Abstract
The nictitating membrane is an anatomic structure exclusively exhibited by Carcharhiniformes, the largest order among sharks. Here we present a detailed description of morphological characteristics of the nictitating membrane through light microscopy (LM) and scanning electron microscopy (SEM) in the following shark species: Carcharhinus limbatus, Galeocerdo cuvier, Prionace glauca, Rhizoprionodon lalandii, R. porosus, Sphyrna lewini and S. zygaena. Differences in the microscopic aspects of dermal denticles from the species studied were observed. P. glauca, a pelagic shark, showed a well-developed protection apparatus when compared with other pelagic species, while coastal sharks showed even higher structural complexity. In the blue shark the denticles are enameled, presenting an extensive pulp cavity and a base inserted in a connective tissue. Moreover, the species exhibits the higher number of ridges (up to nine) of varied size and shape and the muscular tissue is inserted in the ventral region of the connective tissue. Dermal denticles from C. limbatus, R. lalandii, R. porosus, S. zygaena and G. cuvier exhibit up to five ridges with hexagonal ornamentations in the crown. In S. lewini and S. zygaena, the denticles are rounded shaped and glandular cells are present. The patterns observed in the present study suggest a high level of specialization and evolutionary conservation shaped by the function of the structure. In addition, we hypothesize that the morphological simplification observed in the membrane when compared to the dermal denticles from the skin, is an evolutionary trait that evolved to improve the dynamic and biomechanics of this highly mobile structure allowing this way, a rapid and efficient protection against abrasion, mainly during predation events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Carcharhiniformes order is the largest among sharks, with about 55% of all species currently described (~250) (Compagno 2005). The presence of a nictitating membrane distinguishes the sharks of the order (Compagno 1988; Hueter et al. 2004). It is denominated the third eyelid, covering the shark eye completely (Gruber and Schneiderman 1975; Gruber and Myrberg 1977) during the feeding activities as observed in other vertebrates such as felines (Crawford and Marc 1976), canines (Pires et al. 2008), equines (Puff et al. 2008) and primates (Crawford and Marc 1976). The structure protects against mechanic damages, being activated in predation events or as response against changes in pressure around the eyes or head (Bell and Satchell 1963; Gruber and Schneiderman 1975). Recently, the nictitating membrane has been used as a stress indicator during scientific capture by analyzing the imparity reflex activated by external stimulation (Danylchuk et al. 2014; Gallagher et al. 2014).
In sharks, the membrane is thin and opaque, composed by connective tissue and covered externally by dermal denticles. The denticles are also called placoid scales which are also observed across the shark’s body (Bell and Satchell 1963; Raschi and Tabit 1992; Kemp 1999). Dermal denticles exhibit a variety of shapes closely related with function, including protection against predators and ectoparasites (denticles with thorns and mucus), reduction of the hydrodynamic drag (denticles with furrows in the crown), and accommodation of sensory and bioluminescent structures (Reif 1978; Raschi and Tabit 1992). For that reason, the denticles are composed of calcified dentine and covered with a thin layer of enamel (Gravendeel et al. 2002), being more resistant than the cartilaginous skeleton and an effective tool for fossils identification (Gravendeel et al. 2002) and phylogenetic assessment (Cappetta 1987; Marshall 2011).
However, studies describing the detailed morphology of the dermal denticles in a comparative level are scarce (Marshall 2011; Mello et al. 2013; Ciena et al. 2015; Rangel et al. 2016). Since the morphological structure differs between body regions, it is imperative to perform a detailed study in order to identify the unique characteristics, allowing its use as a taxonomic tool (Marshall 2011; Mello et al. 2013). In this context, the present study used light (LM) and scanning electron microscopy (SEM) to describe in details the microstructure and distribution of dermal denticles in the nictitating membrane of seven Carcharhinidae and Sphyrnidae species.
Materials and methods
Animals
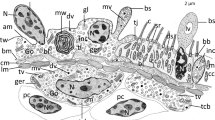
Samples of ten sharks were obtained from commercial fishing in Southern and South Brazil. Carcharhinus limbatus (n = 2), Prionace glauca (embryos n = 3 and adults n = 3), Rhizoprionodon lalandii (n = 2), R. porosus (n = 2), Sphyrna lewini (n = 2), and S. zygaena (n = 2) were captured from December 2014 to November 2015. Samples from Galeocerdo cuvier (n = 2) were obtained in the fish collection from the Laboratory of Ichthyology located in the Institution of Bioscience of University of São Paulo. The samples were obtained using tweezers and a scissor to separate the membrane from the ventral preorbital and the elevating musculatures (Fig. 1).
The work was approved by the Brazilian Ministry of Environment and IBAMA through SISBIO (Sistema de Autorização e Informação em Biodiversidade), permit number 48348-7 and by the Ethics Committee from FMVZ-USP, Protocol Number 4245050214.
Light microscopy (LM)
The nictitating membrane of one blue shark adult (P. glauca) was fixed in 10% formaldehyde solution. The sample was rinsed for 15 min and stored in 70°GL alcohol and then dehydrated in ascending ethanol series (from 70 to 100°GL) and thereafter cleared in xylene for subsequent embedding in paraplast. Paraplast blocks were sectioned using a microtome (Leica, German) and stained with hematoxylin and eosin (HE) for analysis.
Scanning electron microscopy (SEM)
Nictitating membranes from P. glauca, C. limbatus, R. lalandii, R. porosus, S. lewini, S. zygaena and G. cuvier were separated, fixed in 4% formaldehyde solution (overnight) and stored in 70°GL alcohol. The samples were then dehydrated in a series of increasing ethanol density. After dehydration, the samples were dried (overnight) in a Balzers CPD 020 critical-point device mounted onto metal stubs with carbon adhesive and sputtered with gold in an Emitech K550 sputter apparatus and read in scanning electron microscope Leo 435 VP (FMVZ-USP).
Results
The morphological analysis showed that there are relevant differences in the microscopic aspects of dermal denticles from the species studied. It was not possible to define the orientation of the denticles according to the body axis (head–tail).
Prionace glauca
In SEM, it was possible to observe the dermal denticles covering the entire external surface of the nictitating membrane. The denticles vary in shape and size (Fig. 2a, b). The denticle crown is rounded shaped, exhibiting between 3 and 9 ridges, which extend from the crown base to a third or half of the upper region of the crown (Fig. 2a–c).
Nictitating membrane of an adult Prionace glauca. a SEM showing distribution of denticles (dd) in the membrane; b, c SEM of denticles (dd) in the membrane, highlighting the hexagonal ornamentations (ho) and arrows indicating the longitudinal demarcations; d LM showing denticles (d) with wide pulp cavity (pc) a thin layer composed by dentine (d),enamel (e) with connective tissue (ct) and musculature (m); e denticles (dd) overlapped; f a single denticle with hexagonal ornamentations (ho) and arrows indicating the demarcations
In the lateral extremities it was possible to observe the presence of hexagonal ornamentations (Fig. <link rid="fig2">2</link>b, c). Close to the ridges, the ornamentations were bigger, reaching the base of the denticle, while the structures in the upper region were smaller.
Under LM, it was possible to observe the dermal denticles in a transversal angle. The denticles are composed of a thin layer of dentine and enameloid (upper region) presenting a wide pulp cavity and a denticle base inserted in the connective tissue. The musculature is located below the connective tissue (Fig. 2d).
Carcharhinus limbatus
The dermal denticles in C. limbatus are equally distributed in the external surface, exhibiting differentiated crown structure (Fig. 3a). The anterior denticle margin is monocuspid and rounded shaped (Fig. 3b, c). In the dorsal surface, it was possible to observe 3–5 well-marked ridges, which extend from the denticle base until the upper half region of the crown. Hexagonal microstructures are observed, being bigger in the posterior region (Fig. 3b, c). The denticles overlap in the species (Fig. 3b, c).
SEM from nictitating membrane of several shark species. a dermal denticles of nictitating membrane in C. limbatus, b oval-shaped denticles (dd); c oval-shaped denticles with longitudinal demarcations, hexagonal ornamentations (ho) and arrows indicating the demarcations; d dermal denticles distribution in R. lalandii; e oval-shaped denticles (dd) with well-marked demarcations; f denticles with ornamentations (ho) across the surface and demarcations (arrows) restricted to the posterior margin; g dermal denticles in R. porosus of a variety of formats; h oval/rounded-shaped denticles (dd) with restricted demarcations in the posterior margin; i oval-shaped denticles with hexagonal ornamentations (ho) restricted to the posterior margin with arrows indicating the demarcations
Rhizoprionodon lalandii
The dermal denticles in R. lalandii are oval/flattened-shaped with uniform distribution, presenting similar structural characteristics along the crown (Fig. 3d, e). The anterior margin is monocuspid and rounded shaped, as observed in C. limbatus (Fig. 3f). It presents 3–5 slightly marked ridges in the crown, which extend from the base to the upper half region of the structure (Fig. 3e, f). The hexagonal microstructures are observed across the surface, being bigger in the anterior region, where the ridges are located (Fig. 3e, f).
Rhizoprionodon porosus
The dermal denticles of R. porosus are distinct from each other, due to the area analyzed (Fig. 3g, h). Some are monocuspid with a rounded-shaped anterior margin, while others are flattened-shaped (Fig. 3g) or oval-flattened-shaped (Fig. 3h). The monocuspid denticles exhibit uneven distribution, covering all the blank spaces in the surface (Fig. 3G), while the oval-shaped denticles exhibit spaced distribution in some regions (Fig. 3h).
Slightly marked and well-marked ridges are observed (2–5) extending from the denticle base to the upper half region of the crown (Fig. 3g–i). The hexagonal structures are observed across the crown surface, being bigger in the anterior region, where the ridges are located (Fig. 3i).
Sphyrna lewini
The denticles are distributed evenly across the membrane, with scarce spaces in the epithelium and an oval-shape (Fig. 4a). Hexagonal ornamentations in the anterior and posterior extremities are observed. It was possible to identify glandular cells between the denticles (Fig. 4b).
a, b Nictitating membranes of juvenile Sphyrna lewini (c–f) and adult S. zygaena. a Denticles (dd) distribution in the membrane of S. lewini; b glandular structure, wrapped by denticles with hexagonal ornamentations (ho) and epithelium (e) in the structure center; c denticles (dd) distribution in the membrane of S. zygaena, the yellow arrows indicating the empty spaces between the denticles; d close-up in the denticles and spaces (yellow arrows) between them; e close-up in the spaces, showing the epithelium (e) present, the denticles that surrounded this glandular structure and white arrows indicating the glandular cell; f close-up into the denticle epithelium (e) with hexagonal ornamentations (ho)
Sphyrna zygaena
The denticles are distributed evenly, arranged side-by-side, with little or no space between them (Fig. 4c, d). The structures are oval-shaped, with hexagonal ornamentations restricted to the margins and small ridges in the posterior region of the crown (3–6) (Fig. 4e, f). The denticles exhibit differentiated shape, size and position (flat; without longitudinal demarcations) (Fig. 4e, f). Secretory cells are present in the epithelium adjacent to the denticles (Fig. 4e, f).
Galeocerdo cuvier
The denticles are similar to S. lewini and S. zygaena: oval-shaped, evenly distributed (Fig. 5a, b) with hexagonal ornamentations restricted to the crown margins (Fig. 5c). The bigger denticles exhibited up to five ridges, restricted to the posterior margin (Fig. 5c).
Discussion
This is the first description of the microscopic aspects of the nictitating membrane of Carcharhinidae and Sphyrnidae sharks. Through the technique, it was possible describe in details the morphological features of the structure, thus allowing a discussion to better understand the functional and evolutionary importance of the membrane.
As observed in this preliminary study, the denticles found in the membrane are covered by a thin layer composed of dentine and enameloid (apical region), being denser than denticles from other body regions. It has been hypothesized that the presence of denser denticles in the nictitating membrane is related to a higher protection needed during feeding events or stressful situations. Corroborating that, it has been described that during feeding, Carcharhiniformes close the nictitating membrane and open the jaw at the same time (Ritter and Godknecht 2000), showing a close relation between prey capture and eye protection. In Negaprion brevirostris, the nictitating membrane response was tested facing luminous and electric stimulation, showing the conditioning facing uncomfortable/stressful events (Gruber and Schneiderman 1975). This protective function is also corroborated by the morphological conformation of the denticles around the eyes, characterized by thickened, knob-like crowns highly sculpted (Raschi and Tabit 1992).
Among the analyzed species, C. limbatus, P. glauca and Rhizoprionodon spp. presented the most generalized conditions, compared to features exclusively observed in S. lewini, S. zygaena and G. cuvier (oval-shaped denticles distributed evenly, with a lower amount of hexagonal ornamentations and crowns with up to six ridges). Thus, it is possible that the generalized condition in C. limbatus, P. glauca and Rhizoprionodon spp. species is related to a higher eye protection needed during feeding. Since all feature similar head-shape (diamond) and eye angle, it is possible that those morphological conditions are more susceptible to damages during prey capture.
On the other hand, the similarity between hammerheads and tiger sharks (simplified) could be related to a reduced need for protection but for distinct reasons. While hammerhead sharks exhibit eyes at the end of the cephalofoil, the chances of eye damage during capture are reduced, allowing an evolutionary decrease in energetic expenditure for shaping and maintenance of the structure. In G. cuvier, the triangular and robust shape of the head may be a morphological advantage that protects the eyes from direct angles during capture, also reducing the evolutionary cost of shaping and maintenance. Finally, the glandular structure observed only in hammerhead species may be related to a higher need for eye lubrication (Klećkowska-Nawrot and Dziegiel 2007) and increase in water flow through eyes during swimming as a way to reduce the impacts caused by quick maneuvers against the water current during hunting.
The main structural differences between denticles from body and from the nictitating membrane are the ridges that vary in shape, size and density. The ridges are closely related to hydrodynamic adaptations in some species (Marshall 2011), being proposed that the ornamentations are responsible for increase the resistance of the crown, resulting in weight reduction and better performance (Marshall 2011). Study performed with R. lalandii described the skin denticles as specialized/complex structures (three cusps, and well-defined crests and ridges) (Laranjeira et al. 2015), being different from the denticles in the membrane, which are less complex. The same pattern is observed in C. limbatus (Motta et al. 2012), S. lewini, S. zygaena (Mello et al. 2013) and G. cuvier (Dillon et al. 2017), where the complexity of denticles of the body is greater when compared to denticles in the membrane.
Changes in denticle complexity was reviewed by Raschi and Tabit (1992), bringing evidence that ecological aspects such as habits (e.g. benthic species vs. active species) mold the structure of the denticles. In fact, denticles can be separated according to their function (e.g. drag reduction; abrasion strength; defense), being scattered according to the region of the body compatible with its function (Dillon et al. 2017). By comparing our findings with the structures molded for protection against abrasion, it is possible to confirm that in fact the less complex morphology is compatible to a protective function (Dillon et al. 2017).
The sharks analyzed in the present study share an active hunting behavior, which could explain by the highly-preserved morphology shaped over millions of years of selective pressures. However, differences among life habits and environment occupation could explain the specializations observed. We conclude that in terms of function, the dermal denticles in the nictitating membrane are not only related to protection during hunting, but also responsible to facilitate water flow during swimming, as a possible way to reduce the drag in an evolutionary attempt to reduce energy cost by modifying morphological features. Also, the lower complexity when compared to the denticles of the body, may be related to the greater need of mobility of the structure, thus improving the biomechanical versatility of the membrane. Lastly, since the structure seems to vary in details among species, a deeper analysis relating membrane features, head shape and eye angle could elucidate points raised in the present study such as the use of this new characters complex to understanding the evolution of the eye protection during the feeding and predation events in the Carcharhiniformes.
References
Bell JP, Satchell GH (1963) An undescribed unilateral ocular reflex in the dogfish Squalus acanthias. Austr J Exp Biol 41:221–234
Cappetta H (1987) Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii. Gustav Fischer, New York, pp 1–193
Ciena AP, Rangel BS, Bruno CEM, Miglino MA, Amorim AF, Rici REG, Watanabe I (2015) Morphological aspects of oral denticles in the Sharpnose shark Rhizoprionodon lalandii (Muller and Henle, 1839) (Elasmobranchii, Carcharhinidae). Anat Histol Embryol 45:109–114. DOI:10.1111/ahe.12178
Compagno LJV (1988) Sharks of the order carcharhiniformes. Princeton University Press, New Jersey, p 572
COMPAGNO LJV (2005) Checklist of living Chondrichthyes. In: Hamlett WC (eds) Reproductive biology and phylogeny of Chondrichthyes: sharks, batoids and chimaeras. Science Publishes, Inc, United States, pp 501–548
Crawford MLJ, Marc RE (1976) Light transmission of cat and monkey eyelids. Vision Res 16:323–324
Danylchuk AJ, Suski CD, Mandelman JW, Murchie KJ, Haak CR, Brooks AML, Cook SJ (2014) Hooking injury, physiological status and short-term mortality of juvenile lemon sharks (Negaprion bevirostris) following catch-and-release recreational angling. Conser Physiol. doi:10.1093/conphys/cot036
Dillon EM, Norris RD, Dea AO (2017) Dermal denticles as a tool to reconstruct shark communities. Mar Ecol Prog Ser 566:117–134
Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser 496:207–218
Gravendeel R, Neer WV, Brinkhuizen D (2002) An identification key for dermal denticles of Rajidae from the North Sea. Int J Osteoarchaeol 12:420–441. doi:10.1002/oa.645
Gruber SH, Myrberg AA (1977) Approaches to the study of the behavior of sharks. Am Zool 17:471–486
Gruber SH, Schneiderman N (1975) Classical conditioning of the nictitating membrane response of the lemon shark (Negaprion brevirostris). Behav Res Methods Instrum 7:430–434. doi:10.3758/BF03201554
Hueter RE, Mann DA, Maruska KP, Sisneros JA, Demski LS (2004) Sensory biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC, Boca Raton, pp 325–368
Kemp NE (1999) Integumentary system and teeth. In: Hamlett WC (ed) Sharks, skates and rays: the biology of elasmobranch fishes. John Hopkins University Press, Baltimore, pp 43–68
Klećkowska-Nawrot J, Dzięgiel P (2007) Morphology of the third eyelid and superficial gland of the third eyelid on pig fetuses. Anat Histol Embryol 36:428–432. doi:10.1111/j.1439-0264.2007.00780.x
Laranjeira ME, Guimarães JP, Amorim AF, Rotundo M, Rici REG, Mari RB (2015) Ultrastructure of dermal denticles in sharpnose shark (Rhizoprionodon lalandii)(Elasmobranchii, Carcharhinidae). Microsc Res Tech 78(10):859–864. doi:10.1002/jemt.22546
Marshall LJ (2011) The fin blue line: Quantifying fishing mortality using shark fin morphology. Dissertation, University of Tasmania
Mello WC, De Carvalho JJ, Brito PMM (2013) Microstructural morphology in early dermal denticles of hammerhead sharks (Elasmobranchii: Sphyrnidae) and related taxa. Acta Zool-Stockholm 94:147–153. doi:10.1111/j.1463-6395.2011.00547.x
Motta P, Habegger ML, Lang A, Hueter R, Davis J (2012) Scale morphology and flexibility in the shortfin mako Isurus oxyrinchus and the blacktip shark Carcharhinus limbatus. J Morphol 273(10):1096–1110
Pires AG, Algueró MC, Mendes JL, Trindade H, Correia M (2008) Immunophenotyping of lymphocyte subsets in the third eyelid tissue in dogs (Canis familiaris): Morphological, microvascular, and secretory aspects of this ocular adnexa. Microsc Res Tech 7:521–528
Puff C, Herder V, Philipp A, Baumgartner W (2008) Lymphangiosarcoma in the nictitating membrane of a horse. J Veter Diagn Invest 20:108–110
Rangel BS, Ciena AP, Wosnick N, Amorim AF, Kfoury-Junior JR, Rici REG (2016) Ecomorphology of oral papillae and denticles ofZapteryx brevirostris (Chondrichthyes, Rhinobatidae). Zoomorph 135:189–195. doi:10.1007/s00435-016-0304-0
Raschi W, Tabit C (1992) Functional aspects of placoid scales: a review and update. Aust J Mar Fresh Res 43:123–147
Reif WE (1978) Protective and hydrodynamic function of the dermal skeleton of elasmobranchs. Neues Jahrbuch Für Geologie Und Paläontologie 157:33–141
Ritter EK, Godknecht AJ (2000) Agonistic displays in the blacktip shark (Carcharhinus limbatus). Copeia 2000:282–284
Acknowledgements
We would like to thank CAPES (granted ANP and NW) for the support, the postgraduate program of Department of Surgery, Faculty of the Veterinary Medicine and Animal Science, University of São Paulo and the funding provided by FAPESP through contract number 2016/09095-2 (Granted to BRS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poscai, A.N., de Sousa Rangel, B., da Silva Casas, A.L. et al. Microscopic aspects of the nictitating membrane in Carcharhinidae and Sphyrnidae sharks: a preliminary study. Zoomorphology 136, 359–364 (2017). https://doi.org/10.1007/s00435-017-0351-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-017-0351-1