Abstract
Elasmobranchs have an impressive range of highly specialized sensory systems shaped over 400 million years of evolution. The morphological analysis of oral papillae and denticle in elasmobranchs elucidates the biological role that these structures play during feeding and ventilation, bringing important descriptive information about ecological implications in an evolutionary context. The present study provides descriptions of the distribution patterns, histological characteristics and three-dimensional aspects of oral papillae and denticles in the lesser guitarfish Zapteryx brevirostris, through light microscopy and scanning electron microscopy. The presence of oral denticles in the oropharyngeal cavity suggests that this structure may have the following functions: protect against abrasion and parasites, increase the ability to grasp and hold prey and assist in reduction in hydrodynamic drag. The denticles in Z. brevirostris are similar to those found in pelagic sharks with forced ventilation (RAM). The structural conformity of denticles observed in the gill slits may facilitate water flow during prey grasp and food processing. This study supports the hypothesis that these structures may be an adaptive reflection shaped by feeding habits, capture strategies and processing prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sharks and batoids (skates, stingrays, guitarfish and sawfish) have an impressive range of highly specialized sensory systems that have been shaped by over 400 million years of evolution. Every sensory modality has been developed to detect and respond to a different set of biotic/abiotic stimuli, playing a crucial role in animal survival and longevity (Collin 2012; Hart and Collin 2015). The physiological response to chemical stimuli detected by olfaction, gustation and solitary chemosensory cells facilitates social interactions, communication, mating response and the detection of prey and predators (Reutter et al. 1974; Kotrschal 1996; Collin 2012).
The gustatory system is observed in all classes of vertebrates, being mainly involved with the ability evaluation of the palatability of foods, leading to the decision to ingest or reject prey (Atkinson and Collin 2010; Collin 2012). This system is composed of taste buds, which are peripheral sense organs, located in the epithelium of the body parts involved with food intake and handling, such as lips, oral cavity, tongue and pharynx (Atkinson and Collin 2010; Collin 2012). The taste buds consist of modified epithelial cells, comprised of multicellular peripheral chemoreceptors with an axon in the base situated in oral papillae (Whitear and Moate 1994a; Northcutt 2004; Atkinson and Collin 2010). Although gustation is essential for survival, growth, mobility as well as maintenance of neural activity and the immune system, few studies on this system have been performed for this group (Whitear and Moate 1994a, b; Hueter et al. 2004; Atkinson and Collin 2010; Ferrando et al. 2012; Gardiner et al. 2012).
Elasmobranchs present oral denticles in the oropharyngeal cavity, similar to placoid scales, also known as dermal denticles, found across the animal’s body (Nelson 1970; Atkinson and Collin 2012; Ciena et al. 2015). The main functions of oral denticles are to protect against abrasion during food consumption, improve the grasp of prey and assist in hydrodynamic drag (Atkinson and Collin 2010, 2012). These structures exhibit interspecific variation, demonstrating differences in number, size, complexity and distribution (Imms 1905; Fahrenholz 1915; Daniel 1928; Nelson 1970; Atkinson and Collin 2012).
Fossils of guitarfishes (Rhinobatidae) date the Jurassic period, being the oldest family among current batoids (McEachran et al. 1996; Shirai 1996). The family is comprised of four genera: Aptychotrema, Rhinobatos, Trygonorrhina and Zapteryx, with approximately 45 species (Compagno 2005). Zapteryx brevirostris Müller and Henle 1841, commonly known as the lesser guitarfish, distinguishes itself from other guitarfish by presenting a shorter snout. It can be found from Espírito Santo, Brazil, to Argentina (Bigelow and Schroeder 1953; Figueiredo 1977). It is currently classified as vulnerable by the IUCN Red List due to fishing pressure and low fecundity (Santos et al. 2006; Vooren et al. 2006). Recently in Brazil, the species was included in the “National Official List of Endangered Fauna—Fish and Aquatic Invertebrate” proposed by the Brazilian Ministry of Environment (MMA, no. 445, December 2014).
Morphological analysis of oral papillae and oral denticles in elasmobranchs helps to elucidate the biological role of these structures during feeding and ventilation, allowing discussions about the ecological implications in an evolutionary context. The aim of the present study was to: (1) determine distribution patterns of oral papillae and denticles in oral epithelium and (2) elucidate the histological characteristics and three-dimensional aspects of these structures in Z. brevirostris through light microscopy and scanning electron microscopy.
Materials and methods
Animals
Five adults (Z. brevirostris) were collected as bycatch from shrimp trawlers operating in Guarujá, São Paulo, Brazil, in February 2014. The specimens were donated to the Instituto de Pesca/Santos, São Paulo and forwarded to the Department of Anatomy of Veterinary Medicine and Animal Science of the University of São Paulo, where the samples were processed and analyzed. The project was approved by the Ethics Committee on Animal Use (CEUA) (Protocol No 9623050214-FMVZ/USP).
Light microscopy (LM)
Samples of the oropharyngeal cavity from three adult specimens were fragmented and fixed in 10 % formaldehyde. After 10 days, the samples were washed for 15 min and then dehydrated in ascending ethanol series (from 70 to 100 %) and cleared in xylene for subsequent embedding in paraplast. Paraplast blocks were sectioned (5 µm) using a microtome (Leica, German) and stained with hematoxylin and eosin (HE). Analysis was performed using a light microscope (Nikon Eclipse E-800).
Scanning electron microscopy (SEM)
Samples of the oropharyngeal cavity from two adult specimens were fixed in 10 % formaldehyde solution and then dehydrated in series of increasing ethanol concentration (70–100 %). After dehydration, the samples were dried in a Balzers CPD 020 critical-point device mounted onto metal stubs with carbon adhesive and sputtered with gold in an Emitech K550 sputter apparatus. Finally, the samples were photographed in a LEO 435VP scanning electron microscope. The terminology used for oral denticles was based on Thies and Leidner (2012) and Atkinson and Collin (2012) and for papillae was based on Whitear and Moate (1994a, b) and Atkinson and Collin (2010).
Count of papillae and denticles
To estimate the abundance of papillae and denticles in the oral cavity of Z. brevirostris, the photographs obtained by SEM were edited for analysis using ImageJ software (version 1.48). The images analyzed were edited for contrast and sharpness enabling counting using the cell counter available in the program. All photographs were analyzed in triplicate, and the mean was used to estimate the number of papillae and denticles per cm2, by selecting random areas.
Results
The animals examined had total length 423–510 mm and disk width 200–250 mm. The papillae and denticles were detected on the oropharyngeal cavity across the epithelium of the ventral and dorsal regions of the gills arches.
Oral papillae
Oral papillae are well-evidenced epithelial projections with dome shaped and circular edges, observed between the denticles in the ventral and dorsal surface of the oropharyngeal cavity with a diameter of approximately 107 µm and a density of approximately 90 per cm2 (Figs. 1a, c, 2a, b). These structures consist of a dense layer of germ cells, covered with a stratified squamous epithelium. In the lamina propria, it was possible to identify numerous nuclei of papillary fibroblasts and papillary base cells. Bellow the papillae it was possible to observe the presence of a longitudinal arrangement of the nerve responsible to modulate the chemical signal to the central nervous system (Fig. 2b).
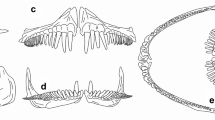
Oral denticles of oropharyngeal cavity. a–c In scanning electron microscopy, the denticles (d) are distributed randomly, among them the oral papillae (p); gill rakers (gr); mucous cells (mc). d, e In light microscopy denticle of the layers, enamel (en) dentin (d), the pulp (pc), apex (a) and bases (asterisk) adjacent the epithelium (e), below connective tissue (ct); denticle (dd) in forming the germ layer (ge); HE coloration. Scale bar a 1 mm; c, e 200 μm; b, e 100 μm
Oral papillae of the oropharyngeal region. a Scanning electron microscopy showing the oral denticles (d) and oral papillae (p). b, c Under light microscopy the presence of oral epithelium with papillae (p), formed by germ cells (ge) and nerve fiber (n), adjacent are oral denticles (d), sustained by connective tissue (ct) and lamina propria (lp); there are regions with a high concentration of mucous cells (mc) in the epithelium (e); HE coloration. d, e In scanning electron microscopy, black arrows indicate the possible taste buds and the yellow arrows to the villi of the chemoreceptors in small groups. Scale bars b 200 µm; c 100 µm; a 30 µm; d 10 µm; e 2 µm
In some regions, with a lower density of oral papillae and denticles it was possible to observe a thickening of the epithelium and the presence of a large number of mucous glandular cells (Fig. 2c). With SEM, it was possible to detect some of these cells opened to the surface, forming holes in the epithelium (Fig. 1c).
On top of the oral papillae, taste buds and solitary chemosensory cells are distributed randomly (Fig. 2d, e). With SEM, the three-dimensional aspects of the papillae reveal the presence of microvilli aggregation (Fig. 2d). We also found isolated microvilli in the epithelium (Fig. 2e). The microvilli identified could be taste buds or solitary chemosensory cells.
Oral denticles
The oral denticles are distributed randomly with a narrow space between them, with approximate size of 107 µm and density of approximately 4500 per cm2. The apex faces the caudal region (Fig. 1a–c), except for denticles inserted on gill arches, where the apex is facing the oropharyngeal cavity (Fig. 1a). The crown of the denticle is mostly monocuspids, with 4–6 prominent folds (Fig. 1c). Distinct denticles were also observed, presenting conformation similar to “fused” denticles (Fig. 1c).
Observations with LM show that the denticle is attached to the epithelium by rods (Fig. 1d). It was possible to observe the rods in a sagittal section, which converge in the superior region forming a rectangular base, with the apex inclined to the caudal region (Fig. 1d). The base lies between the epithelium and the wide area of connective tissue (Fig. 1d, e). Once fully developed, denticle is found below the non-exposed thin epithelium, with a layer of germ cells being formed in the upper structure (Fig. 1e). In Fig. 1c, adjacent to the denticles, oval openings were observed (mucus-secreting cells).
Discussion
The ventral position of the mouth in Z. brevirostris corresponds to the pattern found in batoids. The pattern was molded by benthic feeding habits during evolutionary diversification (Bigelow and Schroeder 1953; Moss 1977; Wilga et al. 2007). The morphology of the oropharyngeal cavity surface and the distribution of oral papillae resemble other species of the same family, while morphological features of oral denticle are closer to those found in sharks (Atkinson and Collin 2012).
Behavioral studies during predation reveal a basic sequence in the mechanisms used for feeding, which may vary according to the foraging strategies and the type of prey, with a combination of behaviors such as biting, sucking, manipulation, forced ventilation (RAM) and filtration (Wilga and Motta 1998; Dean et al. 2007). This set of behavioral strategies was shaped throughout evolution as a consequence of morphological specializations observed in teeth, muscle, oral denticles and oral papillae.
Oral papillae
The gustatory system in vertebrates consists of multicellular peripheral chemoreceptors called oral papillae, which feature a phyletic variation regarding morphology and distribution (Northcutt 2004). Todaro (1872) described, through histology, two types of oral papillae found in the oral cavity and oropharyngeal valves of Trygon pastinaca: (1) cup shaped with a narrow base and (2) inverted bell with a circular base and wide apex. He also described the presence of microvilli in the apex of the taste buds. In Squalus acanthias, the oral papillae are distributed across the oropharyngeal cavity and more numerous in the dorsal region (Cook and Neal 1921). Pevsner (1976) conducted a study using electron microscopy, with T. pastinaca, confirming the presence of basal cells and sensory cells, presenting several apical microvilli. More recently, Whitear and Moate (1994a) carried out a detailed ultrastructural analysis in Scyliorhinus canícula, confirming the association between nerve fibers and receptors. Since then, very little has been studied and published. The most comprehensive study was conducted by Atkinson (2011), describing the morphology, distribution and development of oral papillae and oral denticles in six species of batoids and six species of sharks in Australia, but has not yet been published. However, the correlation between oral denticles and papillae with feeding behavior and diet was neglected.
In Z. brevirostris, the papillae were more abundant in the ventral and dorsal regions of oropharyngeal cavity, but were also found along the gill arches. Since this species uses suction as feeding strategy, a greater number of papillae in the oral cavity could indicate that it should be the main site for handling of prey during ingestion. The final crushing stage also occurs in this cavity, which is advantageous considering the cavity allows a secondary site for food selection. It is possible that the high density of oral denticles observed limits the space available for a high density of papillae, suggesting that the generalist feeding habits presented by this species are directly correlated with reduced tasting capability. The same was observed for other species of batoids (Atkinson and Collin 2012). Otherwise, pelagic sharks have a higher abundance of papillae in the oral valve region, suggesting that the area around the jaw is more efficient for tasting, which increases the efficiency during offshore predation (scarcity of resources).
The microvilli found on the apical surface of the papillae protrude into several clusters, being and indicator of the presence of taste buds in the species. The same morphological and distributional patterns were observed in Scyliorhinus canicula (Whitear and Moate 1994a), Raja clavata (Whitear and Moate 1994b), and other species of batoids and sharks studied by Atkinson (2011). The microvilli identified could be taste buds or solitary chemosensory cells; however, analysis with transmission microscopy needs to be performed in order to truly understand the role of these structures on feeding behavior.
The diameter of the papillae observed in Z. brevirostris (CT = 50 cm) is similar to that found in adults blue sharks (~200 μm) (CT = 200 cm) (Rangel et al. in prep.), showing that the size of the papilla does not correlate with the overall length of the animal, since larger specimens do not necessarily have the largest papillae. However, ontogenetic studies have revealed that this structure does increase in diameter during development.
Oral denticles
The presence of denticles in the oropharyngeal cavity in Z. brevirostris suggests that this structure may have the following functions: protecting against abrasion and parasites, improved grasp and holding of prey and reduced hydrodynamic drag, as suggested for other species (Atkinson and Collin 2012). The morphological characteristics of the denticles in Z. brevirostris (monocuspids, with the presence of prominent folds forming well-defined furrows) are similar to that found in pelagic sharks with RAM ventilation (Atkinson and Collin 2012; Ciena et al. 2015; Rangel et al. in prep.) and also observed for other guitarfish, Trygonorrhina fasciata and Aptychotrema rostrata (Atkinson and Collin 2012). These morphological features suggest that the denticles have a hydrodynamic function during feeding and swimming, since the presence of keels reduces water friction (Lang et al. 2012).
Many morphological characters in Rhinobatiformes are intermediate between sharks and batoids (Compagno 1977; Shirai 1996; McEachran et al. 1996), among them are oral denticles, suggesting that the structure was maintained in guitarfishes due to its feeding behavior combined with ventilation. Wilga and Motta (1998) demonstrated important behaviors through feeding experiments in Rhinobatos lentiginosus that could be extrapolated to all Rhinobatidae including Z. brevirostris, allowing the elaboration of some theories about how the distribution and morphology of oral denticles can influence behavior. For R. lentiginosus, (1) the entire feeding event, comprising the prey capture, manipulation, suction or compression, is longer compared to other elasmobranch species; (2) sequences of movements performed suggest that R. lentiginosus chews their prey, differing from the norm in elasmobranchs; (3) food compression is similar to the tongue lifting phase during swallowing in terrestrial vertebrates; (4) dissociation between the basihyal cartilage and hyomandibula in batoids allows the gill cavity to move independently of the jaws, permitting ventilatory movements simultaneously to the prey processing (Wilga and Motta 1998).
The Z. brevirostris is considered to be a generalist, with a wide range of prey in their diet (Bornatowski et al. 2014). The ingestion of bony fish is uncommon (about 6 % of total stomach content). Mollusk ingestion is also low (7 %). On the other hand, the consumption of polychaetes, such marine soft body worms, is high (about 41 % of stomach content). However, the most abundant prey for the species is crustaceans (45 %) (Bornatowski et al. 2014).
Despite this knowledge of stomach contents, food preference has never been studied in Z. brevirostris. Aforementioned stomach content analyses revealed readily available prey, but did not necessarily reflect preferred diet. Since this species is benthic and often consumes hard shell prey (with chitinous exoskeleton), the buccal apparatus must present morphological and functional adaptations that allow not only the maceration of such hard exoskeletons, but also enable the animal to ingest substrate particles without abrasion or damage to the oral papillae. The presence of keeled monocuspid denticles would be advantageous not only in suction mechanisms and RAM ventilation during prey capture, but also in protection of oral epithelium during prey crushing, compression and ingestion.
Guitarfish and sawfish (Pristiformes), basal taxa among batoids (McEachran et al. 1996), are the only batoids that feature oral denticles in the oropharyngeal cavity (Deynat 2005; Atkinson and Collin 2012; Rangel et al. in prep.). The electrosensory system, represented by ampullae of Lorenzini, is also similar between in Rhinobatidae and Pristiformes (Rangel et al. in prep.; Wueringer et al. 2011), leading us to believe that both groups are in fact closely related (phylogenetically). In derived stingrays (Myliobatiformes order) (McEachran et al. 1996), the oral denticles are absent (Atkinson and Collin 2012), and some Rajiformes such as Atlantoraja cyclophora and A. platana (Arhynchobatidae) possess only oral papillae (Rangel et al. in prep.).
In batoids, there is an increased role of the gill arches during feeding, due to restricted movements of the jaw (Dean et al. 2007). Due to the lack of a lingual structure in elasmobranchs, prey is processed by rhythmic contractions of the gill arches and jaw, creating a coordinated water flow that allows the separation of edible parts (Dean and Motta 2004; Dean et al. 2005). Denticles observed in the gill arches have their apex facing the oropharyngeal cavity, suggesting that the arrangement is advantageous during feeding, with water flow via gills assisting in food manipulation.
Conclusions
The distribution and morphology of oral papillae and the presence of oral denticles in Z. brevirostris may be an adaptive reflection shaped by feeding habits, capture strategies and prey processing. The reduced number of oral papillae may reflect the generalist nature of the species. The presence of denticles in the oropharyngeal cavity and gill arches suggests that this structure can protect and facilitate prey intake. The denticles in this species are similar to those found in pelagic sharks with RAM ventilation, so such conformation may represent between batoids and sharks.
References
Atkinson CJL (2011) The gustatory system of elasmobranchs: morphology, distribution and development of oral papillae and oral denticles. Ph.D. Thesis. University of Queensland
Atkinson CJL, Collin SP (2010) Taste: Vertebrates. In: Breed MD, Moore J (eds) Encyclopedia of animal behavior, vol III. Academic Press, Oxford, pp 386–393
Atkinson CJL, Collin SP (2012) Structure and topographic distribution of oral denticles in elasmobranch fishes. Biol Bull 222:26–34
Bigelow HB, Schroeder WC (1953) Sawfishes, guitarfishes, skates and rays, vol 1 de Memoir. Sears Foundation for Marine Research, New Haven
Bornatowski H, Natascha W, Carmo WPD, Abilhoa V, Corrêa MFM (2014) Feeding comparisons of four batoids (Elasmobranchii) in coastal waters of southern Brazil. J Mar Biol Assoc UK 1:1–9
Ciena AP, Rangel BS, Bruno CEM, Miglino MA, Amorim AF, Rici REG, Watanabe I (2015) Morphological aspects of oral denticles in the Sharpnose shark Rhizoprionodon lalandii (Müller and Henle, 1839) (Elasmobranchii, Carcharhinidae). Anat Histol Embryol. doi:10.1111/ahe.12178
Collin SP (2012) The neuroecology of cartilaginous fishes: sensory strategies for survival. Brain Behav Evol 80:80–96
Compagno LJV (1977) Phyletic relationships of living sharks and rays. Am Zool 17:303–322
Compagno LJV (2005) Checklist of living chondrichthyes. In: Hamlett WC (ed) Reproductive biology and phylogeny of chondrichthyes. Sharks, batoids and chimaeras. Science Publishers Inc, Plymouth, pp 503–548
Cook MH, Neal HV (1921) Are the taste-buds of elasmobranchs endodermal in origin? J Comp Neurol 33:45–63
Daniel JF (1928) The elasmobranch fishes. University of California Press, Berkeley
Dean MN, Motta PJ (2004) Feeding behavior and kinematics of the lesser electric ray, Narcine brasiliensis. Zoology 107:171–189
Dean MN, Wilga CD, Summers AP (2005) Eating without hands or tongue: specialization, elaboration and the evolution of prey processing mechanisms in cartilaginous fishes. Biol Lett 1:357–361
Dean MN, Bizzarro JJ, Summers AP (2007) The evolution of cranial design, diet, and feeding mechanisms in batoid fishes. Integr Comp Biol 47:70–81
Deynat PP (2005) New data on the systematics and interrelationships of sawfishes (Elasmobranchii, Batoidea, Pristiformes). J Fish Biol 66:1447–1458
Fahrenholz C (1915) Über die Verbreitung von Zahnbildungen und Sinnesorganen im Vorderdarm der Selachier und ihre phylogenetische Beurteilung. Jena Z Naturwiss 53:389–444
Ferrando S, Gallus L, Gambardella C, Masini MA, Cutolo A, Vacchi M (2012) First detection of taste buds in a chimaeroid fish (Chondrichthyes: Holocephali) and their Gαi-like immunoreactivity. Neurosci Lett 517:98–101
Figueiredo JL (1977) Manual de peixes marinhos do Brasil—I. Introdução: cações, raias e quimeras. Museu de Zoologia, Universidade de São Paulo, São Paulo, pp 1–105
Gardiner JM, Hueter RE, Maruska KP, Sisneros JA, Casper BM, Mann DA, Demski LS (2012) Sensory physiology and behavior of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives, vol I, 2nd edn. CRC Press, Boca Raton, pp 349–401
Hart NS, Collin SP (2015) Sharks senses and shark repellents. Integr Zool 10:38–64
Hueter RE, Mann DA, Maruska KP, Sisneros JA, Demski LS (2004) Sensory biology of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Boca Raton, FL, pp 325–368
Imms AD (1905) On the oral and pharyngeal denticles of elasmobranch fishes. Proc Zool Soc Lond 1:41–49
Kotrschal KK (1996) Solitary chemosensory cells: Why do primary aquatic vertebrates need another taste system? Trends Ecol Evol 11:110–114
Lang A, Habegger ML, Motta P (2012) Shark skin drag reduction. In: Bhushan B (ed) Encyclopedia of nanotechnology, part 19. Springer, Berlin, pp 2394–2400
McEachran JD, Miyake T, Dunn KA (1996) Interrelationships of the batoid fishes (Chondrichthyes: Batoidea). In: Stiassny MLJ, Parenti LR, Johnson GD (eds) Interrelationships of fishes. Academic Press, New York, pp 63–84
Moss SA (1977) Feeding mechanisms in sharks. Am Zool 17:355–364
Müller J, Henle FGJ (1841) Systematische Beschreibung der Plagiostomen. Veit und Comp, Berlin, i–xxii, pp 103–200
Nelson GJ (1970) Pharyngeal denticles (placoid scales) of sharks, with notes on the dermal skeleton of vertebrates. Am Mus Novit 2415:1–26
Northcutt RG (2004) Taste buds: development and evolution. Brain Behav Evol 64:198–206
Pevsner RA (1976) Electron microscope study of the taste buds of Elasmobranches, Trzgongastinaca and Raja clavota. Tsitologiya 18:560–566
Reutter KW, Breipohl W, Bijvank GJ (1974) Taste bud types in fishes. Cell Tissue Res 153:151–165
Santos C, Cortellete GM, Araújo KCB, Spach HL (2006) Estrutura populacional da raia-viola Zapteryx brevirostris (Chondrichthyes, Rhinobatidae), na Plataforma adjacente à Baía de Paranaguá, PR. Acta Biol Leop 28:32–37
Shirai S (1996) Phylogenetic interrelationships of Neoselachian (Chondrichthyes: Euselachii). In: Stiassny MLJ, Parenti LR, Johnson GD (eds) Interrelationships of fishes. Academic Press, New York, pp 9–32
Thies D, Leidner A (2012) Sharks and guitarfishes (Elasmobranchii) from the Late Jurassic of Europe. Palaeodiversity 4:63–184
Todaro F (1872) Die Geschmacksorgane der Rochen. Zenthl med Wiss No 15: 227–229
Vooren CM, Lamónaca AF, Massa A, Hozbor N (2006) Zapteryx brevirostris. In: IUCN 2014. IUCN red list of threatened species. Version 2014.1. www.iucnredlist.org. Access 26 June 2014
Whitear M, Moate RM (1994a) Microanatomy of taste buds in the dogfish, Scyliorhinus canicula. J Submicrosc Cytol Pathol 26:357–367
Whitear M, Moate R (1994b) Chemosensory cells in the oral epithelium of Raja clavata (Chondrichthyes). J Zool (Lond) 232:295–312
Wilga CD, Motta PJ (1998) Feeding mechanism of the Atlantic guitarfish Rhinobatos lentiginosus: modulation of kinematic and motor activity. J Exp Biol 201:3167–3184
Wilga CD, Motta PJ, Sanford CP (2007) Evolution and ecology of feeding in elasmobranchs. Integr Comp Biol 47:55–69
Wueringer BE, Peverell SC, Seymour J, Squire SL, Kajiura SM, Collin SP (2011) Sensory systems in sawfishes. 1. The ampullae of Lorenzini. Brain Behav Evol 78:139–149
Acknowledgments
We would like to thank CNPq for Scientific initiation scholarship, CAPES for the Ph.D. scholarship, the postgraduate program of Department of Surgery, Faculty of the Veterinary Medicine and Animal Science from University of São Paulo and postgraduate program in Aquaculture and fishing in Instituto de Pesca. We also would like to thank MSc. Julia Whidden for reviewing the English, and the fisherman and professor Jorge Luís Santos for the donation of the animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Rights and permissions
About this article
Cite this article
Rangel, B.d., Ciena, A.P., Wosnick, N. et al. Ecomorphology of oral papillae and denticles of Zapteryx brevirostris (Chondrichthyes, Rhinobatidae). Zoomorphology 135, 189–195 (2016). https://doi.org/10.1007/s00435-016-0304-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-016-0304-0