Abstract
Enterocytozoon bieneusi is the most common microsporidium associated with AIDS patients. Moreover, its detection in increasing numbers of immunocompetent patients has made it an emerging pathogen. This organism was also identified in a wide range of animals, and the zoonotic potential of human infections is of particular interest. In this study, 538 fecal samples from cattle in Korea were analyzed for the presence of E. bieneusi by PCR. Approximately 15% were found to be positive, with higher rates being detected over the summer months. The internal transcribed spacer (ITS) regions of the rRNA gene of ten E. bieneusi positive samples were amplified using nested PCR and sequenced. Genetic polymorphisms, which were represented by six distinct genotypes (CEbA–CEbF), were found among the E. bieneusi isolates. Five isolates from this study had identical ribosomal ITS to the previously known E. bieneusi genotype ITSs in cattle and other animals. Four isolates were previously unreported but were quite similar to the previously known genotypes of E. bieneusi from cattle and other animals. One isolate was identical to the human E. bieneusi type D, which indicated some E. bieneusi isolates from cattle in the country may be of public health importance. To the best of my knowledge, this is the first report of E. bieneusi study in cattle in Asia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microsporidia are intracellular organisms that cause opportunistic infections in a wide range of animals and humans (Didier and Weiss 2006). The phylum Microspora contains almost 100 genera and more than 1,200 species of microsporidia (Mathis et al. 2005). These organisms are defined by the presence of a nucleated sporoplasm, a coiled polar tube, and an anchoring disk, and an absence of several eukaryotic characteristics such as a lack of mitochondria, Golgi membranes, and eukaryotic ribosomes (Weber et al. 1996; Franzen and Muller 1999; Mathis et al. 2005). Several species of microsporidia are becoming increasingly recognized as a cause of significant diseases in humans. Enterocytozoon bieneusi is the most common microsporidium found in AIDS patients and was detected in increasing numbers in immunocompetent patients (Weber and Bryan 1994; Rinder et al. 2000). This organism primarily infects the enterocytes of the small intestine and causes chronic diarrhea (Desportes et al. 1985; Cali and Owen 1990). Since E. bieneusi was first recognized in biopsy specimens from persons with AIDS in 1985 (Desportes et al. 1985), this parasite has been identified in 30 to 50% of AIDS patients suffering from chronic diarrhea, and also causes significant wasting and malabsorption (Shadduck and Orenstein 1993; Weber et al. 1996; Mathis et al. 1999). Moreover, E. bieneusi was recently reported to be associated with hepatobiliary and pulmonary infections and cause papillary stenosis, acalculous cholecystitis, bile duct dilatation, and sclerosing cholangitis (McWhinney et al. 1991; Beaugerie et al. 1992; Weber et al. 1992; Pol et al. 1993). The sources of microsporidia infecting humans and its transmission routes are not completely understood. However, animal is one of the most likely sources of human infections because this organism is released into the environment through an animal’s stool, urine, and respiratory secretions. Since the detection of E. bieneusi in fecal samples from pigs was described in 1996 (Deplazes et al. 1996), the occurrence of E. bieneusi in several other animals such as dogs, cats, rabbits, monkeys, and cattle were reported (Mansfield et al. 1998; del Aguila et al. 1999; Mathis et al. 1999; Rinder et al. 2000). Of these animal studies, epidemiologic research on animals is a critically important parameter for illustrating the sources of human infection and for public health.
It is unclear if E. bieneusi-infected cattle pose a risk of human infection. However, they must be considered a potential source of human infection in cases of cattle harboring zoonotic genotypes (Sulaiman et al. 2004). There are few reports on the characterization of E. bieneusi in cattle, and these reports are in mainly of Europe and North America (Rinder et al. 2000; Dengjel et al. 2001; Fayer et al. 2003; Santín et al. 2004; Sulaiman et al. 2004). There is little information of the characterization of E. bieneusi in cattle in other areas of the world. This study investigated the occurrence of E. bieneusi in cattle in Korea using molecular methods and the sequence analysis to both assess their genotypic characteristics, and determined if they are a potential source of human infection. Recently, the molecular characterization of the 243-bp internal transcribed spacer (ITS) region of the E. bieneusi rRNA was examined in an attempt to determine the differences between E. bieneusi stains (Rinder et al. 2000; Buckholt et al. 2002; Sulaiman et al. 2004). This allows an examination of the differences in E. bieneusi strains and an assessment of the strains found in more than one host type. In this study, sequence analysis of ITS was carried out for molecular typing and a comparison with the available sequences. The analysis revealed six ITS polymorphisms and three new genotypes of E. bieneusi among the isolates examined. More importantly, an isolate from one sample had an identical sequence to that of the human-derived E. bieneusi type D, which was also previously identified in pigs and several wild animals (Rinder et al. 2000; Buckholt et al. 2002; Sulaiman et al. 2003). This suggests that this genotype can be a crossover between hosts and have zoonotic potential.
Materials and methods
Collection of fecal specimens
A total of 538 fecal samples were collected at monthly intervals from beef and dairy cattle at 157 farms located throughout Korea from September 2004 to August 2005 (Table 1). Two to five samples were collected per farm from each of the sampling locations. The feces were collected directly from the rectum of each cow. All the samples were transported immediately to the laboratory in ice-cooled containers and were processed within 2 days of collection.
DNA extraction for PCR
Approximately 200 μl of feces was transferred to a 2-ml screw cap conical tube containing 200 μl of 0.5-mm glass beads (Biospec Products, Bartlesville, OK, USA) and 400 μl of a digestion buffer (100 mM NaCl, 25 mM EDTA, 10 mM Tris–Cl (pH 8.0), 1% sodium dodecyl sulfate (SDS), and 100 μg/ml proteinase K). The sample was then placed in a minibead beater at 5,000 rpm for 2 min and incubated for 1 h at 50°C. The samples were then spun in a microcentrifuge for 2 min at top speed. The supernatant was transferred to a new tube and mixed with an equal volume of phenol/chloroform and 300 μl of the supernatant was added to 50 μl of 5 M NaCl. The mixture was incubated for 10 min at 65°C. After incubation the solution was extracted with an equal volume of chloroform. The DNA was recovered from the resulting supernatant using the Geneclean system (BIO101, OH, USA) according to the manufacture’s protocol for liquid samples, and the DNA was resuspended in 20 μl of distilled water. As PCR template, 1 to 2 μl of the DNA solution was used.
PCR amplification and sequence analysis
The presence of E. bieneusi in the feces was examined by nested PCR amplification. The first PCR amplification was performed using the EBIEF1 and EBIER1 primers as described by De Silva et al. (1996). The cycling parameters consisted of 45 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 40 s. Nested amplification was performed using the primer EBIEF5: 5′-GCGACACTCTTAGACGTAT-3′ and EBIER6: 5′-TGGCCTTCCGTCAATTTC-3′, with 30 cycles of 94°C for 30 s, 57°C for 30 s, and 72°C for 30 s. The amplification of the E. bieneusi templates with the nested primer pair results in a 200-bp DNA fragment. A 350-bp internal control amplified by EBIER1 and EBIEF1 was included in each reaction mixture to test for any false-negative results due to the inhibition of PCR. The amplified products were resolved electrophoretically on a 2% agarose gel and stained with ethidium bromide for visual analysis. The identities of the PCR products were further confirmed by digestion with the MspA1I restriction enzyme (Kondova et al. 1998). Amplification of the ITS region of the rRNA gene of E. bieneusi isolates was also carried out using nested PCR. For primary PCR, a PCR product of 410 bp was amplified using the primers AL4037: 5′-GATGGTCA TAGGGATGAAGAGCTT-3′and AL4039: 5′-AATACAG GATCACT TGGATCCGT-3′. The cycling parameters were 35 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 60 s (Sulaiman et al. 2004). For secondary PCR, the 392-bp DNA fragment was amplified using the primers AL4038: 5′-AGGGATGAAGAGCTTCGGCTCTG-3′ and AL4040: 5′-AATATCCCTAATACAGGATCACT-3′ under identical conditions to those used for primary PCR. The secondary PCR products with the expected size were purified using a PCR DNA purification system (Promega, Madison, WI, USA), and were sequenced using the sequencing service of the Takara (Seoul, Korea). Sequencing was performed on the products from two separate PCRs, which were sequenced in both directions. The sequences were edited and assembled using the consensus sequence. The sequences of the E. bieneusi isolates detected in this study were compared with those from GenBank. The nucleotide sequences of the E. bieneusi ITS region from cattle representing new genotypes (CEbA, CEbD, and CEbF) were deposited in the GenBank database under the accession numbers EF139195, EF139198, and EF139194, respectively.
Results
Five hundred thirty-two cattle were sampled during the 12-month period. Eighty (approximately 15%) PCR products of the expected size (200 bp) were produced for E. bieneusi. Table 1 shows the percentage of positive samples per collection date. There was a trend toward a higher prevalence in the warmer periods with the highest and lowest positive rate being found in August (25.9%) and February(5.5%), respectively. The PCR products of EBIEF5 and EBIER6, as well as AL4038 and AL4040 from the 10 E. bieneusi-positive samples were randomly selected for sequencing to ensure the specificity of the PCR assay. The homologies to the published E. bieneusi sequences ranged from 99 to 100% for the small subunit ribosomal RNA (SSU-rRNA) portions of each PCR fragment amplified by EBIEF5 and EBIER6 (GenBank accession numbers L16868, L07123, AF024657, AF119100, and AF023245). The homology of the ITS sequences amplified by AL4038 and AL4040 ranged from 94 to 100% of the published E. bieneusi ITS sequences (Tables 2 and 3).
An analysis of ITS sequences revealed the presence of six distinct genotypes (CEbA–CEBF) of E. bieneusi among the ten isolates characterized. Sequence heterogeneity among the six genotypes was found in the ITS region of the sequences. Table 2 shows the polymorphic sites from this study. Four genotypes differed from each other by one to three single nucleotide polymorphisms (SNPs). However, one of the genotypes, CEbC, differed by 10 SNPs, and another, CEbD, was even more divergent (14 SNPs).
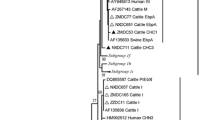
Two isolates of the genotype CEbB were homologous to type J or BEB1, which were originally isolated from cattle and chickens (Table 3). Three isolates of the genotype CEbE were homologous to type I or BEB2, which were originally isolated from cattle. One isolate was identical to E. bieneusi ITS type D, PigEBITS9 or WL8, which was originally isolated from humans, pigs, and wild animals such as foxes, beavers, and raccoons. Four isolates of previously unreported types with three new polymorphic nucleotides of the ITS region were also discovered, and these new types are designated CEbA, CEbD, and CEbF (Tables 2 and 3). The isolates of CEbA, CEbD, and CEbF showed 99% similarity with types I or BEB2, 98% similarity with types F or EbpA, which were originally isolated from cattle and pigs, and 99% similarity with types J or BEB1.
Discussion
The epidemiological information of E. bieneusi has evolved in the recent years. However, The sources of infection and its transmission routes are not completely understood. This organism was isolated from many animals, and the isolates from the animals have revealed high genetic similarity to those of the human isolates; this indicates that the animals are the possible sources of human infections in E. bieneusi (Weber et al. 1996; Mansfield et al. 1998; del Aguila et al. 1999; Mathis et al. 1999, 2005; Rinder et al. 2000; Dengjel et al. 2001; Buckholt et al. 2002; Sulaiman et al. 2003). This study demonstrated, for the first time, the presence of microsporidia in cattle in Asia, and investigated the possible presence of human-related E. bieneusi in these cattle.
Among the 538 fecal samples of cattle analyzed by PCR, approximately 15% of samples tested positive. Ten randomly selected PCR positive specimens were sequenced. The portion of SSU-rRNA coding sequence of this microsporidian was compared with other SSU-rRNA sequences from GenBank. The homologies to the published E. bieneusi sequences were 99 to 100%, which confirms that the isolates from the cattle detected by PCR were E. bieneusi. A recent study of 338 fecal samples of cattle in the United States (Sulaiman et al. 2004) using PCR techniques found 32 of E. bieneusi-positive cattle (9.5%). In a study in Europe, 8 out of 88 (9.1%) cattle tested in Germany were positive (Rinder et al. 2000). The differences in prevalence suggest real geographic variations or differences in the procedures of the samples. The occurrence of E. bieneusi is apparently affected by temperature because there was a trend toward a higher prevalence in the warmer months in this study. Other studies have also reported a higher prevalence in spring and summer (Buckholt et al. 2002). This indicates that the season might be a risk factor for E. bieneusi infections, even though no conclusions can be drawn from this study.
The extent of genetic diversity in E. bieneusi from cattle was measured by the multiple alignment of the ITS sequences. The analysis revealed that heterogeneity among the ITS sequences and genetic polymorphisms, which were represented by six distinct genotypes, were found among the E. bieneusi isolates. Four genotypes differed from each other by only one to three SNPs. However, two genotypes differed by 10 and 14 SNPs. A previous study reported some trends in the nucleotide polymorphisms of the ITS sequences (Buckholt et al. 2002). The isolates of a bovine origin may contain four distinctive polymorphic sites (sites 129, 131, 147, and 158). However, this study revealed that only two genotypes, CEbC and CebD, had the indicated polymorphic sites (Table 2). There are no apparent distinctive polymorphic sites in any of the ITS sequences of the isolates from this study.
Many studies have reported host-specific genotypes and broad host-adapted genotypes of the E. bieneusi. Distinct genotypes, such as WL1, WL2, and WL3, were observed only in raccoon specimens, and genotypes WL4, WL5, and WL6 were found only in muskrat specimens (Sulaiman et al. 2003, 2004). Phylogenetic analysis using neighbor-joining and maximum likelihood analysis also revealed that these genotypes formed two host-specific clusters outside of the major cluster containing all E. bieneusi from humans and domestic animals. This indicates that genotypes WL1, WL2, and WL3 and genotypes WL4, WL5, and WL6 represent only raccoon and muskrat isolates, respectively. Cattle-specific genotypes also exist. Genotype CEbE from this study, along with the previously described genotypes I or BEB2 (Rinder et al. 2000; Sulaiman et al. 2004), was found only in cattle, and phylogenetic analysis showed that they form a cluster consisting of isolates from cattle only, suggesting that this type is host-specific. On the other hand, some genotypes such as CEbC, together with the previously described genotypes D PigEBITS9 or WL8 (Rinder et al. 2000; Buckholt et al. 2002; Sulaiman et al. 2004), were found in many different hosts and were geographically widespread. This type was isolated in humans, pigs, and several wild animals such as foxes, beavers, and raccoons and was detected in Germany, Switzerland, and the United States. Therefore, this genotype has broad host adaptation and can be transmitted from animals to humans. The detection of isolates of this genotype demonstrates the zoonotic potential of E. bieneusi from cattle in this country, and that cattle may be a source of environmental contamination for E. bieneusi infections.
This study also discovered previously unreported types with three new polymorphic nucleotides of the ITS region. These new types showed high similarities (98 to 99%) to previously identified genotypes, which were originally isolated from cattle, chicken, and pigs (Breitenmoser et al. 1999; Rinder et al. 2000; Sulaiman et al. 2004). The new genotypes identified in this study probably represent minor differences in the structures of previously identified genotypes.
In conclusion, these results suggest that E. bieneusi is prevalent in cattle in Korea and there is extensive genetic diversity, as was also reported in other parts of the world. Even though the data suggests that most isolates from cattle are not associated with human infections, cattle can serve as a potential reservoir E. bieneusi due to the possibility of cattle to human transmission.
References
Beaugerie L, Teilhac MF, Deluol A, Fritsch J, Girard P, Rozenbaum W, Le Quintrec Y, Chatelet F (1992) Cholangiopathy associated with Microsporidia infection of the common bile duct mucosa in a patient with HIV infection. Ann Intern Med 117:401–402
Breitenmoser AC, Mathis A, Burgi E, Weber R, Deplazes P (1999) High prevalence of Enterocytozoon bieneusi in swine with four genotypes that differ from those identified in humans. Parasitology 118:447–453
Buckholt MA, Lee JH, Tzipori S (2002) Prevalence of Enterocytozoon bieneusi in swine: an 18-month survey at a slaughterhouse in Massachusetts. Appl Environ Microbiol 68:2595–2599
Cali A, Owen RL (1990) Intracellular development of Enterocytozoon, a unique microsporidian found in the intestine of AIDS patients. J Protozool 37:145–155
Chalifoux LV, Carville A, Pauley D, Thompson B, Lackner AA, Mansfield KG (2000) Enterocytozoon bieneusi as a cause of proliferative serositis in simian immunodeficiency virus-infected immunodeficient macaques (Macaca mulatta). Arch Pathol Lab Med 124:1480–1484
De Silva AJ, Scwartz DA, Visvesvara GS, De Moura H, Slemenda SB, Pieniazek NJ (1996) Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (Microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol 34:986–987
del Aguila C, Izquierdo F, Navajas R (1999) Enterocytozoon bieneusi in animals: rabbits and dogs as new hosts. J Eukaryot Microbiol 46:8S–9S
Dengjel B, Zahler M, Hermanns W, Heinritzi K, Spillmann T, Thomschke A, Loscher T, Gothe R, Rinder H (2001) Zoonotic potential of Enterocytozoon bieneusi. J Clin Microbiol 39:4495–4499
Deplazes P, Mathis A, Mueller C (1996) Molecular epidemiology of Encephalitozoon cuniculi and first detection of Enterocytozoon bieneusi in fecal samples of pigs. J Eukaryot Microbiol 43:93S
Desportes I, Le Charpentier Y, Galian A (1985) Occurrence of a new microsporidian, Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. J Protozool 32:250–254
Didier ES, Weiss LM (2006) Microsporidiosis: current status. Curr Opin Infect Dis 19:485–492
Fayer R, Santín M, Trout JM (2003) First detection of microsporidia in dairy calves in North America. Parasitol Res 90:383–386
Franzen C, Muller A (1999) Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin Microbiol Rev 12:243–285
Kondova I, Mansfield K, Buckholt MA, Stein B, Widmer G, Carville A, Lackner A, Tzipori S (1998) Transmission and serial propagation of Enterocytozoon bieneusi from humans and Rhesus macaques in gnotobiotic piglets. Infect Immun 66:5515–5519
Mansfield KG, Carville A, Hebert D, Chalifoux L, Shvetz D, Link C, Tzipori S, Lackner A (1998) Localization of persistent Enterocytozoon bieneusi infection in normal rhesus macaques to the hepatobiliary tree. J Clin Microbiol 36:3071–3074
Mathis A, Breitenmoser AC, Deplazes P (1999) Detection of new Enterocytozoon genotypes in fecal samples of farm dog and cat. Parasite 6:189–193
Mathis A, Weber R, Deplazes P (2005) Zoonotic potential of the microsporidia. Clin Microbiol Rev 18:423–445
McWhinney PHM, Nathwani D, Green ST (1991) Microsporidia detected in association with AIDS-related sclerosing cholangitis. AIDS 5:1394–1395
Pol S, Romana CA, Richard S (1993) Microsporidia infection in patient with the acquired immunodeficiency virus and unexplained cholangitis. N Engl J Med 328:95–99
Rinder H, Thomschke A, Dengjel B, Gothe R, Loscher T, Zahler M (2000) Close genotypic relationship between Enterocytozoon bieneusi from humans and pigs and first detection in cattle. J Parasitol 86:185–188
Santín M, Trout JM, Fayer R (2004) Prevalence of Enterocytozoon bieneusi in post-weaned dairy calves in the eastern United States. Parasitol Res 93:287–289
Shadduck JA, Orenstein JM (1993) Comparative pathology of microsporidiosis. Arch Pathol Lab Med 117:1215–1219
Sulaiman IM, Fayer R, Lal AA, Trout JM, Schaefer FW, Xiao L (2003) Molecular characterization of microsporidia indicates that wild mammals harbor host-adapted Enterocytozoon spp. as well as human pathogenic Enterocytozoon bieneusi. Appl Environ Microbiol 69:4495–4501
Sulaiman IM, Fayer R, Yang C, Santín M, Matos O, Xiao L (2004) Molecular characterization of Enterocytozoon bieneusi in cattle indicates that only some isolates have zoonotic potential. Parasitol Res 92:328–334
Weber R, Bryan RT (1994) Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis 19:517–521
Weber R, Kuster H, Keller R, Bachi T, Spycher MA, Briner J, Russ E, Luthy R (1992) Pulmonary and intestinal microsporidiosis in a patient with the acquired immunodeficiency syndrome. Am Rev Respir Dis 146:1603–1605
Weber R, Bryan RT, Schwartz DA (1996) Human microsporidia infections. Clin Microbiol Rev 7:426–461
Acknowledgments
This study was supported by grant no. RTI05-03-02 from the Regional Technology Innovation Program of the Ministry of Commerce, Industry and Energy (MOCIE), and by the Brain Korea 21 Project in Republic of Korea. The author wish to thank the technical staff and volunteer students in College of Veterinary Medicine, Chonbuk National University for technical supports and collection of samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J.H. Prevalence and molecular characteristics of Enterocytozoon bieneusi in cattle in Korea. Parasitol Res 101, 391–396 (2007). https://doi.org/10.1007/s00436-007-0468-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0468-0