Abstract
Purpose
To investigate the synergistic effect of resveratrol on the bystander effect of TK/GCV suicide gene system in melanoma cells.
Methods
The effect of resveratrol on the growth of B16 cells and the synergistic effect of resveratrol with or without GCV were detected by MTT assay and high content screening assay. The effect of resveratrol on GJIC function was detected by flow cytometry combined with fluorescence tracer and fluorescence microscope, and the expression of gap junction protein was detected by western blotting. Synergistic killing effect of resveratrol plus TK/GCV was tested in vivo using transplanted melanoma model.
Results
In vitro, resveratrol can enhanced GJ function and upregulated Cx32 and Cx43 protein expression in B16 cells. Resveratrol synergized with GCV to kill mixed B16 melanoma cells (20% TK+ cells and 80% TK− cells) and to improve apoptosis rate of TK− cells (the bystander effect of TK system), and the synergistic action was reversed by the GJ inhibitor AGA. In vivo, when B16 cells were mixed with 30% TK+ B16 cells, significantly reduced tumor weight and volume were observed after combinational treatment with resveratrol plus GCV as compared with GCV or resveratrol treatment alone.
Conclusions
Resveratrol could synergistically enhance the killing effect of TK/GCV suicide gene system in melanoma B16 cells and transplanted melanoma. It might be a promising adjuvant of TK/GCV therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma originates from cells that can produce melanin, and it is a relatively common malignant tumor that is a threat to human health (Luebker and Koepsell 2019). In addition to the conventional surgery, such as radiotherapy and chemotherapy, suicide gene therapy is a promising new approach for treating melanoma (Karjoo et al. 2016; Navarro et al. 2016). The earliest and most mature suicide gene system is herpes simplex virus 1 thymidine kinase/ganciclovir (HSV1-TK/GCV)-mediated cancer therapy, which has entered phase III clinical trials. The mechanism of thymidine kinase (TK) gene function is that TK gene production can catalyze the production of toxic metabolites with the substrate ganciclovir (GCV), leading to cell death. Nonetheless, the low efficiency of TK gene transfection limits the suicide gene therapy strategy, resulting in poor clinical outcome and prognosis (Faneca et al. 2019). Interestingly, the adjacent TK-negative (TK−) cells readily die because the toxic metabolites from TK+ cells can enter the adjacent cells through intercellular gap junctions. This phenomenon is termed the bystander effect (Mesnil and Yamasaki 2000). Unfortunately, tumor cells, including melanoma cells, are usually poorly organized, with less cell–cell communication because of the downregulation of gap junction proteins, especially the connexin protein family. Hence, improving gap junction intercellular communication (GJIC) by promoting connexin protein expression may enhance the bystander effect, leading to improved therapeutic effect of suicide gene therapy (Aasen et al. 2016; Tanaka et al. 2001).
Resveratrol is a major component of Polygonum cuspidatum (Wu et al. 2018), which possesses multiple activities, such as antioxidant activity. Recently, we identified it as an inducer of connexin 43 (Cx43) and connexin 32 (Cx32), the predominantly expressed connexins in melanoma. Hypothetically, resveratrol may enhance the bystander effect of TK/GCV-mediated suicide gene therapy. Here, we conducted a series of experiments to demonstrate that resveratrol can promote GJIC, and that combined with TK/GCV therapy, it has a synergistic killing effect on B16 melanoma cells.
Results
Inhibitory effect of resveratrol on B16 cell growth
We assessed the cytotoxicity of resveratrol to B16 cells via the MTT (3-[4,5-dimethylthiazol-2-yl]-2, 5 diphenyl tetrazolium bromide) assay. As shown in Fig. 1 a, resveratrol dose dependently inhibited the growth of the B16 cells. The half maximal inhibitory concentration (IC50) of resveratrol at 48 h was 40 μM. Low-concentration resveratrol (not exceeding 20 μM) was used in the subsequent studies focusing on the bystander effect promoting function.
Effect of resveratrol on GJIC function in B16 cells
To measure GJIC function, we stained B16 cells with chloromethyl tetramethylrhodamine (CMTMR) and calcein as donor cells and mixed them with the non-stained B16 recipient cells at a 5:95 ratio. CMTMR (red fluorescein) cannot enter the recipient cells because the large molecule cannot cross the GJ channel, while calcein (green fluorescein) can cross from the pre-stained donor cells to the recipient cells through the GJ channel. Thus, the ratio of cells in the G4 quadrant (with green fluorescence only) to those in G3 (without fluorescence) indicated the GJ function. Figure 2a shows that the G4/G3 ratio was increased in a dose-dependent manner following resveratrol treatment, suggesting enhanced GJIC function in the B16 cells. The effect was further confirmed by the results that resveratrol dose dependently upregulated Cx32 and Cx43 protein expression in B16 cells (Fig. 2b).
Resveratrol promoted the GJIC of B16 cells. a Resveratrol-treated B16 cells were stained with CMTMR and calcein as donor cells and mixed with the intact B16 recipient cells by 5:95. Then the cells were detected by flow cytometry analysis and the ratio of cells in G4 to G3 was calculated. G4 (calcein-positive) represents recipient cells containing calcein via the GJ; G3 denotes the double-negative cells; the G4/G3 ratio reflects the GJ-mediated calcein transfer efficiency. b Resveratrol induced upregulation of Cx32 and Cx43 protein in B16 cells in a dose-dependent manner. *p < 0.05, **p < 0.01 vs. control
Synergistic effect of resveratrol plus TK/GCV in inhibiting B16 cell growth
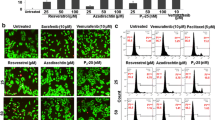
To study the effect of HSV-TK/GCV in B16 cells, we constructed B16TK cells that stably expressed TK gene, and co-cultured these cells with B16 cells. As shown in Fig. 1 b, the inhibitory effect of TK/GCV was significant in the group with 10% of B16TK cells and higher proportions. To mimic low transfection efficiency of suicide gene in clinic, B16TK cells were mixed with B16 cells at the ratio of 1:4 for the subsequent in vitro studies. The MTT results (Fig. 3a) showed that the inhibition rate in cells treated with GCV (15.7 μM) plus resveratrol (5 μM, 10 μM, 20 μM resveratrol) was 30.11 ± 2.75%, 45.89 ± 2.72%, and 55.72 ± 1.99%, respectively, which was significantly increased compared with GCV-alone treatment (10.63 ± 4.18%). The Q value for the GCV plus resveratrol (5 μM, 10 μM, 20 μM) treatment was 2.31, 1.29, and 1.40, respectively, all indicating a synergistic effect. To further confirm the results, the technique of high content screening assay was employed. As shown in Fig. 3b, resveratrol at the indicated doses all significantly improved the inhibitory effect of TK/GCV, and the effect was identified as a synergistic effect as indicated by the Q values of 2.08, 2.05, 1.19, respectively.
Inhibitory effect of resveratrol plus TK/GCV on mixed B16 cells. B16TK cells and B16 cells were co-cultured in a ratio of 1:4 in 96- or 6-well plates for 24 h. Then the cells were treated with indicated concentrations of resveratrol with or without 15.7 μM GCV for 48 h. The cell viability was detected using MTT assay (a) and high content screening assay (b). Cells in 6-well plates were fixed with 70% ethanol, followed by the standard protocol of propidium iodide (PI) staining for analyzing the cell death rate with flow cytometry (c and d). #p < 0.05, ##p < 0.01 vs. control; **p < 0.01 vs. the indicated groups
We also determined the synergistic effect of resveratrol and GCV on the TK suicide system via flow cytometry analysis. Cells with the same treatment were fixed by ethanol and stained with propidium iodide (PI), followed by the standard protocol of flow cytometry analysis. Consistently, the cells treated with GCV plus 5, 10, or 20 μM resveratrol showed a synergistically increased death rate compared with GCV- or resveratrol-alone treatment (Fig. 3c, d) with the Q value of 1.44, 1.45 and 1.52, respectively. The results of the three methods consistently demonstrated that resveratrol played a synergistic role in increasing the inhibitory effect of TK/GCV in B16 cells.
Resveratrol promoted the bystander effect of the TK/GCV system
To clarify if resveratrol promoted the bystander effect of the TK suicide gene, B16 cells stably expressing red fluorescent protein (B16RFP) were mixed with B16 cells stably expressing green fluorescent protein and TK (B16TK−GFP) at a 4:1 ratio. The fused TK-GFP is mainly distributed in the nucleus due to the primary location of TK, whereas RFP is typically expressed in the cytoplasm when the cells were in good condition. By contrast, since the apoptotic or necrotic cells were characterized with less or no pseudopods, and the apoptotic cells are also featured by pyknosis and cell shrinking, RFP in these cells was condensed or aggregated in the apoptotic bodies, and might indicate the apoptotic or necrotic cells. The fluorescence images (Fig. 4a) showed significantly increased apoptotic or necrotic cells in the resveratrol plus TK/GCV group compared with the TK/GCV or corresponding resveratrol group, as indicated by the condensed or shattered red fluorescence. To quantify the effect, the same samples were stained with annexin V, followed by flow cytometry analysis. Since PI and RFP share similar excitation and emission wavelength. PI staining was not applied with annexin V for apoptosis detection in this experiment. The RFP and annexin V double-positive staining indicated the apoptosis of the B16RFP cells, and the increased apoptosis of TK− cells were considered as the result of the bystander effect. Flow cytometry data (Fig. 4b, c) showed that the apoptosis rate of red fluorescent TK− cells treated with TK/GCV plus resveratrol (10 μM and 20 μM) was 35.91 ± 12.97% and 58.01 ± 15.29%, respectively, much higher than that of resveratrol-alone treatment (10 μM: 14.52 ± 6.55%; 20 μM: 28.47 ± 15.18%). Since TK/GCV could not kill TK− cells, the apoptosis of GCV-alone group was caused by its own bystander effect, which was much less obvious due to low proportion of TK+ cells. The synergistic effect of resveratrol (10 μM and 20 μM) plus GCV was confirmed with the Q value, which was 2.414 and 1.552, respectively. The results here indicated that resveratrol could enhance the bystander effect of TK/GCV.
Resveratrol improved the bystander effect of TK/GCV on B16 cells. B16RFP cells were cultured with B16TK−GFP cells at a 4:1 ratio. Cells were subjected to the indicated treatment for 48 h. a GFP fused with TK in B16TK−GFP cells and RFP in B16RFP cells were tracked and images were taken by fluorescence microscopy. White arrows indicate necrotic cells, apoptotic cells or apoptotic bodies. The same samples were harvested and stained with Annexin V for detecting by the flow cytometry (b) and the apoptosis rate of B16RFP cells was calculated (c). #p < 0.05, ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. the indicated groups
GJIC inhibitor antagonized the synergistic inhibitory effect of resveratrol and TK/GCV on B16 cells
Since the function of gap junction intercellular communication (GJIC) was critically involved in the bystander effect of TK/GCV (Matono et al. 2003; Mesnil and Yamasaki 2000), and that resveratrol significantly upregulated Cx32 and Cx43 expression in B16 cells, we hypothesized the synergistic effect of resveratrol might be mediated by improved GJIC. Due to the compensatory effect of connexin isoforms and the fact that knockdown of single connexin does not predominantly block GJIC, AGA, the long-term GJ inhibitor (Ammerpohl et al. 2004; Yan et al. 2017), was employed in this study. Mixed B16 cells containing 20% TK+ B16 cells were treated as described in Methods. MTT result (Fig. 5a) showed that AGA alone had no significant inhibitory effect. Also, resveratrol plus GCV co-treatment synergistically decreased the cell viability with the Q value of 1.363. However, the synergistic effect was significantly reversed by AGA. The Q value was reduced to 0.737, which indicated an antagonistic effect. The inhibitory function of AGA was further confirmed with the technique of high content screening (Fig. 5b, c), and the result was consistent (Q value of 5 μM resveratrol plus GCV was reduced from 1.392 to 0.713, and that of 10 μM resveratrol plus GCV was reduced from 1.487 to 0.839). The data together suggested that resveratrol might promote the killing effect of TK/GCV through GJIC.
The effect of AGA on the killing action of resveratrol plus TK/GCV. B16TK and B16 cells were co-cultured in a ratio of 1:4 in 96-well plates and subjected to the indicated treatments. The cell viability was detected using MTT assay (a) and high content screening (HCS) assay (b and c). For HCS assay, alive cell numbers were determined by nuclei counts (c) with the minimum area of 65 μm2. Nine image fields per well were acquired using the 20× objective. Resulting values were normalized to the fold change in cell numbers compared to the ctrl group. #p < 0.05, ##p < 0.01 vs. control; **p < 0.01 vs. the indicated groups
Synergistic killing effect of resveratrol plus TK/GCV on B16 cells in vivo
To confirm the synergistic effect of resveratrol and TK/GCV, we performed in vivo study with the transplanted melanoma model. To determine the optimal ratio of mixed cells, we did preliminary experiment, in which the cells injected into mice were mixed by B16 cells with 20%, 40%, and 80% B16TK cells, respectively. 7 days later, GCV was administrated for another 7-day period. Then the tumors were isolated. The result showed that, while 20% of B16TK cells did not have obvious inhibitory effect, the tumor mass was significantly reduced by 53.53%, and 80.32% in the group with 40% and 80% of B16TK cells, respectively (data not shown). Since the transduction efficiency of recombinant virus in human is relative low (Rincon et al. 2015), and to better evaluate the combinational effect of resveratrol, the ratio of 3:7 was determined in the subsequent in vivo study. The data in Fig. 6b show that 200 mg/kg/day of resveratrol was able to inhibit the tumor size since the day 13, whereas TK/GCV showed no significant inhibition. However, co-administration of either 100 or 200 mg/kg/day resveratrol with GCV markedly inhibited the tumor size. On the 16th day, the mice were sacrificed, and the tumor was isolated for measuring volume and mass. As shown in Fig. 6c, combination of 200 mg/kg/day resveratrol and GCV significantly inhibited the tumor growth, resulting in smaller size (Fig. 6b) and less weight (Fig. 6c).
Discussion
Resveratrol exists in a variety of plant sources (Rauf et al. 2017) and has multiple functions such as anti-inflammation, cardiovascular protection, and neuroprotection (Dyck et al. 2019; Hou et al. 2019; Oliveira et al. 2017). The accumulating literature has suggested that resveratrol has an inhibitory effect on a series of cancer cells (Sinha et al. 2016; Yousef et al. 2017; Zulueta et al. 2015). In the present study, the preliminary data showed that resveratrol upregulated the expression of connexins, leading to enhanced GJIC. In vitro assay demonstrated that resveratrol plus GCV had a synergistic killing effect on mixed B16 cells composed of 20% TK+ and 80% TK− B16 cells. AGA blocking of GJIC reversed the synergistic action. The data confirmed our hypothesis that resveratrol would promote the killing effect of TK/GCV, which might be mediated by improved GJIC function. Consistently, the in vivo experiments indicated the enhanced inhibitory effect of GCV plus resveratrol as compared with GCV alone. This finding provides a theoretical basis for the combined application of TK/GCV and resveratrol in clinical practice.
The MTT assay showed that resveratrol inhibited B16 cell growth in a concentration-dependent manner. The IC50 was about 40 μM. Since resveratrol could inhibit B16 cell growth at the indicated doses, it is possible that the combinational killing effect of GCV and resveratrol might partly result from the cytotoxicity of resveratrol. Interestingly, based on the Q values, the effect was determined as a synergistic effect, rather than an additive effect. As a critical mechanism medicating the killing effect of TK/GCV suicide gene system (Mesnil and Yamasaki 2000; Robe et al. 2005), the bystander effect was also synergistically improved by the combinational treatment. In addition, connexins play a tumor-suppressive role (Aasen et al. 2017; Pelin et al. 1994), and also contribute to the bystander effect of the TK/GCV system (Cottin et al. 2008; Zhao et al. 2014). Accordingly, the synergistic effect was reversed by AGA, suggesting that resveratrol might enhance the bystander killing effect of GCV by promoting GJ. However, AGA is actually an inhibitor of many different GJs without being Cx subtype specific (Bodendiek and Raman 2010), and the mechanism of AGA in inhibiting GJ is still not completely understood. It was proposed that they may affect the phosphorylation state of Cx43, or modify the ability of connexin subunits to aggregate within the cellular environment (Guan et al. 1996). Unfortunately, we did not detect the phosphorylation state of Cx43 and Cx32 due to unavailability of antibodies. Nevertheless, further study is still required to figure out the specific mechanism by which the bystander effect is synergized.
For the in vivo experiments, we first optimized the ratio between TK+ and TK− B16 cells. As expected, the killing effect of GCV was enhanced with the percentage of TK+ cells in the mixed population increased. In the mice injected with 80% TK+ cells, solid tumor failed to grow in 75% of mice (data not shown). Since the transfection efficiency of recombinant virus is quite limited in clinical trials (Rincon et al. 2015), we simulated the clinical transfection rate by inoculating mixed cells containing 30% TK+ and 70% TK− B16 cells into mice. The data showed that single drug treatment (GCV or resveratrol) had certain inhibitory effects. In comparison, the combination of GCV and resveratrol had an enhanced killing effect. The significant difference between the GCV-alone group and resveratrol plus GCV group (p < 0.01) indicated that resveratrol synergistically promotes the anti-cancer effect of TK/GCV in vivo.
In summary, our findings suggest that resveratrol might be a promising adjuvant of TK suicide gene therapy. It would be perspective to use the natural product to enhance the effect of suicide gene therapy and play a synergistic role in tumor suppression.
Materials and methods
Cell culture and reagents
The B16 melanoma cell line was purchased from the experimental animal center of Sun Yat-Sen University. All cells were cultured in RPMI 1640 medium with 10% fetal bovine serum, penicillin and streptomycin (100 U/mL each). For the in vitro experiments, resveratrol (> 98% pure, high-performance liquid chromatography [HPLC]-grade, R5010) and GCV (≥ 99% pure, SML2346) were purchased from Sigma. For the in vivo experiments, GCV (≥ 99% pure, #1303212) was purchased from Hubei Huatong Qianlong Pharmaceutical Co., Ltd.; resveratrol (≥ 99% pure, #031M5204V) was purchased from the Nanjing Institute of Traditional Chinese Medicine.
Plasmid construction and generation of stable cell lines
To generate the construct with thymidine kinase gene, pLXSN-GFP was firstly constructed by subcloning the GFP gene from pEGFP-N1 into the XhoI–BamHI site of the retroviral vector pLXSN, whereas pLXSN-RFP was established by subcloning RFP gene from pRFP-N1 into the EcoRI–BamHI site of the same vector. Then, the thymidine kinase gene without termination codon from pIC19R/MC1-tk (Mansour et al. 1988) was subcloned into the EcoRI–XhoI site of to establish the construct pLXSN-TK-GFP, which expresses TK protein fused with GFP. The constructs pLXSN-TK, pLXSN -RFP, and pLXSN-TK-GFP, were respectively transfected into the packaging cell line PT67 cells in DMEM for 2 days, followed by G418 screening for 2 weeks. The G418-resistant clones were trypsinized for expanded culture to obtain the PT67 cells with the recombinant constructs, which were named PT67/TK, PT67/RFP, PT67/TK-GFP, respectively. B16 cells were infected by the active retrovirus particle excreted by the packaging cell lines, respectively, followed by G418 screening for 2–4 weeks. The clones with highest resistance to G418 were obtained and identified as B16TK, B16RFP, B16TK−GFP cells, respectively. All recombinant constructs were verified by sequencing. The stable cell lines were verified by PCR with primers of the inserted genes, and the genomic DNA as the template. Location of GFP and RFP, as well as the enzyme activity of TK, were also detected to confirm the result (Du et al. 2004; Zeng et al. 2014).
MTT assay
B16 cells were seeded into 96-well plates at 4 × 103 cells/well and cultured overnight. Adherent cells were treated in sextuplicate with resveratrol (0 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM) for 48 h, followed by the MTT assay. Absorbance values were measured at 490 nm with a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
High content screening (HCS) assay
Cells with the indicated treatments were stained with Hoechst 33342 solution (final concentration: 5 µg/ml; Meilun, Dalian, China, MA0126) for 10 min, followed by rinsed with PBS twice. The images were taken by the IN Cell Analyzer 2000 HCS system (GE Healthcare, USA) and analyzed with the IN Cell Analyzer Workstation. The excitation and emission filters for Hoechst was 350 nm and 461 nm, respectively. The cell numbers were counted by the nuclei counts, parameter of which were set with the sensitivity of 60 and the minimum area of 65 μm2 to exclude the dead and apoptotic cells. Nine image fields per well were acquired using the 20 × objective. Resulting values were normalized to the fold change in cell numbers compared to the ctrl group.
Analysis of GJIC
B16 cells were seeded into 6-well plates and treated with resveratrol (2.5 μM, 5 μM, 10 μM, 20 μM) in duplicate for 48 h. Then the cells of one replicate were staineded with CMTMR (final concentration: 2.5 μM) plus calcein (final concentration: 0.1 µg/ml) in serum-free medium for 1 h, which were deemed as the donor cells. By contrast, cells in another replicate were not subjected to staining, and were referred as recipient cells. The donor cells and recipient cells were trypsinized respectively and mixed at a ratio of 5:95. The mixed cells were seeded into dishes for 4 h, and then trypsinized for assessment by flow cytometry.
Western blotting
B16 cells (2 × 105 cells) were seeded into six-well plates overnight and treated with resveratrol (5 μM, 10 μM, 20 μM) for 48 h. The protein samples of each group were collected in 1 × sodium dodecyl sulfate (SDS) loading buffer, separated with 12% SDS–polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were incubated with primary antibody against Cx32 (ABclonal, Wuhan, China, A10112) and Cx43 (Cell Signaling Technology, Danvers, MA, USA, 3512S) at 4 °C overnight, and with the secondary antibody (Cell Signaling Technology, Danvers, MA, USA, #7074, #7076) at room temperature for 1 h. The bands were visualized with enhanced chemiluminescence.
Combinational effect of resveratrol and TK/GCV
B16TK cells and B16 cells were mixed at a 1:4 ratio and seeded into 96- or 6-well plates overnight. Then, the cells were treated with resveratrol (0 μM, 5 μM, 10 μM, 20 μM) or/and GCV (final concentration: 15.7 μM) for 48 h. Cells in 96-well plates were proceeded with MTT assay for measuring cell viability. Cells in 6-well plates were fixed with 70% ethanol at 4 °C, followed by the standard protocol of propidium iodide (PI) staining for analyzing the cell death rate with flow cytometry (Gong et al. 1994; Krishan and Cabana 2004).
Bystander effect analysis
Mixed cells (20% B16TK−GFP cells and 80% B16RFP cells) cultured in 6-well plates were treated with resveratrol (0 μM, 5 μM, 10 μM, 20 μM) and/or GCV (final concentration: 15.7 μM) for 48 h. Fluorescence signals were captured using a fluorescence microscope. Then the cells were trypsinized and stained with Annexin V for flow cytometry analysis.
AGA treatment
B16TK cells and wild-type B16 cells were mixed in a 1:4 ratio and seeded into 96-well plates. After overnight culture, the cells were treated with resveratrol (MTT final concentration: 10 μM; HCS final concentration: 5 μM, 10 μM), GCV (final concentration: 15.7 μM), AGA (final concentration: 15 μM), or combinational treatment as indicated for 48 h, followed by MTT and HCS assay measurement of cell viability.
Animals
Pathogen-free C57BL/6 J mice (18–22 g) were purchased from the laboratory animal center of Sun Yat-Sen University. The following studies were performed at the Guangzhou University of Chinese Medicine animal center, and all studies were approved by the Institute Research Medical Ethics Committee of the Guangzhou University of Chinese Medicine.
B16TK cells and B16 cells were mixed at a 3:7 ratio and subcutaneously inoculated the right flank of the mice (2 × 105 cells). The mice were randomly divided into six groups (n = 15 per group): control (saline only), GCV only, resveratrol only (100 mg/kg), resveratrol (100 mg/kg) + GCV, resveratrol only (200 mg/kg), and resveratrol (200 mg/kg) + GCV. Resveratrol treatment was initiated on day 2 of cell injection and administered once daily consecutively for 14 days; GCV (50 mg/kg/day) treatment started on day 9 after inoculation and was administered once daily for 7 days. The tumor volume was measured from day 13 to day 16 with Vernier calipers. The tumor volume was calculated as follows: A × B2 (A, long diameter; B, short diameter). On day 16, the tumors were dissected from the sacrificed mice and weighed.
Statistical analysis
SPSS 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis, and the quantitative data were expressed as mean ± standard deviation (SD). Quantitative data between groups were compared using one-way analysis of variance (ANOVA); p < 0.05 was considered statistically significant.
The synergistic effect of resveratrol plus TK/GCV was evaluated using the Q value (Jin 2004), where Q = EAB/[EA + EB (1 − EA)]. In the equation, EA, EB and EAB represent the effect of drug A, drug B and the combination of two drugs, respectively. Q < 0.85 indicates antagonism, 0.85 < Q < 1.15 indicates an additive effect, and Q > 1.15 indicates a synergistic effect.
References
Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW (2016) Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer 16:775–788. https://doi.org/10.1038/nrc.2016.105
Aasen T, Mesnil M, Naus CC, Lampe PD, Laird DW (2017) Gap junctions and cancer: communicating for 50 years. Nat Rev Cancer 17:74. https://doi.org/10.1038/nrc.2016.142
Ammerpohl O, Thormeyer D, Khan Z, Appelskog IB, Gojkovic Z, Almqvist PM, Ekstrom TJ (2004) HDACi phenylbutyrate increases bystander killing of HSV-tk transfected glioma cells. Biochem Biophys Res Commun 324:8–14. https://doi.org/10.1016/j.bbrc.2004.09.016
Bodendiek SB, Raman G (2010) Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem 17:4191–4230. https://doi.org/10.2174/092986710793348563
Cottin S, Ghani K, Caruso M (2008) Bystander effect in glioblastoma cells with a predominant cytoplasmic localization of connexin43. Cancer Gene Ther 15:823–831. https://doi.org/10.1038/cgt.2008.49
Du B, Tan Y, Wu Y, Zhao P, Zhou L, Zhao Y (2004) Retroviral vector introduced in the construction of HSV1-tk/GCV antitumor suicide-gene therapeutic system. J Guangzhou Univ Trad Chin Med 21:395–398
Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA (2019) The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci 20:904. https://doi.org/10.3390/ijms20040904
Faneca H, Duzgunes N, Pedroso de Lima MC (2019) Suicide gene therapy for oral squamous cell carcinoma. Methods Mol Biol 1895:43–55. https://doi.org/10.1007/978-1-4939-8922-5_4
Gong J, Traganos F, Darzynkiewicz Z (1994) A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem 218:314–319. https://doi.org/10.1006/abio.1994.1184
Guan X, Wilson S, Schlender KK, Ruch RJ (1996) Gap-junction disassembly and connexin 43 dephosphorylation induced by 18 beta-glycyrrhetinic acid. Mol Carcinog 16:157–164. https://doi.org/10.1002/(sici)1098-2744(199607)16:3%3c157:Aid-mc6%3e3.0.Co;2-e
Hou CY, Tain YL, Yu HR, Huang LT (2019) The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci 20:535. https://doi.org/10.3390/ijms20030535
Jin ZJ (2004) About the evaluation of drug combination. Acta Pharmacol Sin 25:146–147
Karjoo Z, Chen X, Hatefi A (2016) Progress and problems with the use of suicide genes for targeted cancer therapy. Adv Drug Deliv Rev 99:113–128. https://doi.org/10.1016/j.addr.2015.05.009
Krishan A, Cabana R (2004) Flow cytometric analysis of electronic nuclear volume and DNA content in normal mouse tissues. Cell Cycle 3:380–383
Luebker SA, Koepsell SA (2019) Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol 9:268. https://doi.org/10.3389/fonc.2019.00268
Mansour SL, Thomas KR, Capecchi MR (1988) Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336:348–352. https://doi.org/10.1038/336348a0
Matono S, Tanaka T, Sueyoshi S, Yamana H, Fujita H, Shirouzu K (2003) Bystander effect in suicide gene therapy is directly proportional to the degree of gap junctional intercellular communication in esophageal cancer. Int J Oncol 23:1309–1315
Mesnil M, Yamasaki H (2000) Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: role of gap-junctional intercellular communication. Cancer Res 60:3989–3999
Navarro SA, Carrillo E, Grinan-Lison C, Martin A, Peran M, Marchal JA, Boulaiz H (2016) Cancer suicide gene therapy: a patent review. Expert Opin Ther Pat 26:1095–1104. https://doi.org/10.1080/13543776.2016.1211640
Oliveira ALB et al (2017) Resveratrol role in autoimmune disease—a mini-review. Nutrients 9:1306. https://doi.org/10.3390/nu9121306
Pelin K, Hirvonen A, Linnainmaa K (1994) Expression of cell adhesion molecules and connexins in gap junctional intercellular communication deficient human mesothelioma tumour cell lines and communication competent primary mesothelial cells. Carcinogenesis 15:2673–2675. https://doi.org/10.1093/carcin/15.11.2673
Rauf A, Imran M, Suleria HAR, Ahmad B, Peters DG, Mubarak MS (2017) A comprehensive review of the health perspectives of resveratrol. Food Funct 8:4284–4305. https://doi.org/10.1039/c7fo01300k
Rincon MY, VandenDriessche T, Chuah MK (2015) Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res 108:4–20. https://doi.org/10.1093/cvr/cvv205
Robe PA, Nguyen-Khac M, Jolois O, Rogister B, Merville MP, Bours V (2005) Dexamethasone inhibits the HSV-tk/ganciclovir bystander effect in malignant glioma cells. BMC Cancer 5:32. https://doi.org/10.1186/1471-2407-5-32
Sinha D, Sarkar N, Biswas J, Bishayee A (2016) Resveratrol for breast cancer prevention and therapy: preclinical evidence and molecular mechanisms. Semin Cancer Biol 40–41:209–232. https://doi.org/10.1016/j.semcancer.2015.11.001
Tanaka T, Yamasaki H, Mesnil M (2001) Stimulation of intercellular communication of poor-communicating cells by gap-junction-competent cells enhances the HSV-TK/GCV bystander effect in vitro. Int J Cancer 91:538–542. https://doi.org/10.1002/1097-0215(200002)9999:9999%3c:aid-ijc1080%3e3.0.co;2-z
Wu X, Li Q, Feng Y, Ji Q (2018) Antitumor research of the active ingredients from traditional Chinese medical plant polygonum cuspidatum. Evid Based Complement Altern Med 2018:2313021. https://doi.org/10.1155/2018/2313021
Yan TL, Bai LF, Zhu HL, Zhang WM, Lv PC (2017) Synthesis and biological evaluation of glycyrrhetic acid derivatives as potential VEGFR2 inhibitors. ChemMedChem 12:1087–1096. https://doi.org/10.1002/cmdc.201700271
Yousef M, Vlachogiannis IA, Tsiani E (2017) Effects of resveratrol against lung cancer: in vitro and in vivo studies. Nutrients. https://doi.org/10.3390/nu9111231
Zeng L et al (2014) Construction of recombinant HSV1-tkGFP/GCV fluorescent protein monitoring system and its inhibitory effect on murine melanoma. Trad Chin Drug Res Clin Pharmacol 25:546–551
Zhao Y, de Toledo SM, Hu G, Hei TK, Azzam EI (2014) Connexins and cyclooxygenase-2 crosstalk in the expression of radiation-induced bystander effects. Br J Cancer 111:125–131. https://doi.org/10.1038/bjc.2014.276
Zulueta A, Caretti A, Signorelli P, Ghidoni R (2015) Resveratrol: a potential challenger against gastric cancer. World J Gastroenterol 21:10636–10643. https://doi.org/10.3748/wjg.v21.i37.10636
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81774028, 81873146, 81903606), and Natural Science Foundation of Guangdong Province (2017A030310542).
Author information
Authors and Affiliations
Contributions
YT and BD conceived and designed the study, provided technical guidance during experiments. YC, HL, YW, GZ, JL, PQ and LS carried out in vitro experiments and analyzed experimental results. YW, XL, JL and YT carried out in vivo experiments and analyzed experimental results. YC, HL and YT wrote the paper. YT and JX reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, H., Zhang, G. et al. Synergistic inhibitory effect of resveratrol and TK/GCV therapy on melanoma cells. J Cancer Res Clin Oncol 146, 1489–1499 (2020). https://doi.org/10.1007/s00432-020-03203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03203-z