Abstract
The GLW-amide family is a neuropeptide family found in cnidarian species and is characterized by the C-terminal amino acid sequence -Gly-Leu-Trp-NH2. To detect mammalian peptides structurally related to the GLW-amide family, we examined rat brain by immunohistochemistry with an anti-GLW-amide antibody. GLW-amide-like immunoreactivity (GLW-amide-LI) was observed in thin varicose fibers in some regions of the brain. Most neurons showing GLW-amide-LI were observed in the laterodorsal tegmental nucleus, pedunculopontine tegmental nucleus, and trigeminal/spinal ganglia. These results strongly suggest that the rat nervous system contains as yet unidentified GLW-amide-like peptides, and that GLW-amide-LI in the brain is a good marker for ascending projections from mesopontine cholinergic neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropeptides play important roles in a variety of brain functions. Because of their significance, strategies to isolate novel neuropeptides have been pursued for several decades. Among these, a comprehensive identification of bioactive peptides in a freshwater cnidarian species, Hydra magnipapillata, has proven to be useful (Takahashi et al. 1997). This “peptidome” analysis, which is composed of two steps, has been described in detail elsewhere. Briefly, as a first step, peptides extracted from Hydra were systematically purified to homogeneity without any biological assay. Next, the effects of each isolated peptide on gene expression in Hydra were examined by differential-display polymerase chain reaction (Liang and Pardee 1992). Isolated peptides that affected gene expression were selected as candidate bioactive peptides, and their structures and biological activities were finally analyzed. This approach allowed the isolation of several hundred bioactive peptides from Hydra.
The identification of a number of novel Hydra peptides prompted us to examine the existence of homologs in the mammalian brain. Among the many peptides identified by the peptidome analysis mentioned above, we have focused on Hydra GLW-amide family peptides. The GLW-amide family is characterized by the C-terminus sequence -Gly-Leu-Trp-NH2 (Leviev and Grimmelikhuijzen 1995; Leviev et al. 1997; Takahashi et al. 1997). The first identified member of this GLW-amide family was metamorphosin A, a peptide that regulates metamorphosis of the lower metazoan, the cnidarian, Hydractinia echinata (Leitz et al. 1994). GLW-amide peptides are expressed in neurons and probably function as neuropeptides in cnidaria and Hydra (Gajewski et al. 1996; Leitz and Lay 1995; Takahashi et al. 2003).
Here, we demonstrate that the GLW-amide antiserum intensely labels thin varicose fibers in various regions of the rat brain and spinal cord. The distribution pattern of GLW-amide-like immunoreactivity (GLW-amide-LI) strongly suggests the presence of novel neuropeptides structurally related to the Hydra GLW-amide family.
Materials and methods
Preparation of tissues
Seven adult male Wistar rats (200–300 g) were used for the neuroanatomical mapping of GLW-amide-LI. Two received an intraventricular injection of colchicine (80 µg in 10 µl saline) to enhance the staining of cell bodies (Hökfelt et al. 1977). Colchicine-treated rats were killed 24 h after injection. The colchicine-treated rats were deeply anesthetized by pentobarbital (Nembutal; Abbott Laboratories, Chicago, USA) and perfused through the ascending aorta with saline followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB, pH 7.3). The brains and spinal cords were rapidly removed and postfixed overnight in the same fixative. The tissues were then immersed in 0.1 M PB containing 20% (w/v) sucrose for 24 h at 4˚C for cryoprotection. Whole brains were cut into 50-µm-thick serial sections on a freezing microtome, and two adjacent coronal sections were taken at 250-µm intervals. The sections were stored at 4˚C in phosphate-buffered saline (PBS, pH 7.3). One of the two adjacent sections was stained with 0.25% thionin for nuclear identification, and the other was used for immunohistochemistry.
Immunohistochemistry
Frozen sections were rinsed with PBS containing 0.3% Triton X-100 (PBST) for 30 min and preincubated for 1 h with PBST containing 5% normal goat serum (PBST-NGS). Then, the sections were incubated with rabbit anti-GLW-amide antisera (Takahashi et al. 2003) diluted 1:4000 in PBST-NGS at 4˚C overnight. After several rinses with PBST, the sections were incubated with biotinylated goat anti-rabbit IgG (Vector, Calif., USA) diluted 1:400 in PBST-NGS for 1 h at room temperature, rinsed with PBS several times, incubated with avidin-biotin complex reagent (PK-6100, Vector) for 1 h, and rinsed with PBS for 40 min. The peroxidase reaction product was revealed with 0.06% ammonium nickel sulfate, 0.02% diaminobenzidine, and 0.005% hydrogen peroxide in PBS under visual control. The sections were then mounted on gelatin-coated glass slides, dehydrated, and coverslipped with Permount (Fisher Scientific, PA., USA).
Specificity of GLW-amide antiserum
To determine the specificity and epitope of the GLW-amide antiserum, we treated sections of spinal cord with the secondary antibody and avidin-biotin complex reagent after incubation with primary antibody that had been preincubated for 1 h at 37˚C with an excess (10 µg/ml) synthetic peptides including CGLW-NH2, LW-NH2, W-NH2, GPMTGLW, and GPPPGLW. The latter two peptides are non-amidated forms of the Hydra GLW-amide peptides (Takahashi et al. 1997) Hym-54 and Hym-331, respectively.
Results
To investigate the possibility that GLW-amide-like peptides exist in the rat brain, we immunostained rat brain tissues with anti-GLW-amide antiserum, which had been raised against the synthetic peptide CGLW-NH2 conjugated to hemocyanin (Takahashi et al. 2003). We detected GLW-amide-LI in several regions of the rat central nervous system, including spinal cord.
Evaluation of the specificity of anti-GLW-amide antiserum
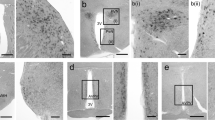
To determine the specificity and epitope of the antiserum, we preabsorbed the antiserum with an excess amount of synthetic peptides, including CGLW-NH2, LW-NH2, and W-NH2 (Fig. 1b-d), and with GPMTGLW and GPPPGLW, which correspond to the Hydra GLW-amide family peptides Hym-54 and Hym-331, respectively, but lack the C-terminal amidation (Fig. 1e, f). Preincubation with 10 µg/ml CGLW-NH2 resulted in a complete loss of staining (Fig. 1b), whereas preincubation with W-NH2, Hym-54-OH, or Hym-331-OH did not change the immunoreactivity compared with the staining pattern seen with non-absorbed antiserum (Fig. 1a). Preincubation with LW-NH2 abolished most, but not all, of the immunoreactivity (Fig. 1c). These data indicated that the anti-GLW-amide antiserum predominantly recognized GLW-NH2 or LW-NH2.
Specificity and epitope determination of the anti-GLW-amide antiserum. In the spinal cord (a), GLW-amide-like immunoreactivity (GLW-amide-LI) is mainly observed in the dorsal horn, especially in Rexed’s laminae I and II. GLW-amide-LI is considerably decreased after preincubation with an excess amount (10 µg/ml) of synthetic peptides CGLW-NH2 (b, +CGLWamide) or LW-NH2 (c, +LWamide), prior to the immunohistochemical procedure. Preincubation with W-NH2 (d, +Wamide), Hym-54-OH (GPMTGLW) (e, +Hym-54-OH), or Hym-331-OH (GPPPGLW) (f, +Hym-331-OH) does not affect the pattern of GLW-amide-LI. Bar 100 µm
GLW-amide-like immunoreactivity in rat brain and spinal cord
We examined the distribution pattern of GLW-amide-LI in the rat brain and spinal cord by immunohistochemistry. In general, GLW-amide-LI was observed as thin fibers with small varicosities in several regions of the brain and spinal cord. The regions in which GLW-amide-LI was detected are listed in Table 1. Because it was difficult to detect somata positive for GLW-amide-LI(hereafter referred to more briefly as GLW-amide-positive cells) in the sections from non-colchicine-treated rats, we immunostained the sections from the colchicine-treated rats and detected cell bodies showing GLW-amide-LI (abbreviations are taken from the rat brain atlas of Paxinos and Watson 1996).
Rhinencephalon
No GLW-amide-positive cell bodies were detected in this region. GLW-amide-positive varicose fibers were occasionally observed in the glomerular layer (Gl), olfactory nerve layer, and external plexiform layer. In the Gl, GLW-amide-positive fibers were distributed in the surrounding glomeruli (data not shown).
Telencephalon
GLW-amide-positive cell bodies were not detected in this area. GLW-amide-positive fibers were observed in the prelimbic cortex (PrL), cingulate cortex, agranular insular cortex, amygdaloid complex, hippocampus, and some of the basal nuclei. A high density of GLW-amide-positive fibers was observed in the PrL, especially in layers II and V (Fig. 2a, b). In the cingulate cortex 1 (Cg1) adjacent to the PrL, we detected fewer immunolabeled fibers than in the PrL (Fig. 2a). In addition to the Cg1, a low density of GLW-amide-positive fibers was observed in the agranular insular cortex (data not shown). We could not find GLW-amide-positive fibers in other areas of the cerebral cortex.
Dark-field photomicrographs showing GLW-amide-positive fibers in the prelimbic region (a, b), central amygdaloid nucleus (c, d), and CA1 region of the hippocampus (e). b Higher magnification of the dashed rectangle in a. A high density of GLW-amide-positive fibers was observed in layers II and V. d Higher magnification of the dotted rectangle in c (Cg1 cingulate cortex area 1, PrL prelimbic cortex, Ce central amygdaloid nucleus, CA1 CA1 region of the hippocampus, Py pyramidal layer, Rad stratum radiatum, Or stratum oriens, I–V layers of the prelimbic cortex). Bars 100 µm
In the amygdaloid complex, the central (Fig. 2c, d) and medial amygdaloid nucleus contained a moderate density of GLW-amide-positive fibers, and the basolateral amygdaloid nucleus contained a low density of GLW-amide-positive fibers. In the hippocampus, a low density of GLW-amide-positive fibers was found in the stratum radiatum and stratum oriens of the CA1 (Fig. 2e), CA2, and CA3 regions.
In the basal nuclei, high densities of GLW-amide-positive fibers were observed in the dorsal taenia tecti, septohippocampal nucleus, lateral septum nucleus, and indusium griseum (Fig. 3). Moderate to low densities of GLW-amide-positive fibers were detected in the ventral taenia tecti, the bed nucleus of the stria terminalis (see below), the nucleus of the horizontal limb of the diagonal band, and the island of Calleja.
Diencephalon
A small number of multipolar GLW-amide-positive cell bodies were detected in the dorsal tuberomammillary nucleus.
In the epithalamus, the highest density of GLW-amide-positive fibers was found in the medial region of the lateral habenula (LHbM) (Fig. 4a, b). In the medial habenula, GLW-amide-positive fibers were found along the dorsal third ventricle (Fig. 4b).
Photomicrographs showing GLW-amide-positive fibers in the lateral habenula (a, b) and interpeduncular nuclei (c, d); b, d higher magnifications of boxed areas in a, c, respectively. A high density of GLW-amide-positive fibers was observed in the medial part of the lateral habenula (b) and the lateral subnucleus of the interpeduncular nuclei (d). cc Corpus callosum, D3V dorsal 3rd ventricle, sm stria medullaris of the thalamus, fr fasciculus retroflexus, HiF hippocampal fissure, MHb medial habenula nucleus, LHbM lateral habenula nucleus (lateral part), LHbL lateral habenula nucleus (medial part), CLi caudal linear nucleus of the raphe, vtgx ventral tegmental decussation, cp cerebral peduncle, ml medial lemniscus, tfp transverse fibers of the pons, IPC interpeduncular nucleus (caudal subnucleus), IPL lateral subnucleus of interpeduncular nucleus, IPDM interpeduncular nucleus (dorsomedial subnucleus). Bars 100 µm
In the thalamus, GLW-amide-positive fibers were predominantly distributed in the intralaminar and midline thalamic nuclei. A moderate density of GLW-amide-positive fibers was observed in the central medial thalamic nucleus and the intermediodorsal thalamic nucleus (Fig. 5a, b). A low density of GLW-amide-positive fibers was found in the paracentral thalamic nucleus, the parafascicular thalamic nucleus, and the mediodorsal thalamic nucleus.
Photomicrographs showing GLW-amide-positive fibers in the central medial thalamic nucleus (a, b), the bed nucleus of the stria terminalis (c), the lateral periaqueductal gray (d), and the nucleus of the solitary tract (e, f). Moderate to low densities of GLW-amide-positive fibers were detected in these regions; b, f higher magnification of boxed areas in a, e, respectively (IMD intermediodorsal thalamic nucleus, CM central medial thalamic nucleus, MD mediodorsal thalamic nucleus, LV lateral ventricle, BST bed nucleus of the stria terminalis, sm stria medullaris of the thalamus, Aq aqueduct, LPAG lateral periaqueductal gray, 4V 4th ventricle, 12 hypoglossal nucleus, Sol nucleus of the solitary tract). Bars 100 µm (a-d, f), 500 µm (e)
In the hypothalamus, a moderate density of GLW-amide-positive fibers was observed in the arcuate nucleus. The periventricular hypothalamic nucleus, suprachiasmatic nucleus, paraventricular hypothalamus nucleus, anterior hypothalamic area, posterior hypothalamic area, lateral hypothalamic area, subincertal nucleus, and supramammillary nucleus all contained a low density of GLW-amide-positive fibers.
Mesencephalon
A small number of GLW-amide-positive cell bodies, which had a unipolar or bipolar dendritic arrangement, were detected in the lateral periaqueductal gray. In addition to these cell bodies, a small number of GLW-amide-positive fibers were detected in the periaqueductal gray (Fig. 5d). Densely packed GLW-amide-positive fibers were observed in the lateral subnucleus of the interpeduncular nuclei (Fig. 4c, d). Low densities of GLW-amide-positive fibers were also detected in the central subnucleus and dorsal medial subnucleus of the interpeduncular nuclei. A moderate density of GLW-amide-positive fibers was found in the interfascicular nucleus and ventral tegmental area. The substantia nigra exhibited a low density of GLW-amide-positive fibers.
Pons and cerebellum
A considerable number of GLW-amide-positive somata, most of which were multipolar and large (about 20 µm), were concentrated in the laterodorsal tegmental area (LDTg) (Fig. 6a, b). In addition, a small number of small GLW-amide-positive cell bodies were observed in the pedunculopontine tegmental nucleus (PPTg). A small number of GLW-amide-positive fibers were found in the dorsal and paramedian raphe nuclei and the superior colliculus. We could not find any GLW-amide-positive cell bodies or fibers in the cerebellum.
Photomicrographs showing GLW-amide-positive cell bodies in the laterodorsal tegmental nucleus (arrow in a) and GLW-amide-positive fibers in the spinal trigeminal tract and the spinal trigeminal nucleus (c, d); b, d higher magnification of boxed areas in a, c, respectively (4V 4th ventricle, LDTg laterodorsal tegmental nucleus, DTg dorsal tegmental nucleus, mlf medial longitudinal fasciculus, PnR pontine raphe nucleus, me5 mesencephalic trigeminal tract, scp superior cerebellar peduncle, PCRt parvicellular reticular nucleus, Sp5 spinal trigeminal nucleus, sp5 spinal trigeminal tract). Bars 500 µm (a, c), 200 µm (b)
Medulla
No GLW-amide-positive cell bodies were found in the medulla oblongata. GLW-amide-positive fibers were observed in the spinal trigeminal nucleus, the spinal trigeminal tract, and the nucleus of the solitary tract. In the spinal trigeminal nucleus, GLW-amide-positive fibers were present in the peripheral region, especially in the most ventral and dorsal part (Fig. 6c). Thick bundles of GLW-amide-positive fibers were observed in the most dorsolateral part of the spinal trigeminal tract (Fig. 6d). A moderate density of GLW-amide-positive fibers was seen in the nucleus of the solitary tract (Fig. 5e, f). Low densities of GLW-amide-positive fibers were detected in the parvicellular reticular nucleus, the lateral paragigantocellular nucleus, the raphe pallidus nucleus, the external cuneate nucleus, and the lateral reticular nucleus.
Spinal cord
No GLW-amide-positive cell bodies were detected in the spinal cord. In all segments of the spinal cord, GLW-amide-positive fibers were concentrated in Rexed’s laminae I and II (Rexed 1954; Fig. 7a, b). Low densities of GLW-amide-positive fibers were detected in Rexed’s laminae III, IV, IX, and X. Interestingly, a small bundle of GLW-amide-positive fibers ran longitudinally just ventral to the central canal (Fig. 7c, d).
Photomicrographs showing GLW-amide-LI in the spinal cord (a-d) and the dorsal root ganglion (e, f); b, c higher magnification of boxed areas in a. b A high density of GLW-amide-positive fibers was observed in laminae I and II. c Around the central canal, a small bundle of GLW-amide-positive fibers runs longitudinally ventral to the canal (arrow). d Sagittal view of the longitudinal bundle of GLW-amide-positive fibers shown in c. f Higher magnification of boxed area in e (arrows GLW-amide-positive cell bodies, asterisks central canal, DH dorsal horn, VH ventral horn, DRG dorsal root ganglion, dr dorsal root, SP spinal cord). Bars 500 µm (a, e), 100 µm (b, f), 50 µm (c, d)
A small number of GLW-amide-positive cell bodies were detected in the dorsal root ganglion (DRG) (Fig. 7e, f). Judging from their size and shape, these cells probably belong to a population of small DRG neurons. We also detected small GLW-amide-positive somata in the trigeminal ganglia (data not shown).
Hypophysis
No GLW-amide-positive fibers or cell bodies were detected in the pituitary gland.
Discussion
Here, we demonstrate immunohistochemical evidence that mammalian peptides structurally related to the Hydra GLW-amide family exist in the rat brain as neuropeptides. Most of the immunoreactive structures labeled with the GLW-amide antiserum were thin varicose fibers, which were generally observed by means of immunohistochemistry of rat brain tissue sections with neuropeptide antibodies. Furthermore, as is the case with other neuropeptides, GLW-amide-positive somata were rarely detected, unless rats were treated with colchicine (Hökfelt et al. 1977). These morphological features of GLW-amide-LI indicated that the GLW-amide antiserum labeled neuropeptide-like molecules. To our knowledge, no known peptide shows a similar distribution pattern to that of GLW-amide-LI. Thus, the GLW-amide antiserum probably labels unknown mammalian neuropeptides.
The GLW-amide antiserum used in this study has been quantitatively characterized by enzyme-linked immunosorbent assay and is reported to react with carboxyl terminal -GLW-NH2 or -LW-NH2 moieties (Takahashi et al. 2003). This result is supported by immunohistochemistry with the GLW-amide antiserum preabsorbed with several synthetic peptides in the present study. Therefore, we speculate that the unknown peptides recognized by the GLW-amide antiserum have the C-terminal sequence -LW-NH2. However, an antiserum raised against FMRF-amide has been reported to recognize neuropeptide Y by immunohistochemistry (Sasek and Elde 1985). Thus, we cannot rule out the possibility that the unknown peptides recognized by the GLW-amide antiserum do not have C-terminal -LW-NH2 sequences.
The functions of rat GLW-amide-like peptides in the rat nervous system are not known, but, judging from their distribution patterns, these peptides may be involved in sensory mechanisms. In the rat spinal cord, the most intense GLW-amide-LI has been observed in laminae I and II as varicose fibers. Because a considerable number of small GLW-amide-positive neurons have been seen in the spinal ganglia, the GLW-amide-positive varicose fibers are most probably derived from the spinal ganglia. Furthermore, we have detected a high density of GLW-amide-positive fibers in the spinal trigeminal nucleus and the spinal trigeminal tract (Fig. 6c, d) and a number of smaller GLW-amide-positive neurons in the trigeminal ganglia (data not shown). These distribution patterns of GLW-amide-positive fibers are similar to that of substance P (Cuello and Kanazawa 1978; Ljungdahl et al. 1978), which is thought to be an important neuropeptide in the processing of nociceptive information (Cao et al. 1998; De Felipe et al. 1998). Thus, we speculate that rat GLW-amide-like peptides are indeed involved in sensory mechanisms, possibly related to nociception.
In the rat brain, most GLW-amide-positive somata have been were detected in the LDTg. In addition, fewer GLW-amide-positive somata have been observed in the PPTg. These two nuclei contain a number of cholinergic neurons and have ascending projections to extensive portions of the brain (Butcher and Woolf 2003). Tract-tracing studies have revealed that LDTg neurons innervate various brain regions, including the medial prefrontal cortex, the septofibrial nucleus, the lateral septal nuclei, the ventral pallidum, the nucleus of the horizontal limb of the diagonal band, the thalamus, the lateral habenula, the lateral hypothalamus, the lateral interpeduncular nuclei, the substantia nigra, and the periaqueductal gray (Cornwall et al. 1990; Satoh and Fibiger 1986; Woolf and Butcher 1986). In these regions, we have consistently detected GLW-amide-positive fibers. The PPTg is also known to innervate a variety of regions, including the lateral septal nuclei, the ventral pallidum, the central and medial amygdaloid nuclei, and the substantia nigra in which GLW-amide-positive fibers have been observed (Hallanger and Wainer 1988; Woolf and Butcher 1986). Thus, most of the GLW-amide-positive fibers in the brain could be derived from the LDTg and PPTg.
The ascending cholinergic projections from the LDTg and PPTg are thought to be a major component of the ascending reticular activating system, which plays a central role in the regulation of arousal and sleep (for a review, see Siegel 2004). Our results demonstrate that GLW-amide-LI is a good marker for the ascending reticular activating system and thus suggest that GLW-amide-like peptides modulate cholinergic neurotransmission and influence arousal and sleep.
Many neuropeptides isolated from the nervous systems of vertebrates are known to exist in invertebrates (Strand 1999). For example, nerve cells of Hydra have been reported to contain cholecystokinin-, substance P-, neurotensin-, bombesin- oxytocin-, vasopressin-, and gonadotropin-releasing hormone-like peptides (Grimmelikhuijzen et al. 1980, 1982; Powell et al. 1996; Schot et al. 1981). Conversely, peptides related to the RF-amide peptide, which was originally isolated from the ganglia of the Venus clam (an invertebrate), exist in the nervous systems of vertebrates (Boer et al. 1980; Yang et al. 1985). These previous findings strongly suggest that neuropeptides are phylogenetically conserved among vertebrates and invertebrates. Thus, the isolation of peptides structurally related to novel invertebrate bioactive peptides may be a useful strategy for the identification of unknown mammalian neuropeptides. We have tried to isolate GLW-amide-like peptides from rat brains and have identified a novel peptide related to GLW-amide family peptides (data not shown). However, the amino acid sequence of the isolated GLW-amide-like peptide (Val-His-Leu-Thr-Asp-Ala-Glu-Lys-Ala-Ala-Val-Asn-Gly-Leu-Trp-NH2) completely corresponds to an N-terminal fragment of rat beta-globin. Although this peptide has an amidated C-terminal end, we cannot rule out the possibility that the peptide is artificially converted, by alpha-amidation activity in plasma or cerebrospinal fluid (Kapuscinski et al. 1993; Wand et al. 1985), from an N-terminal 2–16 fragment of beta-globin with a C-terminal glycine residue during the purification process. Further studies will be required to identify authentic mammalian GLW-amide-like peptides.
References
Boer HH, Schot LP, Veenstra JA, Reichelt D (1980) Immunocytochemical identification of neural elements in the central nervous systems of a snail, some insects, a fish, and a mammal with an antiserum to the molluscan cardio-excitatory tetrapeptide FMRF-amide. Cell Tissue Res 213:21–27
Butcher LL, Woolf NJ (2003) Cholinergic neurons and networks revisited. In: Paxinos G (ed) The rat nervous system. Academic Press, New York, pp 1257-1268
Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI (1998) Primary afferent tachykinins are required to experience moderate to intense pain. Nature 392:390–394
Cornwall J, Cooper JD, Phillipson OT (1990) Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull 25:271–284
Cuello AC, Kanazawa I (1978) The distribution of substance P immunoreactive fibers in the rat central nervous system. J Comp Neurol 178:129–156
De Felipe C, Herrero JF, O'Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP (1998) Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392:394–397
Gajewski M, Leitz T, Schloßherr J, Plickert G (1996) LWamides from Cnidaria constitute a novel family of neuropeptides with morphogenetic activity. Roux's Arch Dev Biol 205:232–242
Grimmelikhuijzen CJ, Sundler F, Rehfeld JF (1980) Gastrin/CCK-like immunoreactivity in the nervous system of coelenterates. Histochemistry 69:61–68
Grimmelikhuijzen CJ, Dierickx K, Boer GJ (1982) Oxytocin/vasopressin-like immunoreactivity is present in the nervous system of hydra. Neuroscience 7:3191–3199
Hallanger AE, Wainer BH (1988) Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol 274:483–515
Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G (1977) Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci USA 74:3081–3085
Kapuscinski M, Green M, Sinha SN, Shepherd JJ, Shulkes A (1993) Peptide alpha-amidation activity in human plasma: relationship to gastrin processing. Clin Endocrinol (Oxf) 39:51–58
Leitz T, Lay M (1995) Metamorphosin A is a neuropeptide. Roux's Arch Dev Biol 204:276–279
Leitz T, Morand K, Mann M (1994) Metamorphosin A: a novel peptide controlling development of the lower metazoan Hydractinia echinata (Coelenterata, Hydrozoa). Dev Biol 163:440–446
Leviev I, Grimmelikhuijzen CJ (1995) Molecular cloning of preprohormone from sea anemones containing numerous copies of a metamorphosis-inducing neuropeptide: a likely role for dipeptidyl aminopeptidase in neuropeptide precursor processing. Proc Natl Acad Sci USA 92:11647–11651
Leviev I, Williamson M, Grimmelikhuijzen CJ (1997) Molecular cloning of a preprohormone from Hydra magnipapillata containing multiple copies of Hydra-LWamide (Leu-Trp-NH2) neuropeptides: evidence for processing at Ser and Asn residues. J Neurochem 68:1319–1325
Liang P, Pardee AB (1992) Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257:967–971
Ljungdahl A, Hökfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat-I. Cell bodies and nerve terminals. Neuroscience 3:861–943
Paxinos G, Watson C (1996) The rat brain in stereotaxic coordinates. Academic Press, New York
Powell JF, Reska-Skinner SM, Prakash MO, Fischer WH, Park M, Rivier JE, Craig AG, Mackie GO, Sherwood NM (1996) Two new forms of gonadotropin-releasing hormone in a protochordate and the evolutionary implications. Proc Natl Acad Sci USA 93:10461–10464
Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Sasek CA, Elde RP (1985) Distribution of neuropeptide Y-like immunoreactivity and its relationship to FMRF-amide-like immunoreactivity in the sixth lumbar and first sacral spinal cord segments of the rat. J Neurosci 5:1729–1739
Satoh K, Fibiger HC (1986) Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol 253:277–302
Schot LP, Boer HH, Swaab DF, Van Noorden S (1981) Immunocytochemical demonstration of peptidergic neurons in the central nervous system of the pond snail Lymnaea stagnalis with antisera raised to biologically active peptides of vertebrates. Cell Tissue Res 216:273–291
Siegel J (2004) Brain mechanisms that control sleep and waking. Naturwissenschaften 91:355–365
Strand FL (1999) Neuropeptides: regulators of physiological processes. MIT Press, Cambridge (Mass.)
Takahashi T, Muneoka Y, Lohmann J, Lopez de Haro MS, Solleder G, Bosch TC, David CN, Bode HR, Koizumi O, Shimizu H, Hatta M, Fujisawa T, Sugiyama T (1997) Systematic isolation of peptide signal molecules regulating development in hydra: LWamide and PW families. Proc Natl Acad Sci USA 94:1241–1246
Takahashi T, Kobayakawa Y, Muneoka Y, Fujisawa Y, Mohri S, Hatta M, Shimizu H, Fujisawa T, Sugiyama T, Takahara M, Yanagi K, Koizumi O (2003) Identification of a new member of the GLW-amide peptide family: physiological activity and cellular localization in cnidarian polyps. Comp Biochem Physiol [B] Biochem Mol Biol 135:309–324
Wand GS, Ney RL, Mains RE, Eipper BA (1985) Characterization of peptide alpha-amidation activity in human cerebrospinal fluid and central nervous system tissue. Neuroendocrinology 41:482–489
Woolf NJ, Butcher LL (1986) Cholinergic systems in the rat brain. III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull 16:603–637
Yang HY, Fratta W, Majane EA, Costa E (1985) Isolation, sequencing, synthesis, and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc Natl Acad Sci USA 82:7757–7761
Acknowledgments
We thank Dr. Toshitaka Fujisawa (National Institute of Genetics) and Dr. Toshio Takahashi (Suntory Institute for Bioorganic Research) for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Ministry of Education, Sports, and Culture, Japan.
Rights and permissions
About this article
Cite this article
Hamaguchi-Hamada, K., Fujisawa, Y., Koizumi, O. et al. Immunohistochemical evidence for the existence of novel mammalian neuropeptides related to the Hydra GLW-amide neuropeptide family. Cell Tissue Res 337, 15–25 (2009). https://doi.org/10.1007/s00441-009-0808-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0808-8