was collected in the peridomiciles of Córrego das Melancias and Caatinguinha, whereas R. nasutus was predominantly collected in its natural habitat, the palm trees C. prunifera, present in the five localities. The feces of 140 triatomines were examined by direct microscopic observation to find out the natural T. cruzi infection. Fifty specimens showed flagellated parasites in their feces and were selected for PCR analysis. Two T. cruzi reference strains were used as controls: Dm28c strain (Z1, T. cruzi I) and Can III strain (Z3, Miles et al. 1980; subgroup IIa, Brisse et al. 2000). The feces aliquots of each positive triatomine were obtained by squeezing the abdomen of live insects, deposited directly on filter paper (FTA Classic Card—Whatman), dried at room temperature, and stored at −20°C until DNA extraction.
Extraction of DNA
A piece filter paper 2 mm in diameter containing feces was removed and transferred into Eppendorf microtubes. The extraction of DNA followed a protocol described previously (Silva et al. 2004; Oliveira et al. 2005).
Polymerase chain reaction
T. cruzi kDNA 330-bp fragment was obtained by PCR with the primers S35 (5′-AAA TAA TGT ACG GG (T/G) GAG ATG CAT GA-3′) and S36 (5′-GGT TCG ATT GGG GTT GGT GTA ATA TA-3′). The reaction mix contained 0.2 mM deoxyribonucleotide triphosphate (dNTP), 1.5 mM MgCl2, 10 mM Tris–HCl, 2.5 IU of Taq polymerase, and 5 pmol/μl of each primer in a total reaction volume of 50 and 3 μl of DNA suspension obtained from fecal filter paper samples. The reaction involved a first step of an initial start temperature of 94°C for 6 min followed by 33 cycles of DNA denaturation (94°C for 1 min), oligonucleotide primer annealing (64°C for 1 min), and elongation (72°C for 1 min), and ended with a final cycle at 72°C for 7 min. PCR products were analyzed by 1.8% agarose gel electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light.
Molecular hybridization
PCR products were transferred to a nylon membrane according to Southern (1975), with the gel having previously been denatured in 1.5 M NaCl/0.5 M NaOH and neutralized in 1 M Tris–HCl at pH 8.0. Each step was carried out for 30 min with mild stirring. Two specific probes to the subgenus Schizotrypanum (Pacheco et al. 1996) and kDNA from Dm28c clone were radiolabeled with α32P 2′-deoxyadenosine 5′-triphosphate by the random primer reaction. After hybridization, the filters were washed three times for 30 min in 0.1× saline-sodium citrate/0.5% sodium dodecyl sulfate at 60°C. Autoradiography was performed using X-ray film (Kodak X-OMAT) at −70°C overnight.
LSSP–PCR
T. cruzi kDNA 330-bp fragments obtained by PCR were purified by Wizard PCR Prep system (Promega) according to the manufacturer’s recommendations. The LSSP–PCR reaction was performed using a specific amplification of the 330-bp fragment by the primer S35 (5′-AAA TAA TGT ACG GG(T/G) GAG ATG CAT GA-3′). LSSP–PCR reaction was carried out in a 25-μl final volume containing 25 pmol of the S35 primer, 200 μM of each dNTP, 10 mM Tris–HCl at pH 8.6, 50 mM KCl, 1.5 mM MgCl2, and 4 IU of Taq polymerase; the DNA template corresponding to 4 μl of the purified 330 bp fragment was added in the reaction. Amplification reaction was carried out at an initial temperature of 94°C for 3 min followed by 44 cycles at 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min, and a final cycle for 5 min. Twelve microliters of the LSSP–PCR products were visualized by electrophoresis in an 8% polyacrylamide gel and stained with ethidium bromide.
Data analysis
Bands ranging in size from 200 to 330 bp were selected for phenetic analyses. The dendrogram was performed by NTSYS program (version 2.0, Exeter Software, Setauket, NY, USA) using the simple matching coefficient and an unweighted pair group method with arithmetic mean algorithm.
Results
All positive fecal samples displayed a diagnostic band of 330 bp obtained by the pair of PCR primers specific for T. cruzi. The molecular hybridization of the PCR products accomplished in high-stringency conditions revealed homology to the subgenus Schizotrypanum and T. cruzi I (not shown).
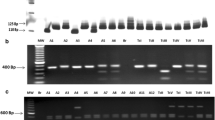
LSSP–PCR analysis showed genetic variability among all T. cruzi strains analyzed. Thirteen T. cruzi genotypes were detected among T. pseudomaculata and R. nasutus (Fig. 2). Phenetic analysis showed the presence of three clusters that diverged by different coefficients of similarity from 0.62 to 1.00 (Fig. 3). The first cluster was formed by five genotypes (1, 2, 3, 4, and 5) separated by coefficient of similarities ranging from 0.74% to 0.82%, represented by T. cruzi strains circulating in T. pseudomaculata and R. nasutus from four localities (Córrego das Melancias, Caatinguinha, Saquinho, and Dió). Twenty-six strains displayed a coefficient of similarity of 1.00 and were distributed in the first cluster. Two strains (167 and 41; genotypes 4 and 5, respectively) obtained from R. nasutus in two different localities (Córrego das Melancias and Saquinho) belong to the same cluster, sharing a coefficient of similarity of 0.83%.
LSSP–PCR profiles showing 13 representative T. cruzi genotypes revealed by 8% polyacrylamide gel electrophoresis and ethidium bromide staining. M 100 bp DNA ladder, 1–4 genotype 1, 5–7 genotype 2, 8 genotype 3, 9 and 10 genotype 4, 11 genotype 5, 12 genotype 6, 13 genotype 7, 14 genotype 8, 15 genotype 9, 16 and 17 genotype 10, 18 genotype 11, 19 and 20 genotype 12, 21–26 genotype 13, 27 Dm28c-reference strain; 28-CANIII reference strain; NC- negative control
Dendrogram using the simple matching coefficient based on genetic profiles of T. cruzi and reference strains obtained from LSSP–PCR. Z1 Dm28c strain, Z3 Can III strain. GEN. 1 genotype 1, GEN. 2 genotype 2, GEN. 3 genotype 3, GEN. 4 genotype 4, GEN. 5 genotype 5, GEN. 6 genotype 6, GEN. 7 genotype7, GEN. 8 genotype 8, GEN. 9 genotype 9, GEN. 10 genotype 10, GEN. 11 genotype 11, GEN. 12 genotype 12, GEN. 13 genotype 13
Five genotypes (6, 7, 8, 9, and 10) belonging to the second cluster were found in five localities, including Perímetro Irrigado. Two T. cruzi strains (162 and 222; genotypes 6 and 7, respectively) from R. nasutus, captured in Córrego das Melancias and Perímetro Irrigado, showed 0.83% similarity in characteristic when compared with the first cluster. The T. cruzi strains (202, 46, and 204; genotype 8) from R. nasutus, captured in Caatinguinha and Saquinho, were found to share 100% of common characteristics with the Can III reference strain. However, T. cruzi genotypes 9 and 10, sharing 0.75% of similarity, were found in T. pseudomaculata and R. nasutus from Caatinguinha, Perímetro Irrigado, and Dió. The T. cruzi reference strain represented by zymodeme 1 was grouped in the same cluster sharing a coefficient of similarity of 0.72%.
Genotypes 11, 12, and 13 grouped into the third cluster represented by T. cruzi strains that circulated between T. pseudomaculata and R. nasutus from two localities (Caatinguinha and Perímetro Irrigado). The different T. cruzi I genotypes found infecting the two species of triatomines under study are shown in Table 1.
Discussion
In the present study, the intra-lineage polymorphism among T. cruzi DNA was investigated using the polymorphic marker LSSP–PCR. Thirteen T. cruzi genotypes grouped into three clusters were found circulating among T. pseudomaculata and R. nasutus. Pacheco et al. (2005) observed ten different genotypes obtained among T. brasiliensis and T. pseudomaculata by randomly amplified polymorphic DNA in rural areas from four different localities of Jaguaruana. These data corroborate the present results.
The 330-bp fragments obtained in the first step of the LSSP–PCR allowed us to specifically detect T. cruzi DNA in triatomines feces collected on filter paper. The use of spotting triatomine feces on the filter paper offered several advantages, mainly in field work, by reducing the risk of transporting live triatomines, as well as by providing a simple method of DNA extraction. The sensitivity of the PCR associated with the Southern hybridization confirmed the subgenus as Schizotrypanum and the species as T. cruzi I. PCR amplification followed by hybridization has been used as a confirmatory test and detects mixed infections, an important tool for molecular epidemiology (Breniere et al. 1995; Madeira et al. 2006).
The localities where entomological surveillance was accomplished were considered urban and peri-urban areas, situated 1 to 5 km from downtown. Naturally infected T. pseudomaculata were captured in the peridomiciles of the two localities called Córrego das Melancias and Caatinguinha, both of which are situated in the urban area where five T. cruzi genotypes (1, 2, 9, 10, and 12) were found.
In recent years, T. pseudomaculata has become adapted to artificial ecotopes, becoming easily captured in peridomiciles, mainly in henhouses (Assis et al. 2007). In Jaguaruana, this triatomine has been found in peridomiciles as well as in intradomiciles, where it can establish colonies (Sarquis et al. 2006), a fact that was also observed in Sobral, a municipality located in the state of Ceará (Frota et al. 1999).
Regarding R. nasutus, it has been found predominantly in the palm tree C. prunifera, its natural habitat, in the five localities studied. Twelve T. cruzi genotypes (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 13) were detected in this species, with several of the genotypes (1, 2, 9, and 10) also found to infect T. pseudomaculata. In all these localities, the soil was greatly altered; however, the palm tree C. prunifera, although intensively reduced, can be found around houses close to sylvatic environment. However, in Jaguaruana, R. nasutus can also be captured colonizing artificial ecotopes, such as henhouses and corrals in peri-urban areas (Sarquis et al. 2006), and even in the tree Licania rigida (Oiticica), another typical tree in the Northeast region (Lima and Sarquis 2008). Artificial ecotopes are very common in this municipality, as well as in the other regions of Ceará state, where there are always several circulating domestic and synanthropic animals. Perhaps, all of these factors have favored the adaptation of the triatomines from their natural habitat to peridomiciles and from these to intradomiciles (Lima and Sarquis 2008). According to Romaña et al. (1999), the association between palm trees and species of the genus Rhodnius is considered as an ecological indicator of a risk area for Chagas disease. In fact, winged specimens have been found inside houses, where they are attracted by light or food, probably due to proximity of the palm trees and the man-made ecotopes in the peridomiciles (Sarquis et al. 2006). However, in our surveys we have never found intradomicile colonies of R. nasutus.
It should be emphasized that in these localities, a considerable number of reservoir hosts, such as the marsupial Didelphis albiventris and synanthropic rodents Rattus rattus, have been captured, with most of them carrying high levels of T. cruzi I infection (unpublished data from our group). These results confirm what was stated by Gaunt and Miles (2000) that there is a close association between palm trees, Rhodnius sp., T. cruzi I, and Didelphis.
Additional results from our group on triatomine blood meal sources revealed that the most frequent was opossum blood, but other sources such as goat, birds, rodent, sheep, human, and hemolymph were detected when tested by enzyme-linked immunosorbent assay (unpublished data).
The genetic variability observed among the T. cruzi I strains in the present study may be due to the wide range of vertebrate hosts coexisting with the triatomine species. In the past, T. cruzi I strains were mainly associated with the sylvatic cycle in different regions from Brazil, and the cases of Chagas disease were contracted from sporadic visits of sylvatic triatomines to human dwellings near the forests or from the eventual contact of the inhabitants of a specific area with the sylvatic cycle of the parasite. Currently, environmental damage has changed the scenario of the transmission cycles, and native species of triatomines have become adapted to the artificial ecotopes in search of food and new shelters.
Our results showed the circulation of different T. cruzi I genotypes in T. pseudomaculata and R. nasutus in distinct ecotopes, which has as a consequence the possible risk of the Chagas disease domestic transmission. Constant epidemiological vigilance in these localities is extremely necessary and new control strategies must be adapted to the peridomicile.
References
Almeida CE, Vinhaes MC, Almeida JR, Silveira AC, Costa J (2000) Monitoring the domiciliary and peridomiciliary invasion process of Triatoma rubrovaria in the state of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz 95:761–768
Assis GFM, Azeredo BVM, La Fuente ALC, Diotaiuti L, Lana M (2007) Domiciliation of Triatoma pseudomaculata (Corrêa e Espínola 1964) in the Jequitinhonha valley, state of Minas Gerais. Rev Soc Bras Med Trop 40:391–396
Breniere SF, Bosseno MF, Revollo S, Rivera MT, Carlier Y, Tibayrenc M (1992) Direct identification of Trypanosoma cruzi natural clones in vectors and mammalian hosts by polymerase chain reaction amplification. Am J Trop Med Hyg 46:335–341
Breniere SF, Bosseno MF, Telleria J, Carrasco R, Vargas F, Yaksic N, Noireau F (1995) Field application of polymerase chain reaction diagnosis and strain typing of Trypanosoma cruzi in Bolivian triatomines. Am J Trop Med Hyg 53:179–184
Brisse S, Barnabé C, Tibayrenc M (2000) Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int J Parasitol 30:35–44
Brito CMM, Sarquis O, Pires MQ, Lima MM, Pacheco RS (2005) Uso da PCR na detecção de DNA de Trypanosoma cruzi em fezes de triatomíneos naturalmente infectados. Rev Soc Bras Med Trop 38(Supl 1):320
Frota FCC, Lima JWO, Braga VSS (1999) Infecção humana pelo Trypanosoma cruzi, num foco urbano de Triatoma pseudomaculata, na cidade de Sobral. Norte do Ceará. Rev Soc Bras Med Trop 32(Supl I):85–86
Gaunt M, Miles M (2000) The ecotopes and evolution of triatomine bugs (Triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz 95:557–565
Hamano S, Horio M, Miura S, Higo H, Iihoshi N, Noda K, Tada I, Takeuchi T (2001) Detection of kinetoplast DNA of Trypanosoma cruzi from dried feces of triatomine bugs by PCR. Parasitol International 50:135–138
Lima MM, Sarquis O (2008) Is Rhodnius nasutus (Hemíptera; Reduviidae) changing its habitat as a consequence of human activity? Parasitol Res 102:797–800
Madeira MF, Schubach A, Schubach TMP, Pacheco RS, Oliveira FS, Pereira AS, Figueiredo FB, Baptista C, Marzochi MCA (2006) Mixed infection with Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi in a naturally infected dog from Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg 100:442–445
Miles MA, Lanham SM, Souza A, Povoa M (1980) Further enzymic characters of Trypanosoma cruzi and their evalution for strain identification. Trans R Soc Trop Med Hyg 74:221–237
Oliveira AWS, Silva IG (2007) Geographical distribution and entomological indicators of synanthropic triatomines captured in the state of Goiás. Rev Soc Bras Med Trop 40:204–208
Oliveira FS, Pirmez C, Pires MQ, Brazil RP, Pacheco RS (2005) PCR-based diagnosis for detection of Leishmania in skin and blood of rodents from an endemic area of cutaneous and visceral leishmaniasis in Brazil. Vet Parasitol 129:219–227
Pacheco RS, Thomaz N, Brandão AA, Pires MQ, Momen H, Degrave W (1996) Synthetic oligonucleotide that discriminates between the subgenera Schizotrypanum and Megatrypanum. Parasite 3:297–299
Pacheco RS, Brito CMM, Sarquis O, Pires MQ, Borges-Pereira J, Lima MM (2005) Genetic heterogeneity in Trypanosoma cruzi strains from naturally infected triatomine vectors in northeastern Brazil: epidemiological implications. Biochemical Genetics 43:519–30
Romana CA, Pizarro JCN, Rodas E, Guilbert E (1999) Palm trees as ecological indicators of risk areas for Chagas disease. Trans R Soc Trop Med Hyg 93:594–595
Russomando G, Arias AR, Almiron M, Figueredo A, Ferreira ME, Morita K (1996) Trypanosoma cruzi: polymerase chain reaction-based detection in dried feces of Triatoma infestans. Exp Parasit 83:62–66
Salazar A, Schijman AG, Triana-Chávez O (2006) High variability of Colombian Trypanosoma cruzi lineage I stocks as revealed by low-stringency single primer-PCR minicircle signatures. Acta Trop 100:110–118
Sarquis O, Sposina R, Oliveira TG, MacCord JR, Cabello PH, Borges-Pereira J, Lima MM (2006) Aspects of peridomiciliary ecotopes in rural áreas of Northeastern Brazil associated to triatomine (Hemíptera, Reduviidae) infestation, vectors of Chagas disease. Mem Inst Oswaldo Cruz 101:143–147
Schmunis GA (2007) Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz 102:75–85
Silva ES, Gontijo CMF, Pacheco RS, Brazil RP (2004) Diagnosis of human visceral leishmaniasis by PCR using blood samples spotted on filter paper. Genet Mol Res 3:251–257
Silveira AC, Vinhaes MC (1998) Doença de Chagas: aspectos epidemiológicos e de controle. Rev Soc Bras Med Trop 31:15–60
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
World Health Organization (2002) Control of Chagas disease. WHO Technical Report Series 811. Second report of the WHO Expert Committee, Geneva
World Health Organization (2007) New global effort to eliminate Chagas disease. http://www.who.int/mediacentre/news/releases/2007/pr36/en/index.html. Cited 10 August 2007
Acknowledgment
This work received financial support from Programa de Apoio a Pesquisa Estratégica (PAPES-III/FIOCRUZ) and Fundação de Apoio a Pesquisa do Estado do Rio de Janeiro (FAPERJ). The authors declare that the experiments comply with the current Brazilian laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brito, C.M.M., Lima, M.M., Sarquis, O. et al. Genetic polymorphism in Trypanosoma cruzi I isolated from Brazilian Northeast triatomines revealed by low-stringency single specific primer–polymerase chain reaction. Parasitol Res 103, 1111–1117 (2008). https://doi.org/10.1007/s00436-008-1102-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1102-5