Abstract
Amoebic keratitis, a sight-threatening, progressive corneal disease, is commonly caused by ubiquitous, pathogenic, free-living Acanthamoeba spp., which are widely distributed in the environment. We investigated clinical findings and histology of Acanthamoeba keratitis in a rat cornea model. Experimental Acanthamoeba keratitis was induced in Wistar rats by intrastromal inoculation of Acanthamoeba castellanii trophozoites. The clinic features of Acanthamoeba keratitis by day 70 are observed. All rats inoculated with Acanthamoeba developed keratitis. Histologically, the eyes displayed blood vessels, edema, and amoebae in stroma. A mixed cellular response, including neutrophils, mononuclear cells, and spindle-shaped cells, was seen. In conclusion, progressive, suppurative Acanthamoeba keratitis can be induced in the rat cornea model. This rat cornea model assists researchers who study the pathogenesis of Acanthamoeba keratitis and devise treatment for this difficult condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acanthamoeba is a free-living amoeba that has been isolated from soil, air, fresh water, and sea water environments (Marciano-Cabral and Cabral 2003; Illingworth and Cook 1998). Acanthamoeba exists in two stages as both the mobile trophozoites and the dormant cysts. The mobile trophozoites (10–25 μm) are characterized by a large central nucleus and prominent nucleolus and normally subsist on bacteria and yeast (Khan 2001). The dormant cyst (8–12 μm) is protected by a bilaminated cellulose wall and resists repeated freeze–thawing cycles, as well as extraordinarily high doses of UV and gamma irradiations (Aksozek et al. 2002).
Human infection with Acanthamoeba is rare and opportunistic. It involves the skin and central nervous system in immunosuppressed patients and patients with acquired immune deficiency syndrome and occasionally involves the cornea in relatively healthy patients by causing Acanthamoeba keratitis (Awwad et al. 2007). Acanthamoeba keratitis is largely restricted to contact lens wearers who have experienced corneal trauma, implicating contact lens wear (Niederkorn et al. 1999a, b). The disease is characterized by severe pain because of radial neuritis and inflammation with redness and photophobia, but this condition is also noted for its wide variety of clinical presentations (Bacon et al. 1993; Illingworth and Cook 1998; Martinez 2001). It is characterized with chronic stromal inflammation as pathology (Kremer et al. 1994; Larkin and Easty 1991; Niederkorn et al. 1999a, b).
The aim of present study was to evaluate clinical findings and histology of Acanthamoeba keratitis in a rat cornea model.
Materials and methods
Animals
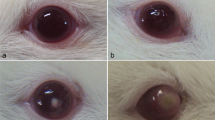
Thirty-three male Wistar rats weighing approximately 125 g were used for the present study. All corneas were examined before inoculation to exclude any abnormality (Fig. 1a). In the first study set for clinical evaluation, 36 eyes of 18 rats were used: 24 eyes of 12 rats for Acanthamoeba infection and 12 eyes of six rats as control. In the second study set for histological evaluation, 30 eyes of 15 rats were used: 20 eyes of ten rats for Acanthamoeba infection and ten eyes of five rats as control.
Amoeba
All studies were performed with Acanthamoeba castellanii strain 1BU, a human corneal isolate (Walochnick et al. 2000).Vegetative forms were obtained from axenic cultures in 25-cm2 corning flasks containing 10 ml protease peptone, yeast extract, and glucose medium (Schuster 2002) and kept at 37°C. Trophozoites in the stage of exponential growth (72 to 96 h) were concentrated by centrifugation at 500 × g for 10 min. The amoebae were washed twice in sterile Neff’s saline solution (1.2 g NaCl, 0.4 g MgSO4·H2O, 0.4 g CaCl2·2H2O, 1.42 g Na2HPO4, 1.36 g KHPO4 in 100 ml distilled water), counted in a hemacytometer, adjusted to a final concentration in Neff’s saline solution at a density of 1 × 106 amoebae/ml (95.0% trophozoites), and used immediately for testing.
Anesthesia
Rompun 10 mg/kg and ketamine HCl mg/kg were given by intramuscular injection, and one drop of preservative-free benoxinate was applied to the right and left eye.
Inoculation technique
The procedure was performed under an operation microscope (Leica-M841). Initially, a half-thickness linear blade incision was made approximately 2 mm from the center of the cornea. With a microliter syringe and 30 G needle, the needle was advanced from the incision through the lamellar of the stroma to the center of the cornea. One microliter of the solution including 1 × 106 amoeba/ml was injected into the stroma. Control animals received a mock inoculum of 1 μl Neff’s saline.
Clinical evaluation
Under general anesthesia, the rats were examined with a slit-lamp microscope on days 1, 3, 5, and 7 after inoculation and weekly thereafter until 70 days. Examination was by retro-illumination, resulting in transillumination of the albino iris and the cornea. The oblique slit beam was used to illuminate lesser degrees of opacity. The following grading scheme, which has been previously described (Larkin and Easty 1990), was used: grade 0 = normal, grade 1 = opacity visible only by oblique slit beam, grade 2 = opacity visible on retro-illumination but not sufficient to obscure iris vessels, grade 3 = opacity visible on retro-illumination, obscuring iris vessel detail. We evaluated clinical findings according to the presence of epithelial edema, punctate epitheliopathy, stromal vascularization, stromal infiltration, and pannus tissue and opacitiy grade (1–3).
Histological evaluation
Under general anesthesia, the rats were examined with a slit-lamp microscope weekly thereafter until 70 days. Then, the eye was enucleated, and the cornea was dissected from the globe and hemisected. The cornea was fixed for 24 h in neutral-buffered formaldehyde, embedded in wax, and 6-μm-thick sections were cut with microtome. All of the tissue sections were stained by hematoxylin–eosin. Eight or more sections were viewed for each eye. We evaluated histological changes according to the presence of stromal edema and neovascularization and trophozoite, neutrophil, eosinophile, and spindle-shaped cells.
Results
Clinical evaluation
The clinic features of Acanthamoeba keratitis at days 1, 3, 5, 7, 14–28, 35, 42, and 49–70 are shown in Table 1. All rats inoculated with Acanthamoeba developed keratitis. In the control eyes, no infection was developed. Slit-lamp examination on day 3 showed corneal edema in 62.5% of the infected eyes (Fig. 1b and c). Fluorescein staining of the cornea on day 3 showed the formation of epithelial defect (Fig. 1d). Opacity was granular in character, with corneal thinning superficial to infiltrates apparent in eyes with more long-standing keratitis. On day 7 after inoculation, grade 1 opacity was observed in 66.6% of the infected eyes (Fig. 2c). On days 14–28 after inoculation, grade 2 opacity was observed in 36.0% of the infected eyes (Fig. 2b). On day 42 after inoculation, grade 3 opacity was observed in 95.8% of the infected eyes (Fig. 2a). Corneal neovascularization was developed in 45.8% of the infected eyes by day 35 (Fig. 3a). In the chronic stage of experimental keratitis, stromal infiltration and pannus developed by day 49 (Fig. 3b and c).
Histological evaluation
The histological features of Acanthamoeba keratitis by weeks 1–10 are shown in Table 2.
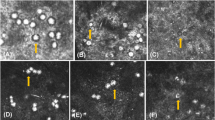
Histological features of normal rat cornea are shown in Fig. 4a. In all examinations, the corneal epithelium was present, but the squamous cells were convoluted and spaced compared with those of the normal rat eye. This was interpreted as being due to edema (Fig. 4b and c). The endothelium was also present, but it was infiltrated and supplanted by neutrophils and mononuclear cells in the central region (Fig. 4c). Hypopyon was observed in some eyes. Amoebae were confined to the stroma (Fig. 4b and c). Monocytes, macrophages, spindle-shaped cells, eosinophils, and blood vessels were also present in the stroma (Fig. 4b and c). Many corneas were seen to have microabscesses, most of which were present in the posterior stroma. Although trophozoites were not shown in the stroma on 5–10 weeks, inflammatory cells are present in the stroma.
Discussion
In this study, the development of Acanthamoeba keratitis was monitored by clinical corneal and histological examination for 70 days postinfection. Epithelial edema was observed on eyes (20.8, 62.5, 29.1, and 16.6%) on days 1, 2, 5, and 7, respectively. After progress of infection from epithelial to stromal tissue, the ratio of epithelial edema gradually decreased to 0 on day 14. Grades 1, 2, and 3 corneal opacity was found on days 5, 7, and 14. Corneal neovascularization was developed in 45.8% of the infected eyes by day 35. In the chronic stage of experimental keratitis, stromal infiltration and pannus developed by day 49.
Acanthamoeba keratitis typically has an indolent but progressive course, with intense discomfort and stromal infiltration (Jones 1986). The disease is often initially misdiagnosed as herpes simplex keratitis or adenoviral keratitis (Tabin et al. 2001). Early signs of Acanthamoeba keratitis clinically include epithelial irregularities, opacities, microerosions, microcystic edema, and patchy anterior stromal infiltrates (Berger et al. 1990; Moore and McCulley 1989). Late during the disease course (prolonged infection), Limbal hyperemia, edema, and ring infiltrate can develop. Ring infiltrate was shown to be present in 18% of Acanthamoeba keratitis cases in the first month and in 83% after 2 months (Bacon et al. 1993). Trophozoites are transformed into cysts (Khunkitti et al. 1998).
The development of an animal model of Acanthamoeba keratitis is essential to the detailed study of this condition and for controlled in vivo testing of new pharmacologic agents. We used rats because they are inexpensive, they are easy to keep in large numbers, and the size of their eyes allows controlled surgical procedures. The inocula were prepared so that most of the amoebae injected were in the trophozoite form. It was thought that inoculation with active amoebae would give the best change of establishing an infection. To our knowledge, the present study is the first model that evaluated clinically and histologically in the rat cornea by 70 days without the need for concomitant immunosuppression by corticosteroid or coinoculation with bacteria. Badenoch et al. (1990) reported that chronic corneal infection was achieved using coinfection of Acanthamoeba and Corynebacterium, a limitation for the evaluation of antiamoebal agents. The anti-inflammatory and immunosuppressive activities of betamethasone may partially control local responses to amoeba-induced necrosis and parasite cytolytic factor release (Mathers et al. 1987). In human cases, local cordicosteroids was also found to promote inhibition of cyst–trophzoite transformation and result in severe corneal lesion (Lindquist 1998).
Most human specimens on which histopathological descriptions are available originate from corneal transplantation and accordingly describe the late stage of the disease, probably modified by drug therapy. Nevertheless, available reports describe changes similar to those described in this report. Disruption and necrosis of stromal lamellae and invasion by amoebae in stroma are observed.
In conclusion, progressive Acanthamoeba keratitis can be induced in rats with these laboratory settings. This Acanthamoeba keratitis rat model will greatly assist researchers who study the pathogenesis and devise treatment of this difficult condition.
References
Aksozek A, McClellan K, Howard K, Niederkorn JY, Alizadeh H (2002) Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol 88:621–623
Awwad ST, Petroll WM, McCulley JP, Cavanagh HD (2007) Updates in Acanthamoeba keratitis. Eye Contact Lens 33:1–8
Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, Wright P (1993) A review of 72 consecutive cases of Acanthamoeba keratitis, 1984–1992. Eye 7:719–725
Badenoch PR, Johnson AM, Christy PE, Coster DJ (1990) Pathogenicity of Acanthamoeba and a Corynebacterium in rat cornea. Arch Ophthalmol 108:107–112
Berger ST, Mondino BJ, Hoft RH, Donzis PB, Holland GN, Farley MK, Levenson JE (1990) Successful medical management of Acanthamoeba keratitis. Am J Ophthalmol 110:395–403
Illingworth CD, Cook SD (1998) Acanthamoeba. keratitis. Surv Ophthalmol 42:493–508
Jones DB (1986) Acanthamoeba. —the ultimate opportunist? Am J Ophthalmol 102:527–530
Khan NA (2001) Pathogenicity, morphology, and differentiation of Acanthamoeba. Curr Microbiol 43:391–395
Khunkitti W, Lloyd D, Furr JR, Russell AD (1998) Acanthamoeba castellanii. : growth, encystment, excystment and biocide susceptibility. J Infect 36:43–48
Kremer I, Cohen EJ, Eagle RC Jr, Udell I, Laibson PR (1994) Histopathologic evaluation of stromal inflammation in Acanthamoeba keratitis. CLAO J 20:45–48
Larkin DF, Easty DL (1990) Acanthamoeba. keratitis: I. Preliminary findings. Br J Ophthalmol 74:551–555
Larkin DF, Easty DL (1991) Experimental Acanthamoeba keratitis. II. Immunohistochemical evaluation. Br J Ophthalmol 75(7):421–424
Lindquist TD (1998) Treatment of Acanthamoeba keratitis. Cornea 17:11–16
Marciano-Cabral F, Cabral G (2003) Acanthamoeba spp. as agents of disease in humans. Clin Microbiol 16:273–307
Martinez AJ (2001) Free-living amebas and the immune deficient host. In: IX International Meeting on the Biology and Pathogenicity of Free-living Amoebae Proceedings, pp 1–12
Mathers W, Stevens G, Rodriguez M, Chan CC, Gold J, Visvesvara GS, Lemp MA, Zimmerman LE (1987) Immunopathology and electron microscopy of Acanthamoeba keratitis. Am J Ophthalmol 103:626–635
Moore MB, McCulley JP (1989) Acanthamoeba. keratitis associated with contact lenses: six consecutive cases of successful management. Br J Ophthalmol 73:271–275
Niederkorn JY, Alizadeh H, Leher H, McCulley JP (1999a) The pathogenesis of Acanthamoeba keratitis. Microbes Infect 1:437–443
Niederkorn JY, Alizadeh H, Leher HF, McCulley JP (1999b) The immunobiology of Acanthamoeba keratitis. Springer Semin Immunopathol 21:147–160
Schuster FL (2002) Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol 15:342–354
Tabin G, Taylor H, Snibson G, Murchison A, Gushchin A, Rogers S (2001) Atypical presentation of Acanthamoeba keratitis. Cornea 20:757–759
Walochnik J, Obwaller A, Aspock H (2000) Correlations between morphological, molecular biological, and physiological characteristics in clinical and nonclinical isolates of Acanthamoeba spp. Appl Environ Microbiol 66:4408–4413
Acknowledgments
We thank Julia Walochnik of the Department of Medical Parasitology, Clinical Institute of Hygiene, University of Vienna, Vienna, Austria, for providing A. castellanii strain 1BU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polat, Z.A., Ozcelik, S., Vural, A. et al. Clinical and histologic evaluations of experimental Acanthamoeba keratitis. Parasitol Res 101, 1621–1625 (2007). https://doi.org/10.1007/s00436-007-0704-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0704-7