Abstract
Phalloidin fluorescence technique, enzyme cytochemistry and immunocytochemistry in conjunction with confocal scanning laser microscopy have been used for the first time to describe the nervous and muscle systems of the viviparous monogenean gill parasite, Macrogyrodactylus clarii. The gross spatial arrangement of muscle and associated cholinergic, peptidergic and aminergic innervations has been examined. The central nervous system (CNS) consists of paired cerebral ganglia from which emanate three pairs of longitudinal ventral, lateral and dorsal nerve cords, connected at intervals by transverse connectives. The CNS is better developed ventrally than dorsally or laterally, and has the strongest reactivity for all neuroactive substances examined. Structural and functional correlates of the neuromusculature of the pharynx, haptor and reproductive tracts have been examined. Results implicate acetylcholine, FMRFamide-related peptides (FaRPs) and serotonin in sensory and motor function in this monogenean, although confirmatory physiological data are obviously required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two species of Macrogyrodactylus (Monopisthocotylea: Gyrodactylidae) have been recorded and described in detail from the freshwater catfish, Clarias gariepinus (syn: C. lazera) inhabiting the Demietta branch of the river Nile. These are M. clarii Gussev 1961 from the gills, and M. congolensis Prudhoe 1957 from the fins and skin surface (see El-Naggar and Serag 1987, and El-Naggar et al. 1999 respectively). El-Naggar et al. (2001a) studied the chaetotaxy of these parasites, mapping the argentophilic elements of their nervous systems. M. clarii is a viviparous monogenean and, in common with all other gyrodactylids, it contains a daughter within its uterus which, in turn, bears a developing embryo. Up to four individuals may be nested within one another, reminiscent of ‘Russian dolls’ (Cable et al. 1998). Consequently, the reproductive organs of gyrodactylids are modified by the twin pressures of viviparity (Llewellyn 1981; Tinsley 1983) and progenesis (Harris 1983).
Many authors have used the method of demonstrating cholinesterase activity as indirect evidence of the presence of acetylcholine in the nervous system of flatworms, including monogeneans, digeneans and cestodes (see review by Halton and Gustafsson 1996, and references therein; Arafa et al. 2002; Reda and Arafa 2002). Also, numerous immunocytochemical studies using confocal imaging have demonstrated aminergic and peptidergic elements in well-differentiated nervous systems in oviparous monogeneans such as Diclidophora merlangi (see Maule et al. 1990a, 1990b), Discocotyle sagittata (Cable et al. 1996b) and Eudiplozoon nipponicum (Zurawski et al. 2001), and have revealed central and peripheral nervous systems which are dominated by peptidergic neurones, especially in the innervation to the female reproductive tract. Combining this methodology with the use of the phalloidin-fluorescence technique for F-actin has added considerably to the understanding of nerve–muscle relationships in these and other parasitic flatworms (Halton et al. 1998). The present work involved a similar approach to explore the neuromusculature of M. clarii and, as far as is known, it is the first study on the nervous system and neuromusculature of a gyrodactylid parasite using enzyme histochemistry and immunocytochemistry in combination with confocal microscopy.
Materials and methods
Specimens of Clarias gariepinus were captured from the Demietta branch of the river Nile, Dakahlia province, Egypt. Fish were kept alive in river water until required, at which time they were killed with a blow to the head. The gills were removed, placed in filtered river water, and examined for M. clarii using a stereomicroscope. The worms were carefully detached from the gills by means of a fine needle and processed as follows. To reveal cholinergic components of the nervous system, specimens (n=20) of M. clarii were flattened between a microscope slide and coverslip, and fixed in 10% neutral formalin for 30 min. They were then washed in distilled water, incubated in a solution of acetylthiocholine iodide (AcThI) according to Rahemo and Gorgees (1987), and examined using a stereomicroscope. As soon as details of the nervous system became visible, the specimens were washed in distilled water. Some of the treated specimens were counterstained with aqueous solutions of toluidine blue (1%), alcian blue (1%), light green (0.2%), fast green (0.2%) or methyl green (2%). They were then dehydrated through ethanol, cleared in terpeniol, mounted in Canada balsam, and viewed using a Leitz Laborlux S light microscope under oil immersion. Control specimens (n=5) of M. clarii were treated as above but incubated without the AcThI substrate.
Serotonin (5-hydroxytryptamine-, 5-HT) and FMRFamide-related neuropeptide (FaRP) immunoreactivities were visualised by the indirect immunofluorescence technique of Coons et al. (1955). Worms (n=20) were flat-fixed between slides in 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS: 0.145 M NaCl; 0.025 M NaH2PO4.2H2O; 0.075 M Na2HPO4; pH 7.4) for 1 h, then transferred to fresh fixative for a further 3 h. Specimens were subsequently placed in 0.1 M antibody diluent (AbD: PBS containing 0.35% (v/v) Triton X-100; 0.1% (w/v) sodium azide; 0.1% (w/v) bovine serum albumin) and immediately airmailed to Belfast. Specimens so treated were then incubated with the following primary antisera for 72 h at 4°C: FaRP neuropeptide antiserum raised in a guinea pig to the flatworm pentapeptide FaRP, GYIRFamide (working dilution of 1:400) or serotonin antiserum raised in a rabbit and used at a working dilution of 1:300. Following overnight wash in AbD, specimens were incubated for 48 h in rabbit anti-guinea pig IgG (1:100) for GYIRFamide or swine anti-rabbit IgG immunoserum for 5-HT. Secondary antisera were conjugated to either fluorescein isothiocyanate (FITC) or tetramethyl rhodamine isothiocyanate (TRITC) as fluorophores for visualisation of binding sites. After an overnight wash, FITC- or TRITC-labelled phalloidin was employed as a counterstain for demonstration of filamentous F-actin of muscle. For this, immunostained specimens were incubated in 200 mg/ml phalloidin-FITC or -TRITC for 24 h, washed overnight in AbD, and finally mounted in PBS/glycerol (1:9 v/v) and viewed using a Leica TCS-NT confocal scanning laser microscope (Leica Microsystems, Milton Keynes, UK). Controls (n=6) used were (1) omission of primary antiserum; (2) replacement of primary antiserum with non-immune serum from the donor species; and (3) pre-adsorption of primary antiserum with 200 mg of appropriate antigen.
Note that all abbreviations shown in the figures are defined in the main text and/or figure captions.
Results
Musculature
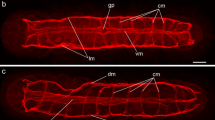
The body wall or subtegumental musculature of Macrogyrodactylus clarii comprises outer circular (cm), intermediate longitudinal (lm) and inner diagonal (dm) myofibres forming distinct layers in a well-organised, lattice-like arrangement encompassing the whole body (Figs. 1 and 2A, F). Additional dorso-ventral muscle fibres extend between the dorsal and ventral borders of the body wall through the parasite. While the circular muscle fibres form a thin layer of close and evenly spaced fibres running perpendicular to the longitudinal body axis, the longitudinal fibres are more widely spaced than the circular fibres and run parallel to the main body axis; moreover, they appear to be concentrated laterally. Most of the diagonal fibres (dm) occur singly but some of them, particularly those found in the region anterior to the pharynx, are arranged in pairs (Fig. 2A, C, F). The diagonal fibres of the dorsal body wall are more extensive and uniformly orientated than those of the ventral surface, especially in the region anterior to the pharynx (Fig. 2F). The two crossing sets of ventral diagonal somatic muscle fibres run at angles of approximately 30° and 120° respectively, with respect to the longitudinal fibres, while those of the dorsal surface are aligned at approximately 60° and 150° respectively (Fig. 1). The anterior extremity of the worm is bilobed, each lobe bearing an adhesive area (aa) and sensory papillae (Fig. 2A). Beneath the subtegumental muscle lie two pairs of ventral extrinsic muscle bands (vmb), each comprising a group of fibres (Figs. 1, 2F). The muscle bands originate from the anterior region of the pharynx and run forwards, such that one pair on each side reaches the base of the head lobe. Four pairs of lateral extrinsic muscle fibres (lem), two on each side, extend from the antero-lateral region of the pharynx to the lateral margins of the body (Fig. 1).
Confocal scanning laser micrographs of whole-mount preparations stained by indirect immunofluorescence using antiserum to the FaRP neuropeptide GYIRFamide and TRITC-coupled secondary antibody (red); muscles stained with FITC-phalloidin (green). A Whole worm. B Inset showing the intestinal caeca (it) supported by a loose arrangement of circular and longitudinal fibres. C Inset showing body wall muscle which comprises outer circular (cm), intermediate longitudinal (lm) and inner diagonal muscle (dm). D Inset showing fine tracts of GYIRFamide-immunoreactive fibres (unlabelled arrows) run in close proximity to the muscle associated with the marginal hooklets. E Muscle fibres (mf) connect the uterine opening to the body wall. Note GYIRFamide-IR was observed in nerve fibres, ventral uterine nerve (vun) branching off from the ventral nerve cords (vnc). F Anterior muscle stained with FITC-phalloidin for filamentous actin. Below the body wall muscle, two pairs of ventral extrinsic muscle bands (vmb) run anteriorly and attach to the base of each head lobe. G Serotonin (5-HT)-immunoreactivity was less extensive in the CNS than FaRPergic staining. Paired cerebral ganglia (cg) are connected by two commissures (anterior and posterior) from which projections (ap) arise to innervate the anterior lobes. 5-HT-IR cell bodies (cb) are in close association with the muscle of the posterior region of the pharynx (pph) and oesophagus
The pharynx is divided into anterior (aph) and posterior portions (pph) by a superficial constriction (Fig. 1). Projecting from the lips of the pharynx are six conical papillae (pp), each endowed with longitudinal muscle fibres (Fig. 1, pp; Fig. 3A). The anterior region of the pharynx (aph) comprises a dense arrangement of five bands of meridional fibres, the most posterior of which are considerably larger than those in the lip region (Figs. 1, 3A). In contrast, the posterior region of the pharynx (pph) is in the form of a relatively large, globular body possessing mainly well-developed radial fibres (rm) which run from the tegumental lining of the pharynx lumen to the outer periphery of the pharynx (Figs. 1, 2G). The pharynx lumen (pl) opens into a short oesophagus (oes) through a conspicuous sphincter (sph) composed of circular muscle bands (Fig. 1). The oesophagus (Fig. 1) is provided with inner longitudinal muscle fibres enveloped within outer circular fibres (not shown). The muscle of the intestinal caeca (it) resembles that of the oesophagus in appearance but presents a more loose arrangement (Fig. 2B).
Confocal scanning laser micrographs of whole-mount preparations stained by indirect immunofluorescence using antiserum to the FaRP neuropeptide GYIRFamide and TRITC-coupled secondary antibody (red); muscles stained with FITC-phalloidin (green). A Anterior region of the body showing cerebral ganglia (cg) with cell bodies (cb), anterior region of the pharynx (aph) and pharyngeal papillae (pp). Mb Muscle band. B Posterior region of the pharynx (pph) with pharyngeal nerve (pn) and cell bodies (cb). C Whole worm treated with acetylthiocholine iodide (AcThI) showing cholinergic components of CNS and PNS
The single testis (t) is surrounded by a diffuse array of muscle fibres and leads to the vas deferens (not shown) which dilates to form a relatively large vesicula seminalis (vs) before entering the male copulatory organ (c); the latter was seen to fluoresce strongly following phalloidin staining, illustrating its highly muscular nature (Fig. 2A). In contrast, the musculature of the female reproductive tract is less well defined. The egg cell-forming region (ECFR) or ootype opens into a large, elongated uterus (ut; Fig. 2A, E). The wall of the former is composed mainly of circular muscle while the latter contains both well-developed outer circular and inner longitudinal fibres (Fig. 2E). Muscle fibres (mf; 6–10 in number) connect the anterior opening of the uterus (ut) to the body wall (Fig. 2E).
The haptor (hp) is a cup-shaped organ delineated from the body proper by a prominent constriction (Fig. 2A). The present study concentrated only on the most prominent haptoral muscles. Below the body wall muscles, two large ventral extrinsic muscle bundles (vmb) of up to 30 individual myofibres, together with two less well-developed dorsal extrinsic muscle fibres (dmb), extend into the haptor (Fig. 2A). The anterior flap (af) of the haptor was seen to fluoresce strongly following phalloidin staining (Fig. 2A, hp), as did the muscle fibres which operate the haptoral sclerites.
Neuroanatomy and neurochemistry
The central nervous system (CNS) comprises paired cerebral ganglia (cg) from which derive anterior and posterior neuronal pathways interlinked by cross connectives and commissures. The peripheral nervous system (PNS) includes innervation of the alimentary tract, reproductive organs, attachment organs (anterior adhesive areas and haptor) and subtegumental muscles. Both the CNS and PNS are bilaterally symmetrical, and better developed ventrally than laterally and dorsally. They display strong staining for cholinesterase (ChE), and extensive immunoreactivity for FaRP. No staining was observed in the absence of ChE substrate or primary antiserum, or following substitution of primary antiserum with non-immune serum; the addition of antigen in liquid phase prior to immunostaining abolished any reactivity.
Cholinergic enzyme histochemical staining
The CNS and PNS (Figs. 3C, 4) were found to be highly reactive for ChE, and stained extensively a dark brown colour. The CNS consists of a thick, curved mass of paired cerebral ganglia (cg) located ventrally just anterior to the pharynx (ph; Fig. 3C). A single anterior ventral commissure (avc) originates from the postero-lateral regions of the cerebral ganglia, and runs in a semicircular manner in the anterior region of the head, just posterior to the head lobes (hl; Figs. 3C, 5). Two considerably thick projections (ap) extend from the anterior median region of the cerebral ganglia, imparting to this organ a butterfly-like appearance. Each projection gives rise to cerebral nerves (cn) which extend anteriorly to enter the head lobes where they innervate the anterior adhesive areas (Figs. 3C, 5).
Two thick ventral nerve cords (vnc) arise one from each lateral region of the cerebral ganglia, and run posteriorly where each joins a prehaptoral ganglion (phg1). Another pair of prehaptoral ganglia (phg2) is located one on each side of the body a short distance posterior to the prehaptoral ganglia (phg1) in the region anterior to the haptor. Both sets of prehaptoral ganglia on each side of the body are connected via two fine ganglionic connectives (gc; Fig. 6). Two thin branches arise, one from each ventral nerve cord, at a point posterior to the pharynx and reconnect to the ventral nerve cord at the level of the anterior part of the uterus. Another, much shorter loop originates from the posterior region of each ventral nerve cord and reconnects to the prehaptoral ganglia (Figs. 3C, 4). Five ventral transverse connectives (vc1–vc5) were also detected, three between the two ventral nerve cords, one between the prehaptoral ganglia (phg1), and one between the prehaptoral ganglia (phg2; Figs. 3C, 4).
From the postero-lateral region of the cerebral ganglia, two lateral nerve cords (lnc) extend posteriorly where they join the prehaptoral ganglia (phg1). At fairly regular intervals along the body, both ventral and lateral nerve cords are cross-linked by ventro-lateral transverse connectives (vlc1–vlc4; Figs. 3C, 4). The posterior median region of the cerebral ganglia gives rise to two dorsal nerve cords (dnc) which extend anteriorly to enter the head lobes, and posteriorly between the ventral nerve cords where they connect with the anterior prehaptoral ganglia (phg1). The two dorsal nerve cords are linked by three dorsal transverse connectives (dc1–dc3; Figs. 3C, 4), while both dorsal and lateral nerve cords are linked by three dorso-lateral connectives (dlc1–dlc3; Figs. 3C, 4).
Staining for cholinergic elements revealed the presence of six pairs of large (approximately 20×30 to 30×44 μm) neurones distributed bilaterally down the main body of the worm. Three pairs of these cells are located on the ventral side of the body (v1–v3) while the other three pairs occur dorsally (d1–d3; Figs. 3C, 4). All of these cells are multipolar, except the first dorsal pair which is bipolar. The first pair of ventral cell bodies (v1) is positioned at the level of the vesicula seminalis, the second pair (v2) at the level of the testis, and the third pair (v3) in the region of the posterior part of the intestinal caeca. The first pair of dorsal cell bodies (d1) is located close to the bases of the head lobes, the second pair (d2) at the level of the anterior part of the uterus, and the third pair (d3) again in the region of the posterior intestinal caeca. Each neurone contains a central nucleus (n) and numerous dense granules (Fig. 7C). Dendritic processes extend from the dorsal and ventral cells to connect with the main nerve cords and connectives (Fig. 4).
Light micrographs of the worm treated with AcThI. A Anterior head region showing cerebral ganglia (cg) with two anterior projections (ap) and anterior ventral commissure (avc). B Innervation of the pharynx. C Ventral nerve cell (v3) with conspicuous nucleus (n). D Middle region of the body showing uterus (ut) containing embryo (e) with more developed nervous system. E Middle portion of the body showing vesicular seminalis (vs), ventral uterine nerve (vun), uterus (ut) containing embryo (e), ventral nerve cell (v1), dorsal nerve cell (d2) and nerve cords (vnc, lnc)
The haptor is extensively innervated by two outer (ohn) and four inner (ihn), relatively thick haptoral nerves. The outer of these arise one from each posterior prehaptoral ganglion (phg2) while the inner members derive from the ventral connective (vc5). The outer and inner haptoral nerves run ventrally in a posterior direction before branching into a plexus of numerous fine nerves in the anterior region of the haptor (Figs. 6 and 8A, B). There are two dorso-lateral haptoral nerves (dhn) arising one from each posterior prehaptoral ganglion (phg2), and these run posteriorly and eventually branch into five pairs of posterior hook nerves (phn) and one pair of antero-lateral hook nerves (ahn). There are also two median nerves originating from the third dorsal connective (dc3), which run posteriorly for a short distance to join each other to form a single, short dorso-median haptoral nerve (mhn). This nerve bifurcates again into two nerves which run posteriorly before branching into two posterior hook nerves (phn) which innervate the muscles associated with the four median posterior marginal hooklets (pmh; Figs. 6, 8C).
The AChThI method did not reveal any peripheral innervation of the subtegumental muscles but did discern innervation of the anterior adhesive areas, pharynx, some reproductive organs and haptoral elements. The pharynx is innervated by two pharyngeal nerves (pn) originating from the first ventral connective (vc1) which are directed anteriorly where they connect three pharyngeal nerve rings (pr; Figs. 4, 5). The pharyngeal nerves are connected via transverse pharyngeal connectives (pc) in the region between the first ventral connective (vc1) and the pharyngeal rings (pr; Figs. 5, 7B). The anterior region of the uterus is innervated by two dorsal uterine nerves (dun) arising one from each dorsal nerve cord, and two ventral uterine nerves (vun) arising one from each ventral nerve cord (Figs. 4, 7E). Innervation of the vesicula seminalis arises from the ventral nerve cords (Fig. 7E).
The cholinergic components of the CNS and PNS of the first embryo (e) were stained and displayed a pattern similar to that obtained for adults (Fig. 7D).
FaRP immunostaining
Immunostaining for FaRP was observed throughout both central and peripheral nerve elements. About 50–60 GYIRFamide-immunoreactive (IR) cell bodies (approx. 15×15 μm in size) were evident in the cerebral ganglia (Fig. 3A). Extensive immunostaining was observed throughout the anterior head lobes, corresponding to the region at the base of each sensillum and presumed adhesive gland duct openings (Fig. 2A). Of the three pairs of posteriorly directed nerve cords, the ventral cords were the best developed (Fig. 2A, B). The three longitudinal nerve cords connect posteriorly, at a site marked by a collection of immunoreactive fibres and associated cell bodies (cb) which are linked by a commisure anterior to the haptor (cc; Fig. 2A). The pharynx is innervated by an extensive network of FaRPergic fibres which subdivide and anastomose throughout the musculature. Its posterior region is provided with a number of FaRPergic cell bodies and two pairs of ventral and dorsal nerve tracts which anastomose among the radial muscle fibres (Fig. 3B). A substantial portion of the reproductive system is IR for GYIRFamide. This includes (1) a fine network of fibres which innervate the copulatory organ, vesicula seminalis, male accessory reservoir (mar) and male accessory glands (mag); and (2) two pairs of uterine nerves (vun) which originate from the ventral nerve cords, one of which provides innervation around the uterine opening (uo) whereas the other innervates the junction between the uterus and egg cell-forming region (ECFR; Fig. 2A, E).
FaRPergic cell bodies (somata) were observed along the ventral nerve cords (Fig. 2A), as well as in the body regions lateral to the posterior portion of the pharynx and in the vicinity of the uterus. Approximately 10 cell bodies form paired nerve plexuses on each side of the prehaptoral region where the longitudinal nerve cords are connected (Fig. 2A). These plexuses are linked by a commissure (cc) comprising several FaRPergic fibres from which the haptor innervations originate. Anterior to this commissure is a cluster of at least three distinct IR cell bodies (cb). A circuit of FaRPergic nerves runs around the margins of the haptor and, at intervals, provides fine tracts of fibres which are associated with the marginal hooklets (mh). Staining was also found in the two rows of haptoral papillae (hpa) which are located ventrally, one on each side of the haptor.
5-HT immunostaining
Compared with the results for the FaRP, staining for 5-HT was considerably less extensive in the brain and CNS in general, although it followed a similar distribution pattern and therefore its description will not be repeated. In addition, immunostaining for 5-HT revealed the presence of 15–20 cell bodies in the cerebral ganglia from which tracts of nerves proceeded in pairs both anteriorly and posteriorly. Those running anteriorly subdivided and anastomosed throughout the anterior lobes where staining was expressed as a carpet of immunoreactivity along the entire antero-ventral margin of the worm. Circular patterns of staining were also observed in the anterior lobes, corresponding to the region at the base of each sensory spine. An elaborate, grid-like pattern of serotoninergic fibres derived from projections originating in the cerebral ganglia innervate the anterior portion of the pharynx (Fig. 2G) while four cell bodies, two dorsal and two ventral, lie either side of the posterior part, just prior to its junction with the oesophagus. Posteriorly, a peripheral nerve ring of 5-HT-IR fibres runs around the lateral margins of the haptor, forming a neural circuit from which fine fibres extend to innervate the terminal reaches of the haptor and musculature of the marginal hooklets. Staining was also observed in the two rows of haptoral papillae which are distributed one each side on the ventral surface of the haptor. Varicosities of 5-HT-IR fibres surround the male copulatory organ and seminal vesicle, and immunostaining was also evident around the testes and in neurons innervating the muscle of the oviduct (not shown). First-born and, to a lesser extent, second-born daughter embryos within the uterus exhibited extensive immunostaining in both the CNS and PNS, mirroring that displayed by the adult.
Discussion
As far as is known, the present study is the first to describe the cholinergic, peptidergic and aminergic components of the nervous system of a member of a genus Macrogyrodactylus, namely M. clarii, a monogenean gill parasite of the Nile catfish Clarias gariepinus. The central nervous system (CNS) of the worm comprises mainly a mass of cerebral ganglia and three pairs of ventral, lateral and dorsal longitudinal nerve cords connected by transverse commissures; it is better developed ventrally than dorsally and laterally. In these respects, the CNS of M. clarii resembles in basic structure that of all previously studied flatworms, including monogeneans, digeneans and cestodes (see Reuter 1987; Halton and Gustafsson 1996; Reuter et al. 1998; Reda and Arafa 2002; Arafa et al. 2002).
The use of phalloidin staining interfaced with confocal microscopy revealed that the body wall of M. clarii is largely composed of an outer layer of compactly arranged, circular muscle fibres, an intermediate layer of paired longitudinal fibres, and an inner layer of well-spaced bands of diagonal fibres arranged in two crossed directions. This arrangement resembles that described for free-living (Rieger et al. 1994) and parasitic flatworms (Halton et al. 1998; Mair et al. 1998; Stewart et al. 2002). El-Naggar et al. (2001b) recorded five patterns of movement for M clarii, namely elongation and shortening, scanning (searching) movement, leech-like locomotion, rotation movement, and self-cleaning movement. It seems likely that elongation and shortening of the worm are brought about by effective contraction of the circular and longitudinal muscles respectively. Prior to translocation to a new attachment site on the gill filament, M. clarii extends its forebody and performs a succession of scanning movements from the distal to the proximal region of the gill filament, and vice versa (El-Naggar et al. 2001b). This movement is probably achieved by the coordinated contraction of longitudinal and circular muscle fibres. Also, it seems most likely that rotation and self-cleaning, during which the body is twisted, could be attributed to contraction and relaxation of the diagonal muscle fibres which are located mainly in the anterior region of the body. In the leech-like locomotion, the adhesive sac everts during attachment, and retracts during detachment. In this respect, the head region was found to contain two pairs of pronounced, ventral muscle bands which extend from the pharynx to the adhesive sacs of the head lobes. It may be that at least retraction of these sacs into the head lobes is brought about by contraction of the ventral muscle bands. A similar contribution of the ventral muscle bands to the retraction of adhesive sacs was suggested by El-Naggar and Kearn (1980) in the monogenean gill parasites, Dactylogyrus amphibothrium and D. hemiamphibothrium.
Application of the phalloidin staining technique in the present study has revealed that the anterior region of the pharynx of M. clarii comprises five bands of muscle fibres, while the posterior region consists mainly of radial muscle fibres. Moreover, six pairs of extrinsic muscles attached to the anterior region of the pharynx were observed. In this respect, the musculature of the pharynx of M. clarii resembles that described by El-Naggar et al. (1999) for M. cogolensis. These extrinsic muscles comprise two pairs on each side which run laterally to the margin of the body. The other two pairs of muscle bands are directed to the head lobes. The anterior portion of the pharynx of M. clarii was reported by El-Naggar and Serag (1987) to protrude during feeding, as has been described for other monogeneans, namely E. soleae and Acanthocotyle sp. (Kearn 1963), M. polpteri (Khalil 1970), Dactylogyrus hemiamphibothrium and D. amphibothrium (see El-Naggar and Kearn 1980), and Quadriacanthus aegypticus (see El-Naggar and Serag 1986). The mechanism by which this is achieved is not known. Protrusion of the anterior region of the pharynx of M. clarii may be controlled by the extrinsic muscles attached to this region. The anterior pharyngeal region of M. clarii accommodates six pharyngeal papillae whose tips protrude during feeding on gyrodactylids (Khalil 1970; El-Naggar and Serag 1987; El-Naggar et al. 1999; El-Naggar and El-Abbassy 2003). This protrusion may be initiated by contraction of the muscle bands in the anterior part of the pharynx, while contraction of the radial muscle of the posterior portion may drive secretion into the pharyngeal papillae.
The haptor is invaded by two pairs of muscle bundles. The first is well developed and located ventrally while the second is less developed and located dorsally. In the posterior two thirds of the body, these muscles insert at points along the body wall and appear to function as retractor muscles in a fashion similar to that described for Diclidophora merlangi (Halton et al. 1998). Thus, it is possible that the rapid contraction of these extrinsic muscles acts to counter sudden water turbulence across the gills by foreshortening the body, thereby minimising its resistance to water currents which may otherwise dislodge the parasite. Contraction of the ventral extrinsic muscle bundles would appear to create a gaffing action by the hamuli. On the other hand, the dorsal extrinsic muscle bundles may neutralise the action of the ventral muscle bundles by withdrawing the hamuli from the host tissue.
The CNS of M. clarii as revealed by cholinergic, peptidergic and serotoninergic staining conforms to the basic orthogonal pattern described for other monogeneans (see Bullock and Horridge 1965; Reisinger 1976; Halton et al. 1993). Typically, the PNS innervates the alimentary system, reproductive organs, attachment organs and subtegumental muscles. These neural pathways and their organisation have previously been described in 10 other monogeneans: Diplozoon paradoxum (see Halton and Jennings 1964), Polystoma integerrimum (Rahemo and Gorgees 1987), Gyrodactylus salaris (Reuter 1987), Pseudodactylogyrus anguillae (Buchmann and Mellergaard 1988; Reda and Arafa 2002), Eudiplozoon nipponicum (Lyukshina and Shishov 1988; Zurawski et al. 2001), Diclidophora merlangi (Maule et al. 1990a, 1990b), Pseudodactylogyrus bini (Reda and Arafa 2002), Entobdella soleae (Marks et al. 1994), Discocotyle sagittata (Cable et al. 1996a), and Protopolystoma xenopodis (McKay et al. 1991).
Cholinergic, FaRPergic and serotoninergic staining revealed numerous pairs of neuronal cell bodies associated with the cerebral ganglia, pharynx, prehaptoral nerve plexuses and prehaptoral commissure of M. clarii, together with scattered cell bodies along the ventral nerve cords and in the vicinity of the uterus. In the related monogenean G. salaris, FaRPergic-IR cell bodies have also been detected in the brain, nerve cords, prehaptoral plexuses, and in the middle region of the body (Reuter 1987). In E. soleae, serotoninergic-IR cell bodies and associated innervation dominate the cerebral ganglia, pharynx, and ootype and ovovitelline duct (Marks et al. 1994). Using the silver nitrate method, El-Naggar et al. (2001a) observed eight pairs of argentophilic, bilaterally distributed nerve cells in M. clarii. The differences in the distribution of cell bodies with argentophilic, cholinergic, peptidergic and serotoninergic staining methods on whole mounts of M. clarii cause some confusion as to the nature of the neuronal cell bodies present. Such may be resolved using transmission electron microscopy. Thus, Shaw (1982) described two types of neuronal cell body on the basis of their ultrastructure within the brain of Gastrocotyle trachuri; Rohde (1972) revealed large, ovoid, membrane-bound secretory granules in the large nerves and axons of Polystomoides asiaticus, and concluded these to be neurosecretory grana; and Bahatanger et al. (1980) reported two types of nerve cells in Ceylonocotyle scoliocoelium, distinguishing them on the basis of their fine structure as neurosecretory (A-type) and non-neurosecretory (B-type).
The cholinergic and peptidergic components of the nervous system of M. clarii are partially overlapped. This conforms with the evidence provided by Halton and Gustafsson (1996) who reported the peptidergic pathways in platyhelminths as following more closely those of the cholinergic system, while those of the serotoninergic system are often quite separate and distinctive in construction, with the staining localised to a different subset of neurons. The same phenomenon has been demonstrated in the nervous systems of other monogeneans—D. merlangi and D. sagittata (see Maule et al.1990a and Cable et al. 1996b respectively). Cable et al. (1996b) suggested a neuronal coexistence of classical neurotransmitters with peptides. Staining for cholinergic and peptidergic components of the nervous system of M. clarii has shown that the former is more extensive than the latter. Acetylcholine (ACh) is considered an inhibitory neurotransmitter in a number of parasitic flatworms (Sukhdeo et al.1984; Ward et al. 1986; Thompson and Mettrick 1989), whereas the function of FaRP neuropeptides in flatworms is yet unclear, although their ubiquitous occurrence throughout the flatworm nervous system implies a fundamental role in nerve muscle physiology (see Halton and Gustafsson 1996). Where examined experimentally, FaRPs have been found to be potently myoactive (see Day and Maule 1999).
In the present study, intense reactions of both cholinergic and FaRPergic components have been observed in the cerebral ganglia which lie immediately anterior to the pharynx, together with the longitudinal nerve cords and nerve fibres which innervate the head lobes, pharynx and haptor. The nerves innervating the head lobes of M. clarii may play a role in coordinating muscular events involved in the release of secretory bodies from the gland cells of the anterior adhesive glands and/or attachment of the adhesive sacs during temporary, leech-like locomotion. Extensive innervation of the head lobes, particularly the adhesive sacs, with FaRPergic-IR nerves is consistent with the presence in these areas of numerous sensilla (El-Naggar 1993; El-Naggar et al. 2001a) which may be sensitive to contact stimulation. Contact perception (and possibly contact chemoreception) by this region is likely to be important during temporary, leech-like locomotion. Cholinergic and FaRPergic fibres of M. clarii subdivide and anastomose throughout the posterior region of the pharynx, and may interact to modulate contraction of the radial muscles which drive the secretion into the papillae, while those fibres supplying the anterior portion may regulate the protrusion of the pharyngeal papillae themselves during feeding. A striking feature of all monogeneans studied hitherto has been the finding of a ring commissure around the mouth (for example, see Rohde 1968; Reuter 1987). However, in the present study, a comparable commissure was not detected. Rohde (1968) suggested an oral commissure is a character which distinguishes the nervous system of the Monogenea from that of Digenea.
It is perhaps noteworthy that some reproductive organs of M. clarii are innervated by cholinergic and FaRPergic nerves, notably the ootype and uterus. It seems probable that cholinergic and FaRPergic nerves innervating the anterior region of the uterus help coordinate events involved in the delivery of offspring (fully mature embryo) during the birth process. The FaRPergic nerves innervating the junction between the ootype and uterus may play a role in controlling movement of the mature oocyte by their directive effect on the muscles found in this region, although Cable and Harris (2002) suggest that the large oocyte proceeds into the uterus through the physical pressure of the oocyte on the adjacent empty uterus. Cable et al. (1996a) suggested that the neuropeptide secretions may influence movement of ova and vitelline cells, release of spermatozoa and Mehlis’ gland secretions, peristaltic contractions of the ootype which shape the egg, and regulate the release of egg from the ootype into the uterus.
The neuromusculature of the haptor of M. clarii is richly innervated with cholinergic, FaRPergic and serotoninergic elements, suggesting a major role in motor function for the haptoral nerves. Similarly, in D. merlangi, all three neuroactive substances have been detected in motor elements innervating the buccal suckers, pharynx and haptoral clamps (Halton and Morris 1969; Maule et al. 1990a), and their action investigated on the motility of worms in vitro (Maule et al. 1989). Some evidence for a sensory role for those nerves connected to the tegumental papillae comes from work on Polystoma integerrimum by Rahemo and Gorgees (1987), but this has yet to be confirmed experimentally.
References
Arafa SZ, Reda ES, El-Naggar MM (2002) Cholinergic components of the nervous system of the digenean parasites, Haplorchoides cahirinus and Acanthostomum absconditum from catfish Bagrus bayad in Egypt. Acta Parasitol 47:272–279
Bahatanger A, Gupta A, Srivastava R (1980) Histological and histochemistry of the neuroendocrine components of Ceylonocotyle scoliocoelium (Digenea: Trematoda). Z Parasitenkd 64:77–84
Buchmann K, Mellergaard S (1988) Histochemical demonstration of the inhibitory effect of Nuvan and Neguvon on cholinesterase activity in Pseudodactylogyrus anguillae (Monogenea). Acta Vet Scand 29:51–55
Bullock TH, Horridge GA (1965) Structure and function in the nervous system of invertebrates, vol I. WH Freeman, San Francisco
Cable J, Harris PD (2002) Gyrodactylid developmental biology: historical review, current status and future trends. Int J Parasitol 32:255–280
Cable J, Harris PD, Tinsley RC (1996a) Ultrastructural adaptations for viviparity in the female reproductive system of gyrodactylid monogeneans. Tissue Cell 28:515–526
Cable J, Marks NJ, Halton DW, Shaw C, Johnston CF, Tinsley RC, Gannicott AM (1996b) Cholinergic, serotoninergic and peptidergic components of the nervous system of Discocotyle sagittata (Monogenea: Polyopisthocotylea). Int J Parasitol 26:1357–1367
Cable J, Harris PD, Tinsley RC (1998) Life history specializations of monogenean flatworms: a review of experimental and microscopical studies. Microsc Res Tech 42:186–199
Coons AH, Leduc EH, Connelly JM (1955) Studies on antibody production. I. A method for histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med 102:42–60
Day TA, Maule AG (1999) Parasitic peptides! The structure and function of neuropeptides in parasitic worms. Peptides 20:999–1019
El-Naggar MM (1993) Scanning electron microscope observations on the head lobes and haptor of the monogenean Macrogyrodactylus clarii Gussev, 1961. J Egyptian German Soc Zool 10:143–155
El-Naggar MM, El-Abbassy SA (2003) Anatomical observations on the viviparous monogenean Gyrodactylus rysavyi Ergens, 1973 from the Nile catfish Clarias gariepinus in Egypt. Egyptian J Zool 40:225–249
El-Naggar MM, Kearn GC (1980) Ultrastructural observations on the anterior adhesive apparatus in the monogeneans Dactylogyrus amphibothrium Wagener, 1857 and D. hemiamphibothrium Ergens 1956. Z Parasitenkd 61:223–241
El-Naggar MM, Serag HM (1986) Quadriacanthus aegypticus n sp., a monogenean gill parasite from the Egypt teleost Clarias lazera. Syst Parasitol 8:129–140
El-Naggar MM, Serag HM (1987) Redescription of Macrogyrodactylus clarii Gussev 1961, a monogenean gill parasite of Clarias lazera in Egypt. Arab Gulf J Sci Res Agric Biol Sci 5:257–271
El-Naggar MM, Kearn, GC, Hagras AE, Arafa SZ (1999) On some anatomical features of Macrogyrodactylus congolensis, a viviparous monogenean ectoparasite of the catfish Clarias gariepinus from Nile water. J Egyptian German Soc Zool 29:1–24
El-Naggar MM, Arafa SZ, El-Abbassy SA, Kearn GC (2001a) Chaetotaxy of the monogeneans Macrogyrodactylus clarii and M. congolensis from the gills and skin of the catfish Clarias gariepinus in Egypt, with a note on argentophilic elements in the nervous system. Folia Parasitol 48:201–208
El-Naggar MM, El-Naggar AM, El-Abbassy SA (2001b) Microhabitat and movement of the viviparous monogeneans Gyrodactylus alberti, Macrogyrodactylus clarii and M. congolensis from the Nile catfish Clarias gariepinus. J Egyptian German Soc Zool Invertebr Zool Parasitol 35(D):169–187
Gussev AV (1961) A viviparous monogenetic trematode from freshwater basins of Africa. Dokl Akad Nauka SSSR 136:490–493
Halton DW, Gustafsson MKS (1996) Functional morphology of the platyhelminth nervous system. Parasitology 113:S57–S72
Halton DW, Jennings JB (1964) Demonstration of the nervous system of the monogenetic trematode Diplozoon paradoxum Nordmann by the indoxyl acetate method for esterases. Nature 202:510–511
Halton DW, Morris GP (1969) Occurrence of cholinesterase and ciliated sensory structures in a fish gill fluke, Diclidophora merlangi (Trematoda: Monogenea). Z Parasitenkd 33:21–30
Halton DW, Maule AG, Shaw C (1993) Neuronal mediators in monongenean parasites. Bull Fr Pêche Piscicult 328:82–104
Halton DW, Maule AG, Mair GR, Shaw C (1998) Monogenean neuromusculature: some structural and functional correlates. Int J Parasitol 28:1609–1623
Harris PD (1983) The morphology and life-cycle of the oviparous Gyrodactylus farlowellae gen. et sp. nov. (Monogenea Gyrodactyidae). Parasitology 87:405–420
Kearn GC (1963) Feeding in some monogenean skin parasites: Entobdella solea on Solae solae and Acanthocotyle sp. on Raia clavata. J Biol Assoc UK 43:749–766
Khalil LF (1970) Further studies on Macrogyrodactylus polypteri, a monogenean on the African freshwater fish Polypterus senegalus. J Helminthol 44:329–348
Llewellyn J (1981) Evolution of viviparity and invasion by adults. Parasitology 82:64–66
Lyukshina LM, Shishov BH (1988) Biogenic amines in the nervous system of Eudiplozoon nipponicum (Monogenea) (in Russian). In: Sakharov DA (ed) Simple nervous systems. Nauka, Moscow, pp 173–176
Mair GR, Maule AG, Shaw C, Johnston CF, Halton DW (1998) Gross anatomy of the muscle systems of Fasciola hepatica as visualized by phalloidin-fluorescence and confocal microscopy. Parasitology 117:75–82
Marks NJ, Halton DW, Kearn GC, Shaw C, Johnston CF (1994) 5-Hydroxytryptamine-immunoreactivity in the monogenean parasite Entobdella soleae. Int J Parasitol 24:1011–1018
Maule AG, Halton DW, Allen JM, Fairweather I (1989) Studies on motility in vitro of the ectoparasitic monogenean, Diclidophora merlangi. Parasitology 98:85–93
Maule AG, Halton DW, Johnston CF, Shaw C, Fairweather I (1990a) The serotoninergic, cholinergic and peptidergic components of the nervous system in the monogenean parasite Diclidophora merlangi. Parasitology 100:255–274
Maule AG, Halton DW, Johnston CF, Shaw C, Fairweather I (1990b) A cytochemical study of the serotoninergic, cholinergic and peptidergic components of the reproductive system of the monogenean parasite Diclidophora merlangi. Parasitol Res 76:409–419
McKay DM, Halton DW, Maule AG, Johnston CF, Shaw C, Fairweather I (1991) Putative neurotransmitters in two monogeneans. Helminthologia 28:75–81
Prudhoe S (1957) Trematoda. Prac National de l’Upema. I. Mission GF et R Witte en collaboration avec W Adam, A Janssens, L Van Meet et R Verheyen (1946–1949), vol 48, pp 1–28
Rahemo ZIF, Gorgees NS (1987) Studies on the nervous system of Polystoma integerrimum as revealed by acetylthiocholine activity. Parasitol Res 73:234–239
Reda ES, Arafa SZ (2002) Cholinergic components of the nervous system of the monogenean gill parasites, Pseudodactylogyrus bini and P. anguillae from the eel Anguilla anguilla in Nile Delta waters. Egyptian J Zool 38:41–54
Reisinger E (1976) Zur Evolution des stomatogastrischen Nervensystem bei den Platyhelminthen. Z Zool Syst Evolutionsforsch 14:241–252
Reuter M (1987) Immunocytochemical demonstration of serotonin and neuropeptides in the nervous system of Gyrodactylus salaris (Monogenea). Acta Zool 68:187–193
Reuter M, Mäntylä K, Gustafsson MKS (1998) Organization of the orthogon-main and minor cords. Hydrobiologia 383:175–182
Rieger RM, Salvenmoser W, Legneti A, Tyler S (1994) Phalloidin-rhodamine preparations of Macrostomum hystricinum marinum (Platyhelminthes): morphology and postembryonic development of the musculature. Zoomorphology 114:133–147
Rohde K (1968) Das Nervensystem der Gattung Polystomoides Ward, 1977 (Monogenea). Z Morphol 62:58–76
Rohde K (1972) Fine structure of the Monogenea, especially Polystomoides Ward. Adv Parasitol 10:1–33
Shaw MK (1982) The fine structure of the brain of Gastrocotyle trachuri (Monogenea: Platyhelminthes) Cell Tissue Res 226:449–460
Stewart MT, Mousley A, Koubková B, Šebelová Š, Marks NJ, Halton DW (2002) Gross anatomy of the muscle systems and associated innervation of Apatemon cobitidis proterorhini metacercaria (Trematoda: Strigeidea), as visualised by confocal microscopy. Parasitology 126:273–282
Sukhdeo MVK, Hsu SC, Thompson CS, Mettrick DF (1984) Hymenolepis diminuta: behavioural effects of 5-hydroxytryptamine, acetylcholine, histamine and somatostatin. J Parasitol 70:682–688
Thompson CS, Mettrick DF (1989) The effects of 5-hydrxyotryptamine and glutamate on muscle contraction in Hymenolepis diminuta (Cestoda). Can J Zool 67:1257–1262
Tinsley RC (1983) Ovoviviparity in platyhelminth life-cycles. Parasitology 86:161–196
Ward SM, Allen JM, McKerr G (1986) Neuromusculature physiology of Grillotia erinaceus metacestodes (Cestoda: Trypanorhyncha) in vitro. Parasitology 93:587–597
Zurawski TH, Mousley A, Mair GR, Brennan GP, Maule AG, Gelnar M, Halton DW (2001) Immunomicroscopical observations on the nervous system of adult Eudiplozoon nipponicum (Monogenea: Diplozoidae). Int J Parasitol 31:779–783
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Naggar, M.M., Arafa, S.Z., El-Abbassy, S.A. et al. Neuromusculature of Macrogyrodactylus clarii, a monogenean gill parasite of the Nile catfish Clarias gariepinus in Egypt. Parasitol Res 94, 163–175 (2004). https://doi.org/10.1007/s00436-004-1198-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1198-1