Abstract
The planula larva of the hydroid Clava multicornis (Forskål, 1775) has a complex nervous system, characterized by the presence of distinct, anteriorly concentrated peptidergic populations of amidated neurons, presumably involved in the detection of environmental stimuli and metamorphic signals. Differently from other hydrozoan larvae in C. multicornis planulae GLW-positive cells with putative sensory role have a peculiar dome-shaped forefront organization, followed by a belt of RF-positive nerve cells. By immunohistochemistry, we investigated the transformation of the peptidergic (GLW-amide and RF-amide) larval neuroanatomy at different stages of metamorphosis and the subsequent development of the primary polyp nervous system. By terminal transferase-mediated dUTP nick end-labeling assay, apoptotic nuclei were first identified in the anterior pole of the settled larva, in the same region occupied by GLW-amide positive putative sensory cells. In primary polyps, GLW-amide positive signals first encircled the hypostome area, later extending downwards along the polyp column or upwards over the hypostome dome, whereas RF-amide positive sensory cells initially appeared at the tentacles base to later extend in the tentacles and the polyp column. In spite of the possession of distinct neuroanatomies, different cnidarian planulae may share common developmental mechanisms underlying metamorphosis, including apoptosis and de novo differentiation. Our data confirm the hypothesis that the developmental dynamics of tissue rearrangements may be not uniform across different taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual reproduction in most cnidarians gives rise to a motile, non-feeding planula larva, that usually undergoes an abrupt metamorphosis with polarity reversal to become a sessile polyp. The metamorphic process consists of at least three distinct phases: (a) the planula gains competence for metamorphosis and change behavior (e.g. negative vs. positive phototaxis, positive geotaxis), (b) a searching phase, when the planula locates appropriate settlement sites driven by physico-chemical cues, and (c) the proper metamorphosis, when drastic anatomical changes occur and the primary polyp develops by reverting the planula anterior and posterior poles, respectively, into polyp aboral and oral poles (Leitz 1997; Grasso et al. 2011).
Beside its general pattern, the extent and duration of metamorphic changes can vary in different cnidarians, independently of any phylogenetic constraint. In the anemone Nematostella vectensis, the transition from the motile larva to the polyp stage is continuous rather than discrete and no specific settlement cues seems to be required (Hand and Uhlinger 1992; Muller and Leitz 2002). On the contrary, in reef-building corals a complex, extensive tissue remodeling occurs, especially at the aboral pole, involving drastic changes in morphology (Grasso et al. 2011). Comparably, the planula of the hydrozoan Eirene hexanemalis undergoes a gradual transition to a pelagic solitary polyp stage (Bouillon 1983), whereas in the colonial hydrozoan Hydractinia echinata, larval metamorphosis is associated with intense apoptotic events and re-differentiation of several cell types, including a dramatic rearrangement of the nervous system (Seipp et al. 2001). Evidence of programmed cell death throughout embryogenesis is missing in the direct development of Hydra spp. (Brumwell and Martin 2002). More generally, early development in different cnidarians appears a highly dynamic process shaped by natural selection toward the adaptation to specific ecological conditions. Thus, any information about the mechanisms of metamorphosis from different cnidarian species can be useful in order to disclose shared or taxon-specific features.
Clava multicornis (Forskål, 1775) is a hydroid species lacking a medusa stage. Egg fertilization occurs directly within the gonophores on female polyp colonies, where embryonic development takes place. Fully formed planulae hatch 48–72 h post fertilization and, differently from most cnidarian larvae, they do not swim in the water column but crawl on the substrate in the search for a suitable site for settlement. Newly hatched planulae of C. multicornis have no competence for metamorphosis, which is generally gained three or more days after liberation (Orlov 1996; Piraino et al. 2011). When a suitable site is recognized, the competent larva settles, attaching to the substrate by the anterior pole. At metamorphosis, the anterior larval pole develops into the basal foot of the polyp, while the larval posterior pole gives rise to the apical polyp structures: mouth and tentacles.
The planula larva of C. multicornis revealed to possess a surprisingly complex nervous system (Piraino et al. 2011). Sensory-motor neurons and ganglionic cells have been recognized by electron microscopy and by immunolocalization: (a) ciliated ectodermal sensory cells; (b) a basiectodermal neuropile with a synaptically dense region, mostly composed by axons and dendrites; (c) distinct sets of bipolar ganglionic cells whose fibers project along the main body axis (Piraino et al. 2011). Distinct populations of neurons have been characterized by the detection of different neuropeptides.
Neuropeptides are internal signal molecules that regulate numerous physiological processes in cnidarians, including metamorphosis, and have been isolated from hydrozoan, scyphozoan, and anthozoan taxa (Grimmelikhuijzen et al. 2002). All neuropeptides have a C-terminal amidated group, which protects against C-terminal degradation and is important also for receptor recognition. A peptide with a Gly–Leu–Trp–NH2 terminal was first isolated in the anthozoan Anthopleura elegantissima and named metamorphosin A (MMA) for its capability to induce metamorphosis (Leitz et al. 1994). Subsequently, it was discovered that MMA was a member of a novel family of neuropeptides, the GLW-amides (Leitz 1998).
GLW-amides are not exclusive cnidarian peptides, as they were detected also in rat brain (Hamaguchi-Hamada et al. 2009) and in Caenorhabditis elegans (Husson et al. 2005), where their function is still unknown.
In the larvae of the hydrozoan H. echinata, GLW-amide is synthesized in ectodermal sensory cells located in a belt-like fashion in the anterior part of the body (Leitz and Lay 1995) to act as a paracrine signal coordinating the metamorphic process (Schmich et al. 1998). Interestingly, the organization of GLW-amide-immunoreactive cells in H. echinata is quite distinct from a typical dome-shaped apical arrangement observed in C. multicornis (Piraino et al. 2011).
Another important family of neurosignals, widely distributed in cnidarians as well as in other invertebrates, is formed by peptides with an Arg–Phe–NH2 carboxy-terminus (RF-amides). RF-amides were first described in mollusks (Price and Greenberg 1977) and lately found in all investigated cnidarians. Immunolabeling studies showed the occurrence of RF-amides in localized areas or throughout entire nerve nets of Hydrozoa, Anthozoa, and Scyphozoa (see Grimmelikhuijzen et al. 2002 for a review). RF-amide positive neurons form a stable, circumferential ring in Hydra’s hypostome (Koizumi et al. 1992). Immunoreactive plexuses were described at the base of cnidocyte assemblages and individual cnidocytes in different cnidarian species. Because of this distribution, it has been suggested that RF-amide positive neurons are involved in chemosensory regulation of cnidocyte discharge (Anderson et al. 2004). In the anthozoan Renilla koellikeri, anti-RF-amide-immunostained neurons were observed in different compartments of the ectoderm, particularly on the oral side of the tentacles and near the mouth of the feeding polyps (Pernet et al. 2004). In this species, it was suggested an involvement of RF-amide in contractile activities associated with tentacle movements during food capture.
RF-amide reactive neurons were also found in planula larvae: in Pennaria tiarella, immunofluorescence was localized in anterior and upper middle regions of the planula, in bipolar and multipolar ganglionic cells, sensory, cells and epitheliomuscular cells (Martin 2000). Plickert and Schneider (2004) surveyed LW and RF immunoreactivity in different hydrozoan, scyphozoan, and cubozoan jellyfish photoreceptor organs and found that RF-amide was involved in the transmission of the photic stimuli to epitheliomuscular cells from the complex eyes of the cubozoan jellyfish Tripedalia cystophora and from some unknown photosensory cells in the H. echinata hydrozoan larvae.
In C. multicornis planulae, two distinct populations of GLW-amide and RF-amide positive cells were described (Piraino et al. 2011): GLW-amide immunoreactive neurons with a fusiform shape are organized in a peculiar dome-like organization in the forefront pole of the larva. On the other hand, a belt-like RF-amide positive cell population occurs just posterior to the GLW-positive cells, a distribution pattern opposite to what is known in H. echinata larvae (Schmich et al. 1998).
Here, we describe the morphological changes, the localization of GLW-amide and RF-amide peptides during the metamorphic transition of C. multicornis planulae, and the occurrence of apoptosis throughout the larval tissue reorganization. Our results provide comparative information to analyze the metamorphic neural rearrangement in planulae larvae of different hydrozoan species and the potential occurrence of shared developmental mechanisms.
Materials and methods
Animals
Colonies of C. multicornis (Hydrozoa, Cnidaria) are common epibionts on Ascophyllum nodosum and Fucus spp. seaweeds along the Northern European rocky shores, where polyp colonies can be collected at low tide and can be easily maintained in laboratory in filtered seawater (FSW). Naturally released planulae, less than 12 h old, were collected and treated in artificial seawater (ASW) with 116 mM CsCl for 3 h to induce metamorphosis (Schmich et al. 1998, 2007). Larvae were kept at 15 °C in a refrigerated incubator throughout the experiments. Starting from 4 h post-induction (hpi) up to 5 days post-induction, different metamorphic larval stages and developing polyp stages were fixed at regular intervals (from 2 to 24 h intervals) in 4 % paraformaldehyde in 0.1 M PBS (pH 7.2 for 16–24 h, at T = 4 °C).
SEM
For scanning electron microscopy (SEM), the planulae were fixed with a mixture of paraformaldehyde and glutaraldehyde in picric acid (SPAFG) (Ermak and Eakin 1976). Transferred onto coverslips coated by poly-l-lysine (to enhance adhesion of specimens: Mazia et al. 1975), specimens were washed in 0.1 M cacodylate buffer (pH = 7.4). Graded dehydration with ethanol was followed by replacement with hexamethyldisilazane (HDMS). Following removal of HDMS, specimens were air-dried over night. The coverslips processed in this way were glued with silver paint to SEM stubs, coated with gold, and observed with a Leo 1430 scanning electron microscope.
Immunohistochemistry
Fixed samples were re-hydrated and then incubated with 0.1 % Tween-20, 0.25 % Triton X-100 in 0.1 M PBS (PBT) for 30 min. After three washes for 10 min each with PBS, the samples were blocked with 10 % normal goat serum in PBS (PBS–NGS) for 2 h and finally incubated overnight at 4 °C with gentle rocking, in the first primary antibody diluted in PBT–NGS. The primary antibodies and the dilutions used were as follows: mouse monoclonal anti-acetylated tubulin (clone 6–11B-1, SIGMA Italy) diluted 1:500; rabbit polyclonal anti-GLW-amide antibody and rabbit polyclonal anti-RF-amide antibody (gifts from Prof. Thomas Leitz, Germany) both diluted 1:700. After the incubation with the first primary antibody and three replicate washes in PBS–NGS, the samples were incubated (T = 4 °C) for 12–14 h with the second primary antibody and then overnight with a mix of the two secondary antibodies (T = 4 °C). The secondary antibodies used were as follows: goat anti-rabbit Alexa Fluor 488 IgG diluted 1:200; goat anti-mouse Alexa Fluor 594 IgG diluted 1:400 (Invitrogen, Italy). Finally, the specimens were washed four times in PBS for 1 h and mounted in 1,4-diazabicyclo[2,2,2]octane (DABCO, Sigma, Italy) on microscope slides. Immunolabeled specimens were visualized using a laser scanning confocal microscopy system (Leica TCS-NT). Fluorescence was detected using the combinations of 488-nm excitation filter and 530/30 nm band pass filter, or 530–560 nm excitation filter and 590/50 nm band pass filter. Multiple optical sections (1 μm) were taken through the depth of the specimen (Z axis). Confocal stack images were recorded and post-processed using a Leica Lite Confocal Software (Leica Hidelberg, Germany). Series of optical sections obtained by scanning whole-mount specimens were projected into one image with greater focal depth. In negative control samples incubated either with the primary or the secondary antibody alone, no fluorescent signal or staining was observed (data not shown).
Whole-mount TUNEL assays
The DNA fragmentation study was performed by using the terminal transferase-mediated dUTP nick end-labeling (TUNEL) assay, according to the protocol recommended by the manufacturer (Boehringer, Mannheim, Germany).
For TUNEL experiments, fixed larvae were washed three times with PBS (pH 7.4), then treated with 500 μl of permeabilization solution (0.1 % Triton X-100 in 0.1 % sodium citrate) for 15 min on ice. Incubations with the enzyme reaction mix containing the terminal desoxynucleotidyl transferase (TdT) and FITC-labeled dUTP were carried out for 1 h at 37 °C in the dark with gentle movement. Planulae covered with TUNEL mix on coverslips were incubated in a humidification chamber. As a positive control, some animals were incubated in DNAse I solution (1 mg/ml in water) for 10 min at room temperature before application of the reaction mix. In some cases, the second wash buffer of the following washes was supplemented with 2 μg/ml propidium iodide and incubated for 3 min to counterstain the nuclei. Larvae were applied to slides, embedded in Mowiol/DABCO (Mowiol/1,4-diazabicyclo-(2,2,2) octane), and analyzed by fluorescence microscopy with filters appropriate for FITC detection.
Results
SEM morphology
By the 116 mM cesium chloride incubation, most competent planulae of C. multicornis rapidly underwent metamorphosis. During this process, the planula morphology abruptly changed by passing through a typical series of distinct morphological stages: elongated crawling larva; shortened settled larva; dish-pile stage; barrel stage; polyp primordium; and finally, the fully formed primary polyp.
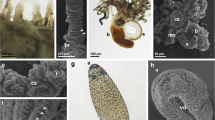
The crawling larva of C. multicornis is 600–800 nm long, cigar-shaped with a club-shaped anterior pole (Fig. 1a). The larval body is entirely covered by a ciliated epithelium (Fig. 1b). The cilia activity supports the gliding, anteriorly directed movement of the larva. Approximately 6 h post-induction (p.i.), the planula arrested its typical crawling behavior and settled by adhering to the substrate by its anterior pole. By 24 h p.i., settling larvae were completely attached and shortened to more than half of their original length. The planula posterior end, facing upward, became rounded. At this stage, the planula ectoderm recalled a “dish-pile” structure (Fig. 1c), with marked ectodermal foldings; at this stage, the epidermal cilia disappeared (Fig. 1d).
Metamorphosis stages of C. multicornis observed by SEM. a Crawling larva, anterior is on the right. b Particular at high magnification of the outer surface of the larva showing the numerous cilia. c “Dish-pile” stage characterized by ectodermal folds. The sample is oriented with the prospective oral opening upwards. d Enlargement of a piece of ectoderm showing the absence of cilia. e “Barrel-shape” stage showing tentacle buds (asterisks). The pedal disk was still attached to a piece of fucus alga (arrowhead). f Developing polyp with recognizable tentacles. The sample is observed from the oral pole. g Primary polyp with eight fully developed tentacles. h Detail of cnidocilia present on the tentacles
Forty-eight hours p.i., the whole planula body gained a rounded barrel morphology, and the ectodermal foldings disappeared. A basal (pedal) adhesion disk and a central (gastric) column could be distinguished, and early tentacle buds appeared at the upper, prospective oral pole (Fig. 1e). Seventy-two hours p.i., the small buds developed in 3–6 tentacles of various lengths, whereas the hypostome was protruding at the center of the tentacle area (Fig. 1f).
Five days after induction, the final shape of the primary polyp was fully recognizable: The hydranth elongated, additional tentacles were developed and the mouth opening broke through at the apical tip of the hypostome, whereas the basal stalk narrowed (Fig. 1g). Functional cnidocytes, characterized by outward-directed cnidocilia, were already positioned along the tentacles epidermis and on the hypostome (Fig. 1h).
Rearrangement of GLW-amide and RF-amide peptidergic subsytems
In order to investigate the fate of the larval nervous system during metamorphosis, C. multicornis larvae induced to metamorphosis by CsCl incubation were harvested for variable periods of time and processed for immunolocalization of neuropeptides by specific antibodies against GLW-amide and RF-amide peptides. As a counterstain, the antiacetylated a-tubulin antibody has been used here because it is known as generalized marker for ciliated ectoderm (Piperno and Fuller 1985) and for cnidarian nerve cells and fibers (see Piraino et al. 2011, and references therein).
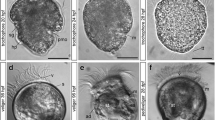
In control larvae, numerous GLW-amide positive cells were organized in a typical dome-shaped sensory array at the anterior pole (Fig. 2a). These elongated cells had slender perikaria arranged perpendicular to the mesoglea and extending toward the outer surface (Fig. 2b). Their basal part extended into immunoreactive nerve processes of the anterior neural plexus located on the mesoglea. Ganglionic interneurons with a triangular shape were observed in the middle and posterior part along positive fibers running longitudinally lengthways the body of the planula (Fig. 2a). Twenty-four hours p.i., newly settled planulae showed a dramatic reduction in their sensory anterior pole: according to the detected immunoreactive pattern, the anterior GLW-amide positive cells lost their elongated shape and the outer projection. Basally projected axons were not detectable anymore. Moreover, the ganglionic positive interneurons previously recorded in the middle-posterior part of the planula and their immunoreactive fibers also disappeared (Fig. 2c, d).
Immunolocalization of GLW-amide neuropeptides (green signal) during metamorphosis of C. multicornis. Acetylated tubulin immunolocalization (red signal) is shown in figures c–l as a counterstaining. Images are sums of 30 optic sections acquired by laser scanning confocal microscopy. a, b Crawling larva. a Whole-mount sample with the anterior sensory pole on the right.Asterisks indicate the ganglionic interneurons. b Magnification of the anterior end of A showing a dome of fusiform GLW-amide positive cells. c, d “Dish-pile” stage. c Whole-mount sample. d Magnification of the prospective pedal disk where GLW-amide signal was still present. e “Barrel-shape” stage. GLW-amide immunoreactive signal was non-detectable. f, g Developing polyp, showing GLW-amide signal at the base of the hypostome. h-l Primary polyp. i, l Details of GLW-amide immunoreactive fibers in the gastric column and in hypostome, respectively
Forty-eight hours p.i., the GLW-amide immunoreactivity was almost completely lost (Fig. 2e). One day later (72 h p.i.), when the polyp tentacles appeared, a few positive cells appeared at the basis of the hypostome region (Fig. 2f, g). Finally, 5 days after settlement, positive cells were newly detectable along the gastric column (Fig. 2h, i) and in the hypostome region (Fig. 2l). GLW-amide positive signals were never observed in the tentacles (Fig. 2h).
RF-amide positive sensory neurons in the control larvae formed a belt in the anterior pole of the larva, shortly beyond the GLW-amide positive cell dome. These RF-amide cells were elongated with the distal end reaching the outer surface (Fig. 3a, c). Several positive fibers run along the main axis of the larval body, connecting to the anterior neural plexus. Along these fibers, few ganglionic positive cells were present (Fig. 3a). Twenty-four hours p.i., a diffuse signal was present in the formerly anterior region, where the basal disk will differentiate, but cell bodies were unrecognizable (Fig. 3b, d). Moreover, the RF-amide positive fibers disappeared (Fig. 3b). Forty-eight hours p.i., RF-amide positive cells were still lacking from the barrel-shaped polyp primordium (Fig. 3e). Seventy-two hours p.i., some RF-amide positive cells appeared at the basis of the tentacle buds (Fig. 3f, g). Five days after settlement, positive cells were scattered all along the tentacles and in the upper region of the hypostome, near the mouth opening, with fibers longitudinally extending toward the base of the polyp column (Fig. 3h, i).
Immunolocalization of RF-amide (green signal) neuropeptides through metamorphosis of C. multicornis. Acetylated tubulin immunolocalization (red signal) is shown as a counterstaining. Images are sums of several optic sections acquired by laser scan confocal microscopy. a Anterior part of a crawling planula, showing the belt of positive fusiform cells. b “Dish-pile” stage; RF-amide signal was faint but still recognizable at the former anterior pole. c Detail of the RF-amide positive cells of a crawling planula. d Detail of basal disk of a “dish-pile” stage sample. e “Barrel-shape” stage, RF-amide signal was non-detectable. f, g Developing polyp, RF-amide signal appeared in the tentacles and in the hypostome. h, i Primary polyp, an intense signal was present in the upper region of the hypostome, near the oral opening. i Positive fibers were observed along the tentacles
Apoptosis
TUNEL analysis has been used to identify apoptotic cells in both crawling and metamorphosing larvae (Fig. 4). TUNEL reaction was negative in control planulae (Fig. 4a), whereas a positive TUNEL signal was evident in settled planulae (24 h p.i.) in a large population of cells, mostly concentrated at the anterior pole of the larva (Fig. 4b). The distribution of staining for apoptosis mostly coincides with the anterior location where GLW-amide and RF-amide immunoreactivity is progressively disappearing (Figs. 2c, d, 3b–d) supporting the hypothesis of a degeneration of the neurosensory and ganglionic cells rather than an interruption of the synthesis of the two peptides. Forty-eight hours p.i., the apoptotic signal spread across the planula ectoderm and endoderm toward the posterior pole, confirming the occurrence of a substantial epithelial remodeling involving programmed cell death (Fig. 4c). In the “barrel-shape” stage (72 h p.i.), apoptotic nuclei were still scattered across large part of the larval ectoderm (Fig. 4d), while in the primary polyp primordium (characterized by the appearance of tentacle buds), apoptosis was restricted into a belt-like pattern in the middle portion of the newly formed gastric column (Fig. 4e). When polyp tentacles were fully developed (5 day p.i.), apoptosis was limited to few cells in the hypostome midline, where the mouth opening was formed (Fig. 4f).
Apoptotic cells during metamorphosis identified by TUNEL staining. a Swimming larva counterstained with propidium iodide (red signal). Only endodermic cells show an apoptotic signal. b Attached planula, 24 h post-induction (p.i.), the apoptotic signal was evident in a large population of cells concentrated at the anterior pole. c In “dish-pile” stage, 48 h p.i., apoptotic signal was spread in all the ectoderm. d In barrel-shape stage, 72 h p.i., apoptotic nuclei were still diffused in all the ectoderm. e Developing polyp in which TUNEL signal was present in a belt of cells in the middle of the gastric column (arrow). f Five day p.i., apoptosis was limited to few cells within the hypostome, where the mouth opening was formed (arrow). Insertions (in d–f show transmission microscope images of corresponding stages
Discussion
Stages and morphology of metamorphosis
We classified metamorphosis stages of C. multicornis according to the morphological changes observed by SEM as for the description of this process in the hydroid H. echinata (Seipp et al. 2007). The most strikingly observations during the first stage of settlement were the disappearance of the cilia from the ectoderm and the “dish–pile,” rugose appearance of the ectoderm. As it was observed in a comparable stage in H. echinata, this peculiar shape may be not simply attributed to an artifact during fixation, but it could reflect a first signal of the metamorphic rearrangement of the ectoderm. The shortening continued in the following stage, when the posterior end became rounded, the ectoderm lost the foldings and a smooth surface of the gastric column was then recognizable in the primary polyp primordium. Five days post-induction, a primary polyp was fully formed. Throughout metamorphosis, immunoreactive signals for both GLW- and RF-amidated peptides gradually disappeared from the anterior sensory cells of the larva and reappeared in newly formed oral structures of the polyp, in particular in the hypostome and in the tentacles. It may be possible that neural cells (a) interrupt the synthesis of neuropeptides during metamorphosis, or (b) that at least part of GLW- and RF-amide positive larval cells are eliminated and novel neural populations are differentiated de novo in the polyp, in accordance with what observed in H. echinata (Seipp et al. 2010). To corroborate this second hypothesis, apoptosis signals were particular evident from anterior pole of the settled larva, coincidently with the distribution of GLW- and RF- amide positive cells in the crawling larva.
In the succeeding stages, apoptotic nuclei were found also on both epithelia of the posterior end of planula. The intense apoptosis observed in the “barrel-shaped” stage is certainly not restricted to the nerve cell populations, but it deals with other cell types involved in the reorganization of the entire larval body. This can be considered as the stage undergoing the most substantial developmental reprograming.
Apoptosis is the canonical way of neuronal cell death in vertebrates. In lower invertebrates, information regarding the fate of neuronal cells of embryonic and larval stages is scarce. Recently, by double-labeling with anti-GLW-amide and anti-RF-amide antibodies together with TUNEL, Seipp et al. (2010) convincingly demonstrated that in H. echinata, a number of the larval anterior neurons are eliminated by apoptosis during the early stages of metamorphosis. Later, the majority of the ectoderm undergoes massive apoptosis, preventing any distinction of cell types involved in the planula-polyp tissue remodeling. This major apoptotic event starts from the posterior tip of the larva and eventually spread anteriorly (Seipp et al. 2001, 2010). The anterior neural architecture of the C. multicornis planula seems to be more conspicuous than in H. echinata (Piraino et al. 2011), and this difference may explain the intense apoptotic signal observed in the anterior pole of C. multicornis (Fig. 4b). In disk stage, grossly corresponding to barrel-shape stage of C. multicornis, the apoptotic staining dramatically diminished. Differently, in the scyphozoan Aurelia aurita, it is the planula endoderm, but not ectoderm, which becomes strongly caspase-3 immunoreactive (Yuan et al. 2008). Although caspase-3 detection did not unambiguously testify occurrence of apoptosis, it can be hypothesized that cells of Aurelia planula endoderm may undergo apoptosis during metamorphosis, and that the primary polyp endoderm may therefore originate by a “secondary gastrulation” from a pool of poorly differentiated cells of the planula ectoderm. However, compared with the FMRF-amide-immunoreactive nervous system of the Aurelia planula, the apical sensory cells in C. multicornis appear to be more abundant, located in a narrower belt and mostly clustered into two contralateral domains (Piraino et al. 2011). Thus, not only the larval neuroanatomies, but also the developmental dynamics of tissue rearrangements during metamorphosis do not appear to be uniform across different cnidarian planulae.
GLW-amidated and RF-amidated peptides distribution is different in the larva and in the polyp
There is a substantial discrete distribution of GLW-amide and of RF-amide neurons both in the planula and in the primary polyp. This distinctive pattern of distribution of the two neuropeptides suggests the existence of two neural populations with different morphological and biochemical properties. In C. multicornis planulae, GLW-amide positive cells are arranged in a dome-like organization in the anterior most region. Since several reports indicate the ability of GLW-amide peptides to induce metamorphosis in planulae of many cnidarian species (Leitz and Lay 1995; Iwao et al. 2002; Muller and Leitz 2002; Plickert et al. 2003), it is conceivable that the anterior GLW-amide cells are the ones receptive of the external environmental inducer, and GLW-amide is the internal signal coordinating the metamorphic process.
However, 3 days after settlement, when the first tentacles and the hypostome start to differentiate, the GLW-amide-positive cells are concentrated in the hypostome, near the future mouth, and this discrete localization remains the same after two more days. Since a metamorphosis-inducing peptide is no longer necessary in the adult polyp, it could be hypothesized that GLW-amide-positive cells retain a role as chemoreceptors, surrounding the polyp mouth.
Instead, in the polyp, RF-amide positive cells appear concentrated in the developing tentacles, both 3 days after settlement, and also when metamorphosis is more advanced.
RF-amide neuropeptides were first discovered in mollusk nervous systems, but were soon recognized to occur widely throughout the invertebrates (from cnidarians to nematodes to insects) and vertebrates too, with multiple roles, from neuromuscular transmission to the control of feeding behavior both in invertebrates and in vertebrates. This would seem to be a remarkable example of conservation of chemical structure and biological function throughout nervous system evolution (reviewed in Dockray 2004).
Neurons reactive to anti-RF-amide antibodies were found in the tentacles of species of all the cnidarian classes (Anderson et al. 2004): immunoreactive nerve plexuses were usually found localized in the proximity of cnidocyte assemblages at the tentacle bases. This observation suggested that such peptidergic neurons were involved in the chemosensory regulation of nematocyst discharge. In the nervous system of the colonial anthozoan Renilla koellikeri, anti-RF-amide immunostained neurons were observed in different compartments of the ectoderm, particularly on the oral side of the tentacles, the oral disk and the pharynx of the feeding polyps (Pernet et al. 2004). This suggested an involvement of RF-amide peptides in food capture.
A general role for the control of movement may be highly reasonable also for the planulae. In C. multicornis planulae, RF-amide sensory cells might be involved in photoreception and/or transmission of the light stimuli to the myoepithelial cells that mediate the planula lateral bending, as also hypothesized for H. echinata planulae (Plickert et al. 2003).
The distribution of the RF-amide-positive cells around the oral pole and in the developing tentacles of C. multicornis polyps is similar to the distribution observed in polyps and medusae of other cnidarian species. Further observations are needed in order to state whether these cells play a role in feeding or in the control of neuromuscular transmission or both.
The planulae and the metamorphosis stages of C. multicornis show morphological specializations (like primitive cephalization, the presence of two different neuropeptidergic populations of neurons) that differ from traditional cnidarian model system and may help for tracing the evolution of the cnidarian and bilaterian nervous systems (Piraino et al. 2011). Our results show a strong reduction in the activity of the peptidergic system of the planula larva of C. multicornis during metamorphosis, and the combination with apoptotic signals in the larval ectoderm suggests that at least part of the nervous system of the larva may degenerate at metamorphosis. In this framework, the developmental plasticity of cnidarians is paradigmatic. Besides high regenerative and asexual reproduction potentials, a number of cnidarians can undergo ontogeny reversal, or reverse development and morph rejuvenation (Piraino et al. 2004; Schmich et al. 2007) by a combination of diverse cellular processes, such as programed cell death, proliferation of undifferentiated cells, and cell transdifferentiation. Indeed, regenerative potential is not solely associated with the proliferation and differentiation of cycling embryonic cells (interstitial or I-cells). Regeneration is known be due to the remarkable transdifferentiation potential of fully differentiated cells, including sensory cells, as demonstrated for tissues of the medusa of Hydractinia carnea (reviewed in Schmid 1992; Piraino et al. 1996). In C. multicornis larvae, at least part of the metamorphosed nervous system is formed by de novo differentiation and construction of two new peptidergic subsystems, with the architectural features of a polyp nerve net. Further research, including double-labeling experiments and cell proliferation assays, will elucidate the relative contributions of newly differentiated cells and the fate of pre-existing larval cells for the formation of the primary polyp in different taxa. Metamorphosis in cnidarians is a highly dynamic event involving complex developmental processes and an unexpected array of cellular interactions. For the above reasons, understanding the metamorphic rearrangements of the nervous system in early eumetazoans would contribute to elucidate mechanisms of neural plasticity in higher metazoans, a matter of interest to a large scientific community in the fields of evolutionary biology and development as well as neurogenesis and clinical research.
References
Anderson PAV, Thompson LF, Moneypenny CG (2004) Evidence for a common pattern of peptidergic innervation of cnidocytes. Biol Bull 207:141–146
Bouillon J (1983) Sur le cycle biologique de Eirene hexanemalis (Goette, 1886) (Eirenidae, Leptomédusae, Hydrozoa, Cnidaria). Cah Biol Mar 24:421–427
Brumwell GB, Martin VJ (2002) Immunocytochemically defined populations of neurons progressively increase in size through embryogenesis of Hydra vulgaris. Biol Bull 203:70–79
Dockray DJ (2004) The expanding family of -RF-amide peptides and their effects on feeding behaviour. Exp Physiol 89:229–235
Ermak TH, Eakin RM (1976) Fine structure of the cerebral and pygidial ocelli in Chone ecaudata (Polychaeta: Sabellidae). J Ultrastruct Res 54:243–260
Grasso LC, Negri AP, Fôret S, Saint R, Hayward DC, Miller DJ, Ball EE (2011) The biology of coral metamorphosis: molecular responses of larvae to inducers of settlement and metamorphosis. Dev Biol 353:411–419
Grimmelikhuijzen CJP, Williamson M, Hansen GN (2002) Neuropeptides in Cnidarians. Can J Zool 80:1690–1702
Hamaguchi-Hamada K, Fujisawa Y, Koizumi O, Muneoka Y, Hamada S (2009) Immunohistochemical evidence for the existence of novel mammalian neuropeptides related to the Hydra GLW-amide neuropeptide family. Cell Tissue Res 337:15–25
Hand C, Uhlinger KR (1992) The culture, sexual and asexual reproduction, and growth of the sea-anemone Nematostella vectensis. Biol Bull 182:169–176
Husson SJ, Clynen E, Baggermann G, DeLoof A, Schoofs L (2005) Discovering neuropeptides in Caenorhabditis elegans by two-dimensional liquid chromatography and mass spectrometry. Biochem Biophys Res Commun 335:76–86
Iwao K, Fujisawa T, Hatta M (2002) A cnidarian neuropeptide of the GLW-amide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 21:127–129
Koizumi O, Itazawa M, Mizumoto H, Minobe S, Javois LC, Grimmelikhuijzen CJ, Bode HR (1992) Nerve ring of the hypostome in hydra. I. Its structure, development, and maintenance. J Comp Neurol 326:7–21
Leitz T (1997) Induction of settlement and metamorphosis of Cnidarian larvae: signal and signal transduction. Invertebr Reprod Dev 31:109–122
Leitz T (1998) Metamorphosin A and related peptides: a novel family of neuropeptides with morphogenetic activity. Ann NY Acad Sci 839:105–110
Leitz T, Lay M (1995) Metamorphosin-A is a neuropeptide. Rouxs Arch Dev Biol 204:276–279
Leitz T, Morand K, Mann M (1994) Metamorphosin A, a novel peptide controlling development of the lower metazoan Hydractinia echinata. Dev Biol 163:440–446
Martin VJ (2000) Reorganization of the nervous system during metamorphosis of a hydrozoan planula. Invertebr Biol 119:243–253
Mazia D, Schatten G, Sale W (1975) Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol 66:198–200
Muller WA, Leitz T (2002) Metamorphosis in the Cnidaria. Can J Zool 80:1755–1771
Orlov D (1996) Observations on the settling behaviour of planulae of Clava multicornis Forskål (Hydroidea, Athecata). In: Piraino S, Boero F, Bouillon J, Cornelius PFS, Gili JM (eds) Advances in hydrozoan biology. Sci Mar 60:121–128
Pernet V, Anctil M, Grimmelikhuijzen JP (2004) Antho-RF-amide-containing neurons in the primitive nervous system of the anthozoan Renilla koellikeri. J Comp Neurol 472:208–220
Piperno G, Fuller MT (1985) Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101:2085–2094
Piraino S, Boero F, Aeschbach B, Schmid V (1996) Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa). Biol Bull 190:302–312
Piraino S, De Vito D, Schmich J, Bouillon J, Boero F (2004) Reverse development in Cnidaria. Can J Zool 82:1748–1754
Piraino S, Zega G, Di Benedetto C, Leone A, Dell’Anna A, Pennati R, Candia Carnevali D, Schmid V, Reichert H (2011) Complex neural architecture in the diploblastic larva of Clava multicornis (Hydrozoa, Cnidaria). J Comp Neurol 519:1931–1951
Plickert G, Schneider B (2004) Neuropeptides and photic behavior in Cnidaria. Hydrobiologia 530:49–57
Plickert G, Schetter E, Verhey-Van-Wijk N, Schlossherr J, Steinbuchel M, Gajewski M (2003) The role of alpha-amidated neuropeptides in hydroid development—LWamides and metamorphosis in Hydractinia echinata. Int J Dev Biol 47:439–450
Price DA, Greenberg MJ (1977) Structure of a molluscan cardioexcitatory peptide. Science 197:670–671
Schmich J, Trepel S, Leitz T (1998) The role of GLW-amides in metamorphosis of Hydractinia echinata. Dev Genes Evol 208:267–273
Schmich J, Kraus Y, De Vito D, Graziussi D, Boero F, Piraino S (2007) Induction of reverse development in two marine hydrozoans. Int J Dev Biol 51:45–46
Schmid V (1992) Transdifferentiation in medusae. Int RevCytol 142:213–261
Seipp S, Schmich J, Leitz T (2001) Morphogenesis of Cnidaria: programmed cell death during metamorphosis of Hydractinia echinata. Zoology (Jena) 103:13
Seipp S, Schmich J, Kehrwald T, Leitz T (2007) Metamorphosis of Hydractinia echinata-natural versus artificial induction and developmental plasticity. Dev Genes Evol 217:385–394
Seipp S, Schmich J, Will B, Shetter E, Plickert G, Leitz T (2010) Neuronal cell death during metamorphosis of Hydractinia echinata (Cnidaria, Hydrozoa). Invertebr Neurosci 10:77–91
Yuan D, Nakanishi N, Jacobs DK, Hartenstein V (2008) Embryonic development and metamorphosis of the scyphozoan Aurelia. Dev Genes Evol 218:525–539
Acknowledgments
This study was funded by PRIN Italian Ministry for research and education (grant number 2007-5WCPWM) and by the ASSEMBLE project (remote access Station Biologique de Roscoff, and on-site access at the Sven Lovén Centre for Marine Sciences, Kristineberg). The publication of this paper has been also supported by CONISMA, the Italian National Inter-University Consortium for Marine Sciences. We thank two anonymous reviewers and Dr Andreas Schmidt-Rhaesa for valuable advices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Roberta Pennati and Stefano Piraino equally contributed to this work.
Rights and permissions
About this article
Cite this article
Pennati, R., Dell’Anna, A., Pagliara, P. et al. Neural system reorganization during metamorphosis in the planula larva of Clava multicornis (Hydrozoa, Cnidaria). Zoomorphology 132, 227–237 (2013). https://doi.org/10.1007/s00435-013-0188-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-013-0188-1