Abstract.
Cholinergic, serotoninergic and neuropeptidergic components of the nervous system were examined and compared in the progenetic metacercaria and adult gasterostome trematode, Bucephaloides gracilescens in order to provide baseline information on neuronal control of the musculature involved in egg-assembly. Enzyme cytochemistry and indirect immunocytochemical techniques interfaced with confocal scanning laser microscopy demonstrated all three classes of neuroactive substance throughout the central and peripheral nervous systems. A comparable orthogonal arrangement of the central nervous system (CNS) and peripheral array of nerve plexuses was observed in both metacercaria and adult. Staining patterns for cholinergic and peptidergic substances showed significant overlap, while the serotoninergic system was confined to a separate set of neurons. Immunostaining for FMRFamide-related peptides (FaRPs) was strong in the CNS and peripheral innervation to the attachment apparatus of metacercaria and adult but was only found in the innervation of the ootype in ovigerous adults, implicating FaRPs in neuronal control of the muscle of the female reproductive tract during egg-assembly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bucephaloides gracilescens is a common gasterostome parasite of the intestine and pyloric caeca of the angler fish, Lophius piscatorius. The metacercarial stage has been reported from nine species of gadoid fish (Matthews 1974), showing a marked predilection to encyst in the central nervous system (CNS), notably in the brain or on the course of the cranial and spinal nerves. In whiting (Merlangius merlangus), Johnston and Halton (1981) reported a prevalence of Bucephaloides as high as 95% in fish caught in the Irish Sea, with most cysts occurring in the intracranial fluid around the brain and in the auditory capsules, eye muscles, orbit and spinal nerves. The nutritional level in the brain cavity was presumed to be sufficient to allow metacercariae to develop to partial progenesis, in that the testes, cirrus, ovary and uterus became fully differentiated. In the most advanced specimens examined, spermatozoa were found in the testes and seminal vesicles but the vitellaria, while containing shell precursors (notably phenols), failed to react for phenolase which is essential for scleritisation (Smyth and Clegg 1959). Nutritive levels in the sites of infection in whiting therefore seem to be high enough to satisfy the demands of gametogenesis but insufficient for egg production. Following ingestion by the angler fish, metacercariae excyst, feed and mature to adulthood in the intestine and pyloric caeca, presumably because of the increased levels of nutriment available here (Halton and Johnston 1983).
The neuromusculature of organs of insemination and egg production in helminths presents an attractive target for chemotherapeutic intervention. A family of invertebrate neuropeptides, the FMRFamide-related peptides (FaRPs), have been shown to be responsible for consistent immunostaining throughout the nervous systems of all helminth species investigated (Halton et al. 1999). In the pig nematode, Ascaris suum, FaRPergic neurons provide rich innervation to the muscular vagina vera which controls the external passage of eggs; and physiological studies of the effects of Ascaris FaRPs on isolated tissue preparations show them all to be myoactive (Fellowes et al. 2000). Similar data on the role of FaRPs in the reproduction of flatworms are less well established, due in part to the technical difficulties of isolating their much smaller, tissue-embedded reproductive structures for experimentation. However, two immunocytochemical studies have implicated FaRPs in co-ordinating the activity of the flatworm ootype during egg formation. First, Armstrong et al. (1997) found FaRP expression in the ootype innervation of the monogenean Polystoma nearcticum coincided with the parasite′s brief period of egg production. Second, in a study of the in vitro development of the strigeid trematodes Apatemon proterorhini cobitidis and Cotylurus erraticus, metacercariae were successfully cultured to ovigerous adults in a matter of a few days (Stewart et al. 2003a). During this time, FaRPs were expressed in the innervation of the ootype only at the onset of egg production, while immunostaining in the CNS was consistently strong and independent of the reproductive state of the worm. Moreover, neuronal staining for 5-hydroxytryptamine (5-HT, serotonin) was observed in close proximity to the egg-assembly apparatus throughout development and remained unchanged during oviposition. Both of these strigeids have metacercariae that are sexually undeveloped and possess only genital primordia, thereby requiring extensive myo- and neurogenesis to reach maturity. In contrast, the metacercaria of Bucephaloides exhibits advanced progenesis, with genitalia differentiated up to late gametogeny, differing from the adult only by the absence of eggs. It therefore presents useful research material for studying not only gametogenesis, vitellogenesis and egg-shell formation, but also (as in the present study) nerve–muscle changes associated with egg-assembly in flatworm parasites.
Materials and methods
Encysted Bucephaloides metacercariae (n=200) removed from the nasal and cranial cavities of locally caught whiting (Merlangius merlangus) were stored in Hanks′ saline overnight and then excysted by sequential action of 0.5% pepsin (pH 2.0, 10 °C, 1 h) followed by an increase in environmental pH (to pH 7.2; after Halton and Johnston 1981). Adult Bucephaloides were recovered from the intestine and pyloric caeca of angler fish (Lophius piscatorius) caught locally and known as monkfish.
For examination of general morphology, metacercariae and adults were flattened and fixed under a number 1 coverslip in 70% ethanol for 30 min, transferred to fresh 70% ethanol and left overnight. Specimens were then rinsed with distilled water, stained with aqueous Gower′s carmine for general morphology and with Fast Red salt B for egg-shell material and finally dehydrated through alcohol, cleared in xylene and mounted in Gurr′s DPX for examination, using a Leitz Orthomat microscope.
For the demonstration of cholinesterase activity as indirect evidence of acetylcholine (ACh), specimens were flat-fixed between two microscope slides in 4% (w/v) paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 0.1 M, pH 7.4) for 2 h at 4 °C before being washed in PBS, rinsed in distilled water and incubated with 5-bromo-chloro-indolyl acetate (after Pearse 1960) and examined as above. Controls consisted of: (1) omission of substrate and (2) pre-incubation of specimens in 1×10−5 M eserine sulphate in PBS for 1 h to inactivate cholinesterase activity.
Serotonin (5-HT) and neuropeptide immunoreactivities were visualised by the indirect immunofluorescence technique of Coons et al. (1955). Excysted metacercariae were washed vigorously in Hanks′ saline, flat-fixed in 4% PFA in PBS for 1 h and then transferred to fresh fixative for a further 3 h. Specimens were subsequently washed at 4 °C overnight in antibody diluent [AbD; 0.1 M PBS, pH 7.4, 0.1% (w/v) Triton X-100, 1% (w/v) bovine serum albumin, 0.1% (w/v) NaN3]. Specimens so treated were incubated with the following primary antisera for 72 h at 4 °C: (1) neuropeptide antiserum raised to the flatworm FaRP, GYIRFamide in a guinea pig at a working dilution of 1:400 and (2) 5-HT antiserum [448(1)] raised in a New Zealand white rabbit and used at a dilution of 1:500. Following an overnight wash in AbD, specimens were incubated for 48 h in rabbit anti-guinea pig IgG (1:100) for GYIRFamide and swine anti-rabbit IgG (1:100) for 5-HT. Secondary antisera were conjugated to either fluorescein isothiocyanate (FITC) or tetramethylrhodamine isothiocyanate (TRITC) for visualisation of binding sites. After washing overnight, FITC- or TRITC-labelled phalloidin was employed as a counterstain for demonstrating the F-actin of muscle. Finally, worms were mounted in PBS/glycerol (1:9 v/v) and examined using a confocal scanning laser microscope (Leica TCS-NT; Leica Microsystems, Milton Keynes, UK).
For dual-labelling of GYIRFamide and 5-HT specimens (n=20) were fixed and washed as described above. These were incubated first with anti-GYIRFamide (72 h), washed overnight in antibody diluent and treated with TRITC-conjugated rabbit anti-guinea pig (48 h). Following an overnight wash in antibody diluent, specimens were then incubated with anti-GYIRFamide (24 h) and again washed overnight. Finally, they were incubated with anti-5-HT (72 h), exposed to FITC-conjugated swine anti-rabbit IgG (48 h), washed, mounted and viewed as described.
Controls comprised: (1) omission of primary antiserum, (2) replacement of primary antiserum with non-immune serum from the donor species, (3) pre-adsorption of primary antiserum with 100–250 ng of appropriate antigen [GYIRFamide was purchased from Sigma-Genosys Biotechnologies (Europe) Limited].
Results
Gross morphology of the metacercaria
The average dimensions of flattened metacercariae were 850×340 μm (Fig. 1A). The metacercaria has a large sucker-like holdfast situated at the anterior tip of the body and a smaller oral sucker or pharynx which opens ventrally about one-quarter of the distance along the body and leads to a simple sac-like intestine. A large excretory bladder runs from the level of the mouth posteriorly and opens terminally via an excretory pore. All of the metacercariae examined were progenetic, with the best developed specimens having fully differentiated genitalia. A small ovoid ovary (60×40 μm) leads to an oviduct which joins the vitelline duct to form the ovo-vitelline duct. Vitelline follicles are situated bilaterally just below the tegument in the anterior half of the worm. The ootype opens to the uterus, which proceeds posteriorly before looping anteriorly and descending to enter the genital atrium via the uterine pore in a long cirrus pouch. There is a common genital pore near the posterior extremity. The male reproductive organs comprise a pair of rounded testes situated in tandem behind the ovary, from which vasa deferentia lead to a seminal vesicle located in the proximal part of an elongate cirrus.
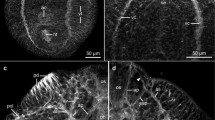
Light micrograph of excysted Bucephaloides gracilescens metacercaria stained with Gower′s carmine and Fast Red salt B. as Anterior sucker, ci cirrus, ga genital atrium, gt gut, os oral sucker, ov ovary, ts testis, vi vitellaria. B–F Confocal micrographs of Bucephaloides metacercariae demonstrating immunostaining for 5-hydroxytryptamine (5-HT) and GYIRFamide and phalloidin staining. B Gross arrangement of the nervous system stained green for the FMRFamide-related (FaRP) neuropeptide, GYIRFamide. The cerebral ganglia (asterisks) give rise to three pairs of posterior nerve cords interconnected along their lengths by cross-connectives. FaRPergic nerve fibres innervate the anterior sucker (as) and oral sucker/pharynx (os). ci Cirrus, ep excretory pore. C Detail of the immunolabelling with tetramethylrhodamine isothiocyanate conjugate (red) for FaRP and fluorescein isothiocyanate conjugate (green), showing FaRP-immunoreactive cell bodies (Fcb), serotoninergic cell bodies (Scb), cross-connectives (cc) between branches of the ventral nerve cord and the sub-tegumental nerve plexus immunoreactive to 5-HT (arrow). D Spatial arrangement of body wall muscle comprising circular (cm), longitudinal (lm) and diagonal muscle (dm). Insert: Image taken from the margin of the metacercaria. E Radial muscle fibres (rm) of the anterior sucker. sc Sucker cavity, asterisk cerebral ganglia. F Dual-staining showing 5-HT-IR (green) and FaRP-IR (red) neurons in the cerebral ganglia ( asterisks) and connective (co). Anterior projections (ap) from the brain innervate the anterior sucker (as)
Neuromusculature of the metacercaria
Cholinesterase-, serotonin (5-HT)- and GYIRFamide-staining was evident within neuronal cells and fibres of both the CNS and peripheral nervous system (PNS) of the metacercaria. Typically, the CNS comprises a pair of cerebral ganglia, connected by a dorsal commissure, from which paired ventral, dorsal and lateral nerve cords extend posteriorly for much of the length of the body (Fig. 1B). Of these, the best developed are the ventral nerve cords and, together with the dorsal cords, terminate around the excretory pore (Figs. 1B, 2C). All three pairs of nerve cords are interconnected at intervals by a series of regularly spaced cross-connectives (Fig. 1B, C). In places, nerve fibres and associated cell bodies originating in the CNS and collectively forming the PNS extend from the nerve cords to innervate the oral sucker/pharynx, the hermaphroditic reproductive system and the subsurface body layers.
Confocal images of 5-HT- and FaRP-IR neurons in the nervous system of Bucephaloides metacercariae. A FaRPergic fibres derived from the ventral nerve cord (vnc) innervate the musculature of the oral sucker/pharynx (os), oesophagus (oe) and gut (gt). Eight paired bands of muscle (mb) link the body wall to the mouth (mo). B Dual-staining of 5-HT (green) and FaRP (red), demonstrating the intricate pattern of innervation to the oral sucker/pharynxos, derived from the ventral nerve cord (vnc). C Optical section through the proximal wall of the cirrus pouch (cp), showing the seminal vesicle (sv) and muscular cirrus (ci), which may be everted through the terminally located gonopore (gp). ep Excretory pore, arrows ventral nerve cords. D Spatial arrangement of the testes (ts), ovary (ov) and ootype (ot) with strong FaRPergic staining (green) in the central nervous system. E FaRP-immunoreactivity is absent from the innervation associated with the egg-assembly apparatus in the metacercaria. ot Ootype, ov ovary, ovd oviduct, ts testis, ut uterus. F 5-HT-IR fibres and cell bodies (cb) surrounding the musculature of the ootype (ot)
Staining for cholinesterase activity approximated that for the FaRP, GYIRFamide, but without the varicosities. In contrast, while 5-HT-immunoreactive (IR) and GYIRFamide-IR fibres were often seen running in close proximity, their respective patterns of staining were quite distinct (Figs. 1C, 2B). Thus, nerve fibres IR to GYIRFamide were varicose in appearance with cell bodies smaller (5–7 μm in diameter) and more numerous than those IR to 5-HT (14–16 μm in diameter). 5-HT-IR cell bodies were more symmetrical in arrangement, particularly along the longitudinal nerve cords, and were also a more prominent feature of the networks of fine fibres that formed plexuses beneath the body wall musculature.
The body wall muscle itself presents a regular array of outer circular, intermediate longitudinal and inner diagonal fibres (Fig. 1D). Longitudinal and circular fibres are compactly arranged (ca. two and four per micron, respectively) as separate bundles of muscle that run perpendicular and parallel, respectively, to the main body axis. Diagonal fibres are arranged into muscle bands of two or more parallel fibres, each band separated by a ca.15-μm space and orientated at angles of 45°and 135° with respect to the longitudinal fibres; and individual circular and longitudinal muscle fibres are more regularly spaced. Circular and diagonal fibres are approximately equal in diameter (ca. 0.5 μm), while longitudinal fibres on average are twice as thick (ca. 1 μm in diameter). Somatic muscle forms a relatively uniform sub-tegumental layer throughout the body and is only markedly modified in the regions of the gonopore or suckers.
Attachment and feeding
The anterior and oral sucker/pharynx are comparable in structure, exhibiting three types of muscle fibres derived in part from the somatic musculature (Figs. 1E, 2A). Longitudinal fibres of the body wall become the meridional fibres of the suckers, while circular fibres form the equatorially arranged muscle. Radial fibres run between the inner and outer surfaces of the sucker but do not appear to be derived from the body wall. Bands of diagonal body wall muscle bend and insert at intervals along the base of the anterior sucker.
Three pairs of cholinergic and FaRPergic fibres extend anteriorly from the cerebral ganglia and subdivide and anastomose throughout the musculature of the anterior sucker (Fig. 1F). Its anterior portion is innervated mainly by cholinergic and FaRPergic fibres, while staining for all three neuronal substances was evident in the innervation of the posterior region. Large cholinergic fibres extend from the ventral nerve cord to innervate muscle of the oral sucker/pharynx with numerous associated small cell bodies located externally to the ganglia and nerve trunks. Projections of GYIRFamide-IR and 5-HT-IR fibres, emanating from the ventral nerve cord and ventral/lateral nerve cords, respectively, also innervate the oral sucker/pharynx (Fig. 2A, B). These fibres form a nerve-ring around the sucker, from which numerous fibres splay out and enter the sucker musculature.
Eight paired bands of muscle attach the anterolateral openings of the oral sucker/pharynx to the body wall (Fig. 2A). In places, the posterior lip of the oral sucker/pharynx is linked to portions of the body wall by numerous paired bands of muscle fibres. The oral sucker/pharynx is separated from the lattice-like arrangement of longitudinal and circular fibres of the sac-like intestine by a short oesophagus comprising tightly arranged bands of circular muscle. The region between the oral sucker/pharynx and intestine was reactive only for FaRP, with at least eight GYIRFamide-IR bi-polar cell bodies of neurons that originate off the ventral nerve cord. A thin neuromuscular layer surrounds the intestine and was IR for FaRP.
Male reproductive system
The paired testes are invested with a diffuse arrangement of muscle and open into the vas deferens, each comprising mainly loosely arranged longitudinal muscle in continuity with the seminal vesicle situated in the proximal region of the cirrus pouch (Fig. 2C–E). The wall of the cirrus pouch is divided into a proximal region in which there is a compact arrangement of predominately longitudinal fibres with a less organised array of longitudinal and circular fibres in the distal region. The cirrus itself is composed of regularly spaced circular and longitudinal fibres with a double band of especially dense circular muscle forming a sphincter at the junction with the genital atrium. Although FaRP-immunoreactivity was not found in the male system, 5-HT-IR fibres innervated the wall of the cirrus pouch and encircled the genital pore.
Female reproductive system
Circular and longitudinal muscle occur throughout the ducting of the female system, but with regional differences in its arrangement (Fig. 2E). Thus, densely packed circular muscle forms a sphincter at the base of the ovary where it opens into the oviduct, the vitelline duct has a loose arrangement of muscle in its wall before joining the oviduct to form the ovo-vitelline duct in which circular and longitudinal fibres become increasingly more tightly spaced in the vicinity of the ootype and the ootype itself displays large bands of longitudinal muscle. While individual circular fibres dominate the musculature of the uterus, larger longitudinal fibres, although fewer in number, run the extent of the uterus to the uterine pore. In common with the ducts of the male system, diagonal muscle fibres are not observed. Immunostaining of the innervation of the female tract was confined to 5-HT, with additional IR fibres in close proximity to the muscle of the ovo-vitelline duct, ootype and uterus (Fig. 2D–F).
Gross morphology of the adult
Development of adults is accompanied by somatic growth and pronounced elongation of the body to an average dimension of 1,800×400 μm (Fig. 3A). Vitelline follicles become more coarsely follicular and arranged in four main groups, again lying bilaterally in the anterior half of the body. Although little change was observed in the size of the testes, there was a marked increase in the dimensions of the ovary from approximately 60×40 μm (metacercaria) to 140×120 μm (adult). In the gravid form, the uterus is much more convoluted and is filled with thousands of eggs with unusually thick brown shells. These appear to be released through the uterine pore in a single but continuous stream.
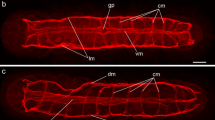
Light micrograph of adult Bucephaloides stained with Gower′s carmine and Fast Red salt B. ci Cirrus. B Confocal image of an egg-producing adult stained for FaRP neuropeptide, showing an orthogonal arrangement to the central nervous system. vnc Ventral nerve cord, lnc lateral nerve cord. C Light micrograph demonstrating cholinesterase activity (blue) in the anterior region of adult Bucephaloides. The paired cerebral ganglia (asterisks) give rise to three pairs of anterior nerve cords which subdivide throughout the anterior sucker (as), while innervation to the oral sucker (os) is derived from the ventral nerve cord. D Confocal image of the posterior region of an ovigerous adult stained for GYIRFamide (green) and muscle (red). The wall of the cirrus pouch is divided by a constriction into a proximal region (pc) comprising a compact arrangement of longitudinal fibres and a distal section (dc) consisting of less tightly packed longitudinal and circular fibres. Large FaRPergic cell bodies (cb) lie in close proximity to the distal wall of the cirrus pouch
Neuromusculature of the adult
The basic orthogonal pattern of nerve cords and cross-connectives in the adult was comparable with that of the metacercaria, although there was a general decrease in the intensity of staining (Fig. 3B). Reactivity associated with the anterior end and oral sucker/pharynx was also similar to that of the metacercaria, with cholinergic, serotoninergic and peptidergic nerves subdividing and anastomosing among the muscle fibres in these regions (Figs. 3B, C). One notable difference was that 5-HT-IR nerve fibres of the oral sucker/pharynx in adults were derived mainly from the ventral nerve cord, rather than the lateral cord as was the case in the metacercaria. Despite the increase in the size of adult worms, the number of cross-connectives remained the same, as did the overall arrangement and dimensions of muscle fibres.
Fine FaRPergic and serotoninergic fibres ran in close association with the wall of the cirrus pouch (Fig. 3D). In all specimens examined, a peculiar pattern of varicose nerve-staining was noted within the proximal third of the cirrus pouch encircling the cirrus proper (Fig. 4A). Large cell bodies IR for FaRP were also noted close to the uterine pore (Fig. 4A). Four to six FaRPergic cell bodies marked the region surrounding the pre-terminal complex.
Confocal micrographs of the reproductive system of adult Bucephaloides stained for GYIRFamide. A Optical sectioning (z-series) below the wall of the cirrus pouch (cp) reveals a highly muscularised cirrus (ci) composed of circular and longitudinal fibres surrounded by staining in a gland-like structure (green). Insert: A large FaRPergic multipolar cell body (arrow) lies proximal to the uterine pore (up) as seen through the wall of the cirrus pouch into the genital atrium. B FaRP staining (green) in association with the egg-assembly apparatus. Nerve tracts derived from the ventral nerve cord subdivide, running ventrally and dorsally around the sphincter-like opening at the junction of ovary (ov) and oviduct (ovd). A large cell body (cb) IR to FaRP lies ventral to the junction of the ootype (ot) and uterus (ut). vi Vitelline duct. C GYIRFamide-IR neurons surround the opening of the ovo-vitelline (ovi) duct into the ootype (ot), which comprises dense bands of circular and longitudinal muscle fibres. D Image of FaRP-IR neurons (light blue) encircling the ducts of the female reproductive system in an egg-producing adult. ot Ootype, ovd oviduct
The highly organised musculature of the female reproductive system was better defined in adults, allowing the arrangement of inner longitudinal and outer circular fibres to be more easily resolved than in the metacercaria, their density again being dependent on the region examined (Fig. 4B). A sphincter of compact circular muscle now marks the entrance to the ootype, in which a lattice-like arrangement of regularly spaced longitudinal and circular fibres extends to the opening into the uterus. From here, the arrangement of muscle fibres becomes progressively less well organised up to the uterine pore.
Branches from the ventral nerve cord run in close apposition to the female reproductive system. FaRPergic fibres subdivide and anastomose around the opening of the ovary (Fig. 4B). Where these fibres run posteriorly, a large FaRP-IR cell body is positioned proximal to the ootype. Varicose nerves IR for peptide surround the sphincter that presumably controls entry of oocytes into the egg chamber and run along the muscle of the ootype wall itself (Fig. 4C, D). Dual-localisation studies demonstrated an intricate pattern of FaRP- and 5-HT-IR fibres innervating the muscle of the egg-assembly apparatus, but with little evidence of co-localisation.
Discussion
Bucephaloides gracilescens metacercariae are progenetic and transform into sexually active worms only after they enter the definitive host. This barrier to full sexual development of the worm was previously assumed to be a nutritional one, with the level of development achieved in the whiting intermediate host being regulated by the nutriment available there (Smyth 1994). However, when removed from the whiting, all metacercariae examined were encysted and thus unable to ingest food orally. The question arises therefore: would the time available to the pre-encysted metacercaria be sufficient for it to attain the observed level of development? Halton and Johnston (1982) showed encysted metacercariae are capable of absorbing radiolabelled substrates ([3H]thymidine, [3H]tyrosine, [3H]proline) across the cyst wall and subsequently incorporating these into tissue. Ruus et al. (2001) found that larger molecules, such as lindane (mol. wt. 290.85), diffuse through the cyst wall from adjacent host tissues. As such, it is possible the encysted metacercariae receive a level of nutriment via the cyst wall sufficient to attain spermatogenesis. The cyst is bilayered, comprising an inner granular layer of parasite origin and an outer cellular, fibrous capsule derived from the host. The dimensions of the cyst wall differ regionally, such that cysts in the orbit/nasal cavity have thicker walls than those in the cranial cavity. If a correlation between levels of available nutriment and development exists, site-specific variation would be expected in the degree of progenicity of metacercariae, which was not the case in the present study. Whether further development is inhibited by a nutritional barrier is unclear. Undernourished adult Schistosoma mansoni have smaller morphometric values (length, width) of the reproductive system than nourished specimens (Neves et al. 2001). However, a number of species of trematode metacercariae, such as Diplostomum spathaceum, which invade the eyes of salmonid species, inhabit immunologically privileged sites and as such remain unencysted. Although free to graze on lens tissue and aqueous humour in the eye, these larvae are non-progenetic, raising the question whether it is indeed the lifting of a nutritional barrier or some other cue from the definitive host that triggers egg production. Encysted metacercariae of Microphallus papillorobustus can self-fertilise and produce eggs when their gammarid intermediate host dies without being eaten by the definitive bird host (Wang and Thomas 2002). Egg production in this situation is at a significantly reduced rate to that in the bird intestine, implying that nutrition influences fecundity but is not necessarily the cue to reproduce. Clearly, further work is required in this area if the triggers for egg production are to be determined.
The nervous system of Bucephaloides is typically orthogonal in design and is comparable in metacercariae and adults. An overall decrease in the intensity of immunostaining in the adults as compared with the metacercariae may be associated with reduced penetration of antibody as a result of the development of the glycocalyx, required to fend off the anglerfish′s immune response. Cholinergic, serotoninergic and peptidergic staining was widespread and displayed an intricate association within the nervous system. However, dual-labelling experiments demonstrated differences in detail and suggest separate neuronal pathways for 5-HT and FaRP, in accordance with that previously described by Halton et al. (1998) and Zurawaski et al. (2001) for other flatworm parasites. 5-HT-IR fibres are generally slimmer with large, well defined cell bodies and nuclei, display marked bilateral symmetry in their disposition and are often arranged in pairs. The varicose nature of GYIRFamide-IR fibres may indicate ″pulses″ of peptidic secretion en route from their site of synthesis in the cell body along the axon for release at the terminus, or they may be sites of paracrine-like release of neuropeptide (Halton and Gustafsson 1996).
ACh appears to serve as an inhibitory neurotransmitter in trematodes, following motility studies in which, together with cholinomimetics and cholinesterase inhibitors, it was shown to blunt flatworm muscle contractility, leading to flaccid paralysis. In contrast, physiological data suggest 5-HT has an excitatory transmitter role in flatworm parasites (Maule et al. 1989). In addition, this biogenic amine is believed to be important in maintaining neuroplasticity in invertebrates and in the generation of muscle and nerve (Mitchell et al. 2001). Although still not well understood, physiological studies so far have shown FaRPs to be myoexcitatory, inducing dose-dependent contractions in vitro of isolated muscle fibres from S. mansoni (Day et al. 1994) and the marine turbellarian, Bdelloura candida (Johnston et al. 1996) and muscle strips from Fasciola hepatica (Marks et al. 1996; Graham et al. 1997).
A PNS provides motor and sensory innervation to the musculature of the body wall, suckers and mouth. An extensive sub-tegumental network IR to 5-HT, especially noticeable in the anterior and lateral margins of the worm, supports the view that serotonin (5-HT) is involved as a neuronal component in contact perception.
Muscle arrangement in the anterior and oral suckers is similar to that described for the digeneans F. hepatica (Mair et al. 1998) and Apatemon cobitidis proterorhini (Stewart et al. 2003b), comprising meridional, radial and equatorial fibres that act sequentially to draw up host mucosa and secure attachment. Whereas the meridional and equatorial fibres are derived in part from the longitudinal and circular body wall musculature, the more extensive radial fibres appear to be intrinsic to both suckers. Diagonal fibres of body wall muscle are skewed anteriorly and insert at points along the base of the anterior sucker, which appears to be the main source of attachment.
Large bands of extrinsic muscle connected to the body wall facilitate opening of the mouth which is particularly well invested with 5-HT-IR neurons. The immunocytochemical distribution of fine aminergic nerve endings in this region correlates well with the surface pattern of numerous unciliated nerve endings observed by scanning electron microscopy (Halton, personal communication), further implicating serotonin (5-HT) in contact perception. A sphincter-like arrangement of dense bands of circular muscle seem likely to control entry of food into the gut. Considering the extensive nature of FaRP-immunostaining in the innervation of the oesophagus, FaRPs may also be involved in co-ordinating peristaltic muscle contractions to conduct food into the sac-like intestine. No FaRP-immunostaining was found associated with the muscle investment of the intestine in either F. hepatica (Mair et al. 1998) or S. mansoni (Mair et al. 2000). The pattern of immunostaining observed in Bucephaloides is more comparable with that observed in the microturbellarian Macrostomum hystricinum marinum (Mair et al. 1996), in which the entire sac-like gut is invested with a nerve-net immunopositive for the turbellarian FaRP, GYIRFamide (Bdelloura candida), suggesting neural input in the regulation of digestion, perhaps in controlling gland release, or gut motility.
Contraction of the dense array of longitudinal muscle in the proximal wall of the cirrus pouch may facilitate rapid release of secretion into the lumen while forcibly driving the cirrus into the genital atrium. The arrangement of circular and longitudinal muscle in the cirrus likely provides a high degree of manoeuvrability in this organ. The extensive array of 5-HT-IR fibres in close association with the wall of the cirrus pouch suggests 5-HT may be involved in controlling the musculature involved in copulation. The only FaRP-staining in close proximity to the male reproductive system was the unusual pattern of immunoreactivity surrounding the proximal region of the cirrus of adults; and this may be non-specific staining associated with glandular secretions in this region. However, given the strategically placed FaRP-IR cell bodies at the uterine pore, FaRPs would appear to be involved in modulating release of eggs into the genital atrium.
The present cytochemical study exposes well defined staining differences between the metacercarial stage and adult, enabling examination of changes in the expression of neuroactive substances in the innervation of the egg-producing apparatus. Previous immunocytochemical studies on serotonin (5-HT) and FaRPs revealed a distinct temporal pattern of GYIRFamide levels within cells innervating the ootype in the monogenean Polystoma nearcticum (Armstrong et al. 1997) and the strigeid trematodes A. c. proterorhini and Cotylurus erraticus (Stewart et al. 2003a, 2003b). GYIRFamide was expressed only at the onset of egg production, while serotonin (5-HT) levels were unaffected and appeared to be expressed consistently within nerve cells innervating the egg-assembly apparatus. These findings are corroborated in the present study, in which persistently strong staining for 5-HT was seen in the innervation of the ootype in both metacercaria and adult, while GYIRFamide (or a related FaRP) was expressed only in the egg-producing adult. In contrast, FaRP-immunoreactivity in the CNS and innervation of the suckers was consistently strong and independent of the reproductive state of the worm. FaRPs have been implicated in the regulation of ovulation and egg-shell formation in ″higher″ invertebrates, such as the cephalopod Sepia officinalis, in which egg production is accompanied by changes in a so-called neuropeptide cocktail in the innervation of the oviduct (Henry et al. 1999). In flatworms, assembly of ectolecithal eggs in the ootype and their release into the uterus also appears to be modulated by FaRPs, most likely through the innervation of the circular and longitudinal muscle of these ducts.
References
Armstrong EP, Halton DW, Tinsley RC, Cable J, Johnston RN, Johnston CF, Shaw C (1997) Immunocytochemical evidence for the involvement of a FMRFamide-related peptide (FaRP) in egg production in the flatworm parasite, Polystoma nearcticum. J Comp Neurol 377:41–48
Coons AH, Leduc EH, Connelly JM (1955) Studies on antibody production. I. A method for histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med 102:42–60
Day TA, Maule AG, Shaw C, Halton DW, Moore S, Bennett JL, Pax RA (1994) Platyhelminth FMRFamide-related peptides (FaRPs) contract Schistosoma mansoni (Trematoda: Digenea) muscle fibres in vitro. Parasitology 109:455–459
Fellowes RA, Maule AG, Marks NJ, Geary TG, Thompson DP, Halton DW (2000) Nematode neuropeptide modulation of the vagina vera of Ascaris suum: in vitro effects of PF1, PF2, PF4, AF3 and AF4. Parasitology 120:79–89
Graham MK, Fairweather I, McGeown JG (1997) The effects of FaRPs on the motility of isolated muscle strips from the liver fluke, Fasciola hepatica. Parasitology 114:455–465
Halton DW, Gustafsson MKS (1996) Functional morphology of the platyhelminth nervous system. Parasitology 113:S47–S72
Halton DW, Johnston BR (1981) Excystation in vitro of Bucephaloides gracilescens metacercaria (Trematoda: Bucephalidae). Z Parasitenk 65:71–88
Halton DW, Johnston BR (1982) Functional morphology of the metacercarial cyst of Bucephaloides gracilescens (Trematoda: Bucephalidae). Parasitology 85:45–52
Halton DW, Johnston BR (1983) Development in vitro of the metacercaria of Bucephaloides gracilescens (Trematoda: Bucephalidae). Int J Parasitol 13:157–164
Halton DW, Maule AG, Mair GR, Shaw C (1998) Monogenean neuromusculature: some structural and functional correlates. Int J Parasitol 28:689–697
Halton DW, Maule AG, Marks NJ, Fellowes RA, Shaw C (1999) FMRFamide-related peptides (FaRPs) in helminth parasites. Acta Parasitol 44:11–18
Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansel A, Clarke IJ (1999) Central administration of lectin ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for dissociation of effects on appetite and neuroendocrine function. Endocrinology 3:1175–1182
Johnston BR, Halton DW (1981). Occurrence of Bucephaloides gracilescens metacercariae in three species of gadoid fish. J Fish Biol 18:685–691
Johnston RN, Shaw C, Halton DW, Verhaert P. Blair KL, Brennan GP, Price DA, Anderson PAV (1996) Isolation, localisation and bioactivity of the FMRFamide-related neuropeptides GYIRFamide and YIRFamide from the marine turbellarian Bdelloura candida. J Neurochem 67:6564–6570
Mair GR, Johnston RN, Halton DW, Shaw C, Johnston CF, Reiter D, Rieger RM (1996) Localisation of GYIRFamide immunoreactivity in Macrostomum hystricinum marinum (Platyhelminthes, Macrostomida). Zoomorphology 14:73–76
Mair GR, Maule AG, Shaw C, Johnston CF, Halton DW (1998) Gross anatomy of the muscle systems of Fasciola hepatica as visualised by phalloidin-fluorescence and confocal microscopy. Parasitology 117:75–82
Mair GR, Maule AG, Day T, Halton DW (2000) A confocal microscopical study of the musculature of adult Schistosoma mansoni. Parasitology 121:163–170
Marks NJ, Johnston S, Maule AG, Halton DW, Shaw C, Geary TG, Moore S, Thompson DP (1996) Physiological effects of platyhelminth RFamide peptides on muscle-strip preparations of Fasciola hepatica (Trematoda: Digenea). Parasitology 113:393–401
Matthews RA (1974) The life-cycle of Bucephaloides gracilescens (Rudolphi, 1819) Hopkins, 1954 (Digenea: Gasterostomata). Parasitology 68:1–12
Maule AG, Halton DW, Allen J, Fairweather I (1989) Studies on motility in vitro of an ectoparasitic monogenean, Diclidophora merlangi. Parasitology 98:85–93
Mitchell GS, Martin KC, Edwards DH, Duman RS (2001) Serotonin-induced neuroplasticity: similar mechanisms in diverse neural systems. Abstr Soc Neurosci 27:903
Neves RH, Machado-Silva JR, Pelajo-Machado M, Oliveira SA, Coutinho EM, Lenzi HL, Gomes DC (2001) Morphological aspects of Schistosoma mansoni adult worms isolated from nourished and undernourished mice: a comparative analysis by confocal laser scanning microscopy. Mem Inst Oswaldo Cruz 97:1013–1016
Pearse AGE (1960) Histochemistry, pure and applied, 2nd edn. Churchill, London
Ruus A, Skaare JU, Ingebrigtsen K (2001) Accumulation of the lipophilic environmental contaminant lindane in metacercariae of Bucephaloides gracilescens (Trematoda, Bucephalidae) in the central nervous system of bullrout Myoxocephalus scorpius. Dis Aquat Org 48:75–77
Smyth JD (1994) An introduction to animal parasitology, 3rd edn. Cambridge University Press, Cambridge
Smyth JD, Clegg JA (1959) Egg-shell formation in trematodes and cestodes. Exp Parasitol 8:286–323
Stewart MT, Mousley A, Koubková B, Šebelová Š, Marks NJ, Halton DW (2003a) Development in vitro of the neuromusculature of two strigeid trematodes, Apatemon cobitidis proterorhini and Cotylurus erraticus. Int J Parasitol 33:413–424
Stewart MT, Mousley A, Koubková B, Šebelová Š, Marks NJ, Halton DW (2003b) Gross anatomy of the muscle systems and associated innervation of Apatemon cobitidis proterorhini metacercaria (Trematoda: Strigeidea), as visualised by confocal microscopy. Parasitology 126:273–282
Wang CL, Thomas F (2002) Egg production by metacercariae of Microphallus papillorobustus: a reproductive insurance? J Helminthol 76:279–281
Zurawski TH, Mousley A, Mair GR, Brennan GP, Maule AG, Gelnar M, Halton DW (2001) Immunomicroscopical observations on the nervous system of adult Eudiplozoon nipponicum (Monogenea: Diplozoidae). Int J Parasitol 31:783–792
Acknowledgements.
This work was supported in part by a European Social Fund grant to M.T.S. The authors would like to thank the staff at F.B. Mawhinney and Sons in Portavogie for their kind help in providing fish.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stewart, M.T., Marks, N.J. & Halton, D.W. Neuroactive substances and associated major muscle systems in Bucephaloides gracilescens (Trematoda: Digenea) metacercaria and adult. Parasitol Res 91, 12–21 (2003). https://doi.org/10.1007/s00436-003-0896-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-003-0896-4